2021 年 44 巻 3 号 p. 422-430
2021 年 44 巻 3 号 p. 422-430
Glucosyl hesperidin (GH) is a water-soluble derivative of hesperidin, a citrus flavonoid. GH has various pharmacological effects, such as hypolipidemic and hypouricemic effects, and may therefore be a useful supplement or drug. In the present study, we evaluated the effects of long- and short-term intake of GH on hyperglycemia and macrophage infiltration into the adipose tissue of high-fat diet (HFD)-fed mice. Long-term (11-week) consumption of GH tended to reduce body weight and the fasting blood glucose concentration of the HFD-fed mice, and ameliorated glucose intolerance and insulin resistance, according to glucose and insulin tolerance tests. Additionally, although GH did not affect fat pad weight, it reduced HFD-induced macrophage infiltration into adipose tissue. Short-term (2-week) consumption of GH did not affect the HFD-induced increases in body weight or fasting blood glucose, and it did not ameliorate glucose intolerance or insulin resistance. However, short-term intake did reduce the HFD-induced macrophage infiltration and monocyte chemotactic protein 1 (MCP-1) expression in adipose tissue. Furthermore, hesperetin, which is an aglycone of GH, inhibited MCP-1 expression in 3T3-L1 adipocytes, 3T3-L1 adipocytes co-cultured with RAW264 macrophages, and tumor necrosis factor-α-treated 3T3-L1 adipocytes. The present findings suggest that daily consumption of GH may have preventive and/or therapeutic effects on obesity-related diseases, such as diabetes mellitus.
Hesperidin is a citrus flavonoid that has various pharmacological effects, including antioxidative, anti-inflammatory, antidiabetic, antihypertensive, and antihyperlipidemic effects.1) However, its use as a drug or supplement is limited because it has low solubility in water and poor bioavailability after oral administration. Nonetheless, glucosyl hesperidin (GH), a hesperidin derivative, which is synthesized from hesperidin and α-glucosyl saccharide by a saccharide-transferring enzyme, is about 10000 times more soluble in water2) and is absorbed more rapidly and efficiently than hesperidin.3) GH is readily hydrolysable by α-glucosidases in vivo, yielding hesperidin and D-glucose.4) Furthermore, hesperidin is hydrolyzed by β-glucosidases to form a hesperetin aglycone before its absorption5,6) (Fig. 1). Previous studies have shown that GH ameliorates hypertriglyceridemia, hyperuricemia, and the progression of atherosclerosis in animal models and patients.7–10) Therefore, GH may be a new preventive and/or therapeutic agent for various diseases.
Obesity is a key underlying cause of type 2 diabetes mellitus. Adipose tissue is an endocrine organ, and balancing the secretion of inflammatory and anti-inflammatory factors is important for good health. However, obese adipose tissue overproduces inflammatory factors, such as tumor necrosis factor (TNF)-α, which contribute to the development of insulin resistance and type 2 diabetes mellitus.11) High-fat diet (HFD) induces adipocyte hypertrophy and increases chemokines expression. Interestingly, the increase in chemokines expression in adipose tissue is observed as early as a few days after the start of HFD-feeding in mice.12) Chemokines attract various immune cells, and the adipocyte-immune cells interaction augments the inflammation of the adipose tissue. Of these, macrophage infiltration into adipose tissue induced by monocyte chemoattractant protein-1 (MCP-1) and the subsequent interaction between adipocytes and macrophages play a crucial role in the development obesity-related insulin resistance.13–16) Therefore, a reduction in macrophage infiltration and MCP-1 expression in adipose tissue during the early stages of obesity may help prevent obesity-related diseases.
In the present study, we aimed to determine the effects of long-term (11-week) and short-term (2-week) intake of GH on hyperglycemia and macrophage infiltration into adipose tissue of HFD-fed mice, and to elucidate the mechanisms involved.
GH was kindly provided by HAYASHIBARA CO., LTD. (Okayama, Japan). We purchased hesperetin, dexamethasone (DEX), and isobutylmethylxanthine (IBMX) from Sigma-Aldrich (St. Louis, MO, U.S.A.), dimethyl sulfoxide (DMSO) from Nacalai Tesque (Kyoto, Japan), insulin from the Cell Science & Technology Institute (Sendai, Japan), Dulbecco’s modified Eagle’s medium (DMEM), bovine serum, fetal bovine serum (FBS), and phosphate-buffered saline (PBS) from Invitrogen (Carlsbad, CA, U.S.A.), and 50% glucose solution from Otsuka Pharmaceutical (Tokyo, Japan).
Animals and Animal CareThe experiments were approved by the animal care committee of Kyushu University of Health and Welfare (Approval Nos. 27-1-12, 28-1-09). Male C57BL/6J mice (age, 7 weeks; body weight, 20–22 g) were purchased from the Kyudo Animal Laboratory (Kumamoto, Japan). The mice were housed 5/cage, maintained at 24 ± 2 °C on a 12-h light–dark cycle, and acclimated to their environment for 1 week prior to experimental use. At 8 weeks of age, they were randomly allocated to the following groups: (1) standard-diet (STD)-fed mice that were administered distilled water (STD + water group); (2) HFD-fed mice that were administered distilled water (HFD + water group); (3) HFD-fed mice that were administered 0.063 (w/v)% GH solution (HFD +0.063% GH group); and (4) HFD-fed mice that were administered 0.125 (w/v)% GH solution (HFD +0.125% GH group).
Preliminary experiments revealed that the mice drank 3.21 ± 0.06 mL of water per day and that HFD-feeding increased the body weight of the mice from 21.42 ± 0.24 to 42.08 ± 1.31 g in 10 weeks. Additionally, in our previous studies, we have shown that the citrus flavonoid, naringenin (100 mg/kg/d), dissolved in methylcellulose reduced macrophage and neutrophil infiltration into the adipose tissue of mice.17,18) Therefore, we chose to use 0.063 and 0.125% (w/v) GH. This is because when the mouse weight is 20 and 40 g, respectively, the GH dose will be about 100 mg/kg/d. Mice were fed an STD (D12450B; Research Diets, New Brunswick, NJ, U.S.A.) or an HFD (D12492; Research Diets) and provided with distilled water or GH solution ad libitum for 2 or 11 weeks. The food and liquid intakes of the mice were measured every 2 or 3 d and their body weight was measured weekly. Their epididymal fat pads were collected after they were euthanized.
Oral Glucose Tolerance Test (OGTT)Mice were fasted for 18 h prior to OGTT. A blood sample was initially obtained from the lateral caudal vein and the fasting blood glucose concentration was measured. The mice were then administered glucose orally (2 mg/g) and further blood samples were collected 30, 60, 90, and 120 min after gavage. Blood glucose concentrations were determined using a GLUCOCARD G+ meter (Arkray, Kyoto, Japan). The fasting blood glucose concentrations of the STD group before the OGTT in the long-term and short-term intake experiments were 90.0 ± 6.7 and 76.6 ± 3.4 mg/dL, respectively.
Insulin Tolerance Test (ITT)The mice were fasted for 4 h before ITT. A blood sample was initially obtained from the lateral caudal vein and the fasting blood glucose concentration was measured. The mice were then administered insulin intraperitoneally (20 ng/g) and further blood samples were collected after 30, 60, 90, and 120 min. The fasting blood glucose concentrations of the STD group before the ITT in the long-term and short-term intake experiments were 135.4 ± 18.0 and 181.0 ± 17.2 mg/dL, respectively.
Preparation of the Stromal Vascular Fraction (SVF) of Adipose Tissue and Flow Cytometry AnalysisThe SVF was prepared from adipose tissue as previously described.17,19) Briefly, epididymal fat pads were cut into small pieces and rinsed in an N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid-based Ringer’s solution. The fat pieces were then incubated at 37 °C for 30 min in a collagenase type 1 solution. The SVF was collected by centrifugation and resuspended in Stain Buffer (containing FBS; BD Biosciences, Franklin Lakes, NJ, U.S.A.). After filtration through a 100-µm strainer, the SVF cells were counted and adjusted to 1 × 107 cells/mL. Aliquots containing 106 cells were then incubated with Fc block CD16/32 (clone 2.4G2; BD Biosciences) at 4 °C overnight, and then stained with fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated antibodies at 4 °C for 30 min. The labeled cells were analyzed by a FACSCalibur (BD Biosciences). The antibodies used were FITC rat anti-mouse CD11b (M1/70), PE rat anti-mouse F4/80 (T45-2342), FITC rat immunoglobulin G (IgG)2b κ isotype control, and rat IgG2a κ isotype control (all from BD Biosciences).
Real-Time PCR AnalysisEpididymal fat pad tissue (100 mg) and cultured cells were lysed with TRIzol reagent (Invitrogen) to isolate RNA. Reverse transcription and PCR amplification were performed using the following reagents: ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan) and TaqMan Fast Advanced Master Mix (Applied Biosystems, Carlsbad, CA, U.S.A.). The primers and TaqMan probes were: MCP-1 (CCL2), Mm00441242-m1; MCP-2 (CCL8), Mm01297183-m1; MCP-3 (CCL7), Mm00443113_m1; MIP-1α (CCL3), Mm00441259_g1; regulated upon activation, normal T cell expressed and presumably secreted (RANTES; CCL5), Mm01302427_m1; and 18S (Rn18s), Mm03928990-g1 (all from Applied Biosystems). The relative mRNA expression was determined using the comparative Ct method and was normalized to the 18S ribosomal RNA value as a reference gene.
Cell Culture and Treatments3T3-L1 cells (Health Science Research Resources Bank, Osaka, Japan) and RAW264 cells (RIKEN BioResource Center, Tsukuba, Japan) were maintained in DMEM supplemented with bovine serum or FBS as previously described.18) 3T3-L1 pre-adipocytes were differentiated into mature adipocytes by 0.5 mM IBMX, 0.25 µM DEX, and 5 µg/mL insulin.18) Adipocytes and macrophages were co-cultured in a contact system, as described previously.15) Briefly, RAW264 cells (1 × 105 cells/mL) were seeded into 12-well plates with differentiated 3T3-L1 adipocytes. In each experiment, we treated the cells with hesperetin or TNF-α and used 0.5% DMSO as the vehicle control.
Enzyme-Linked Immunosorbent Assay (ELISA)MCP-1 concentrations were measured using an ELISA kit (88-7391, eBioscience, Santa Clara, CA, U.S.A.), according to the manufacturer’s instructions. The lower detection limit of the kit was 15 pg/mL.
The data are presented as mean ± standard error and analyses were performed using Prism 8 (GraphPad, San Diego, CA, U.S.A.). Differences between groups were assessed using one-way ANOVA or two-way ANOVA with post-hoc Bonferroni’s tests. Differences between groups were considered significant when p < 0.05.
To determine whether long-term GH intake has anti-diabetic effects, we administered the HFD-fed mice GH for 11 weeks. HFD feeding significantly increased the body weight of the mice compared with the STD feeding. Consumption of 0.063% GH did not affect this HFD-induced increase in body weight, but consumption of 0.125% GH tended to reduce the gain (Fig. 2A). Additionally, 0.125% GH reduced the mean daily liquid intake of the mice (Fig. 2B). However, there was no significant difference in the mean daily food intake among the groups (Fig. 2C). After 11 weeks of HFD-feeding the fasting blood glucose concentration of the mice was high, but GH significantly ameliorated this increase (Fig. 2D). Furthermore, the long-term consumption of GH ameliorated the glucose intolerance and insulin resistance of the HFD-fed mice, as shown by the OGTT and ITT data (Figs. 2E, F). Next, we examined the effects of GH on macrophage infiltration into the epididymal fat pad. HFD-feeding significantly increased the fat pad weight. However, GH did not affect this increase (Fig. 3A). Additionally, HFD-feeding increased macrophage infiltration into this adipose depot, and this effect was significantly ameliorated by long-term GH consumption (Figs. 3B, C).
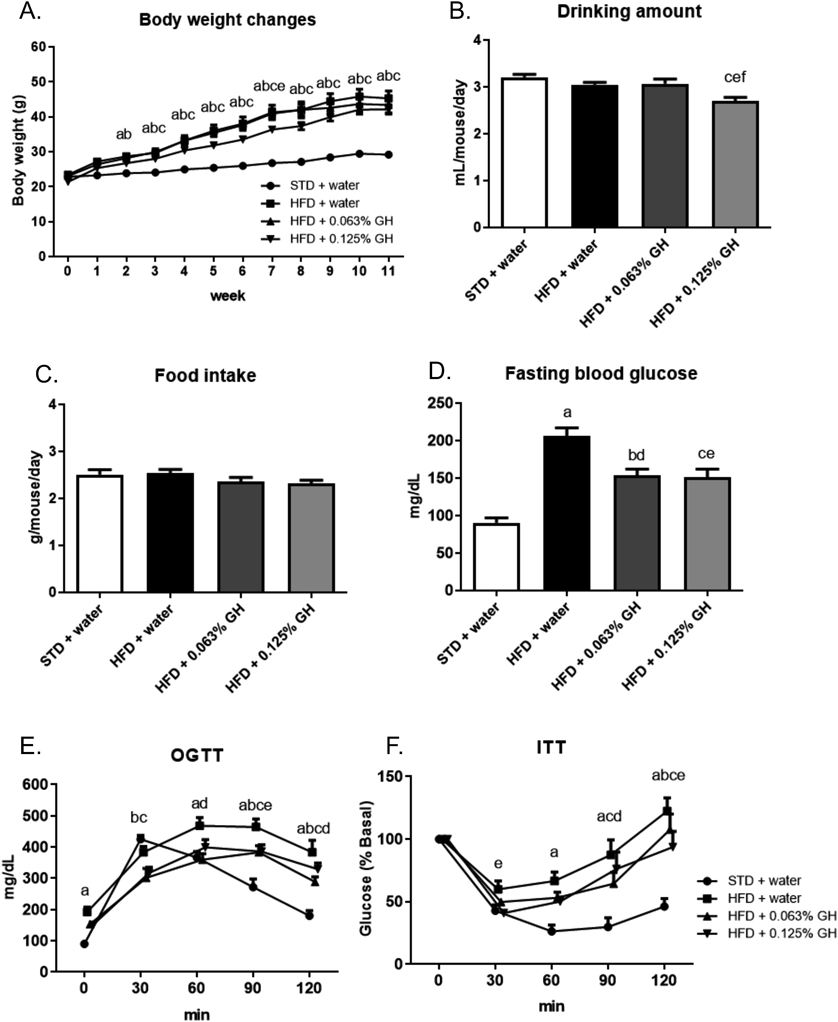
C57BL/6J mice (n = 5) were used. (A) Changes in body weight during the experimental period. (B) Mean daily water intake. (C) Mean daily food intake. (D) Fasting blood glucose concentration after 11 weeks of HFD-feeding and GH consumption. The fasting period was 18 h. (E) OGTT data. (F) ITT data. The blood glucose concentrations are expressed as percent of basal glycemia. a p < 0.05 for STD + water vs. HFD + water. b p < 0.05 for STD + water vs. HFD +0.063% GH. c p < 0.05 for STD + water vs. HFD +0.125% GH. d p < 0.05 for HFD + water vs. HFD +0.063% GH. e p < 0.05 for HFD + water vs. HFD +0.125% GH. f p < 0.05 for HFD +0.063% GH vs. HFD +0.125% GH. STD, standard diet; HFD high-fat diet; GH, glucosyl hesperidin; OGTT, oral glucose tolerance testing; ITT, insulin tolerance testing.

C57BL/6J mice (n = 5) were used. (A) Epididymal fat pad weight after 11 weeks of HFD-feeding and GH consumption. (B) Flow cytometry analysis of macrophages from the SVF of epididymal fat. (C) Flow cytometry analysis data. a p < 0.05 for STD + water vs. HFD + water. b p < 0.05 for STD + water vs. HFD +0.063% GH. c p < 0.05 for STD + water vs. HFD +0.125% GH. d p < 0.05 for HFD + water vs. HFD +0.063% GH. e p < 0.05 for HFD + water vs. HFD +0.125% GH. STD, standard diet; HFD high-fat diet; GH, glucosyl hesperidin.
To determine the effects of GH in the early stages of obesity, we administered mice HFD and GH for 2 weeks. HFD-feeding increased their body weight, but this parameter was not affected by GH (Fig. 4A). In addition, neither HFD-feeding nor GH affected the mean daily water intake of the mice (Fig. 4B) and there was no significant difference in the mean daily food intake among the groups (Fig. 4C). HFD-feeding significantly increased fasting blood glucose, but this change was not affected by GH administration (Fig. 4D). Furthermore, HFD-feeding induced hyperglycemia and insulin resistance in the mice, but there were no significant differences in the OGTT or ITT glucose curves between GH-treated and untreated HFD-fed mice (Figs. 4E, F). Finally, HFD-feeding increased the fat pad weight, but GH did not affect this parameter (Fig. 5A). However, 0.125% GH intake significantly reduced the HFD-induced increase in macrophage infiltration into adipose tissue (Figs. 5B, C).
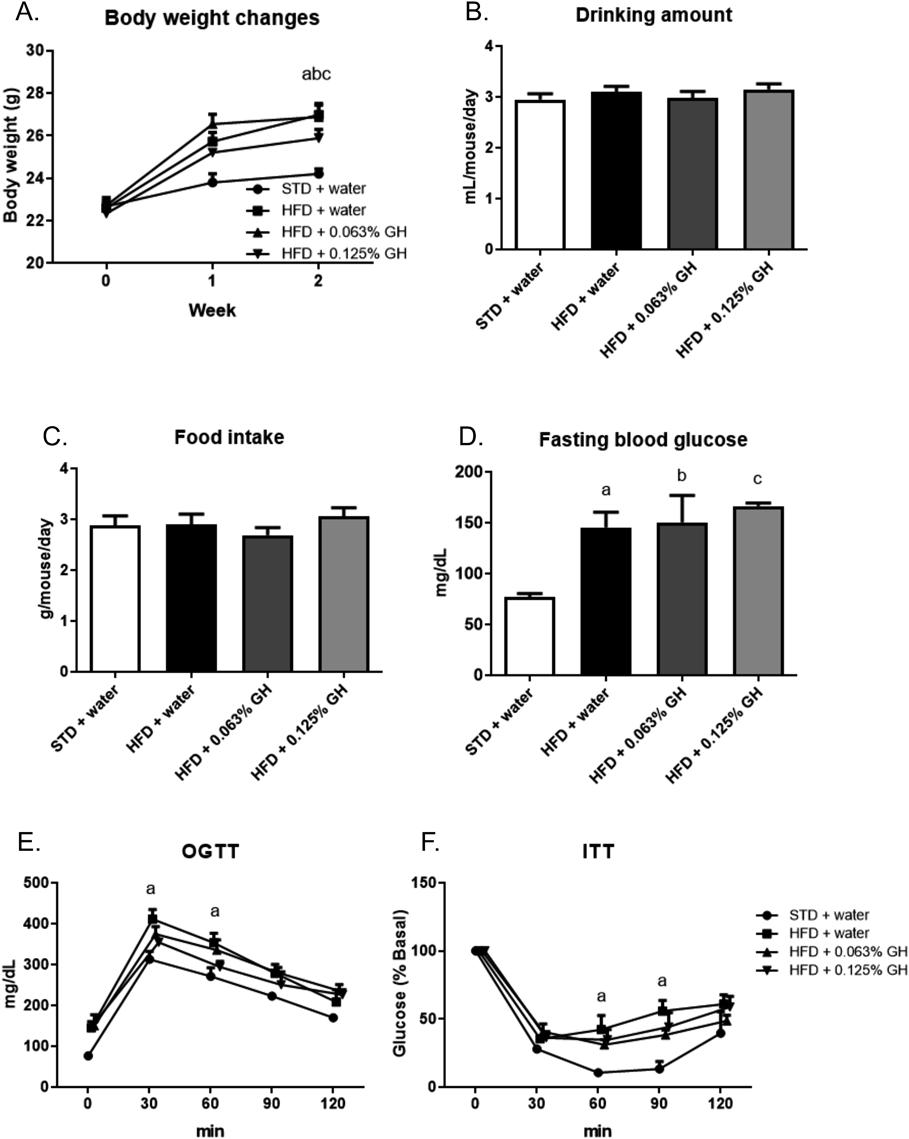
C57BL/6J mice (n = 5) were used. (A) Changes in body weight during the experimental period. (B) Mean daily water intake. (C) Mean daily food intake. (D) Fasting blood glucose concentration after 2 weeks of HFD-feeding and GH consumption. The fasting period was 18 h. (E) OGTT data. (F) ITT data. Blood glucose concentrations are expressed as percent basal glycemia. a p < 0.05 for STD + water vs. HFD + water. b p < 0.05 for STD + water vs. HFD +0.063% GH. c p < 0.05 for STD + water vs. HFD +0.125% GH. STD, standard diet; HFD high-fat diet; GH, glucosyl hesperidin; OGTT, oral glucose tolerance testing; ITT, insulin tolerance testing.
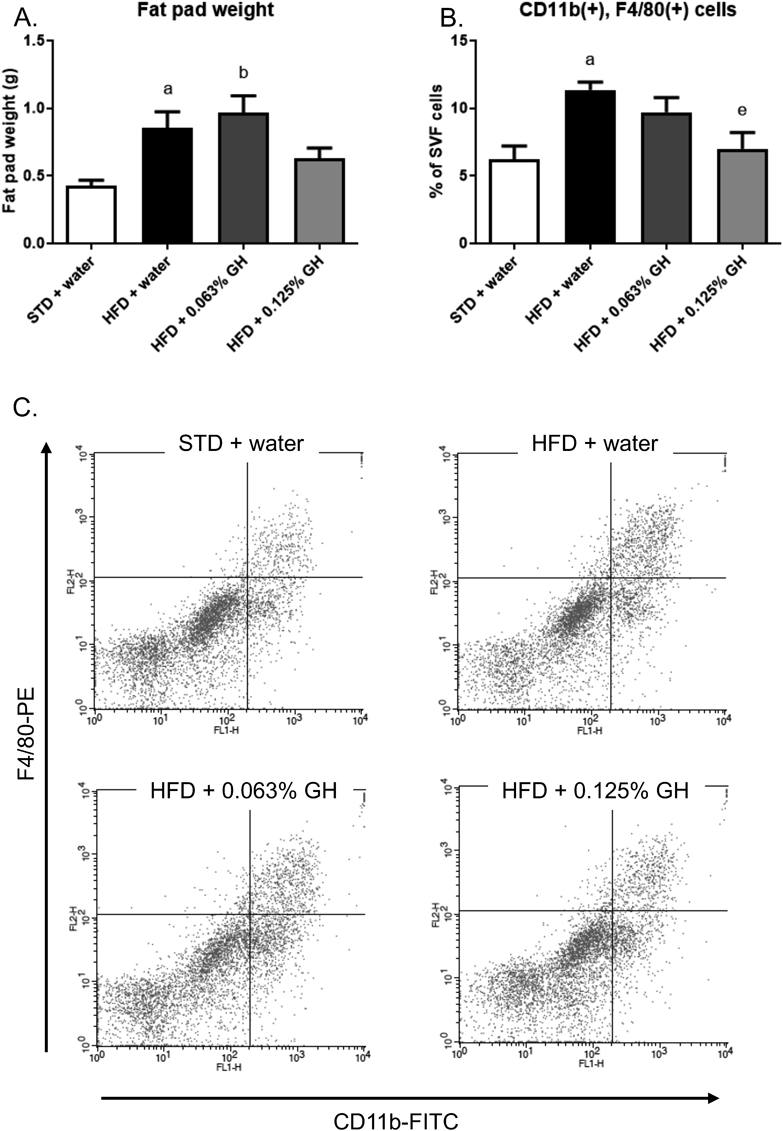
C57BL/6J mice (n = 5) were used. (A) Epididymal fat pad weight after 2 weeks of HFD-feeding and GH consumption. (B) Flow cytometry analysis of macrophages from the SVF of epididymal fat. (C) Flow cytometry analysis data. a p < 0.05 for STD + water vs. HFD + water. b p < 0.05 for STD + water vs. HFD +0.063% GH. e p < 0.05 for HFD + water vs. HFD +0.125% GH. STD, standard diet; HFD high-fat diet; GH, glucosyl hesperidin.
To elucidate the mechanism by which GH ameliorates the HFD-induced macrophage infiltration into adipose tissue, we examined the effects of GH on the mRNA expression of chemokines (MCP-1, MCP-2, MCP-3, MIP-1α, and RANTES). As shown in Fig. 6, HFD-feeding for 2 weeks induced MCP-1 and MCP-3 expression in adipose tissue, but GH significantly reduced this expression. The expression levels of MCP-2 and MIP-1α were lower than that of MCP-1, and HFD-feeding did not further induce MCP-2 and MIP-1α expression. Finally, although the expression level of RANTES was similar to that of MCP-1, there were no significant differences in expression between the STD- and HFD-fed groups.
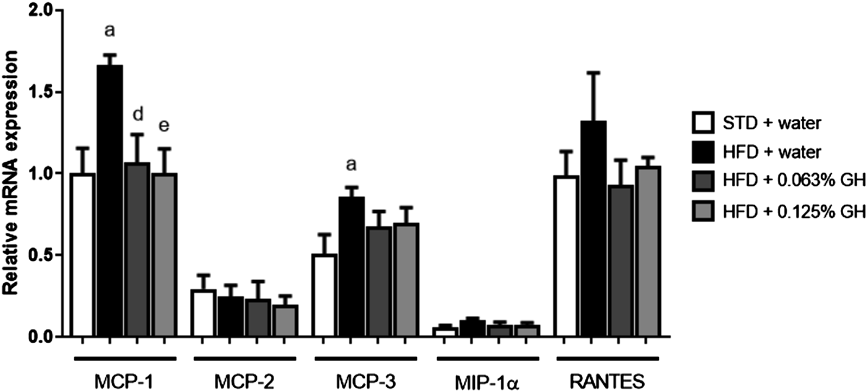
C57BL/6J mice (n = 5) were used. Real-time PCR analysis of MCP-1, MCP-2, MCP-3, MIP-1α, and RANTES mRNA expression. a p < 0.05 for STD + water vs. HFD + water. d p < 0.05 for HFD + water vs. HFD +0.063% GH. e p < 0.05 for HFD + water vs. HFD +0.125% GH. STD, standard diet; HFD high-fat diet; GH, glucosyl hesperidin.
To examine the effects of GH on MCP-1 expression in cellular level, we treated 3T3-L1 adipocytes with hesperetin, an aglycone of GH. Hesperetin reduced MCP-1 mRNA expression and protein secretion in a dose-dependent manner (Figs. 7A, B). In addition, to determine the effects of hesperetin under conditions reminiscent of obese adipose tissue, we studied its effects in a co-culture of 3T3-L1 adipocytes and RAW264 macrophages and in TNF-α-treated 3T3-L1 adipocytes. The co-culture was associated with an induction of MCP-1 expression, but hesperetin significantly suppressed this expression (Figs. 7C, D). Furthermore, hesperetin suppressed the TNF-α-induced expression of MCP-1 in 3T3-L1 adipocytes (Figs. 7E, F).
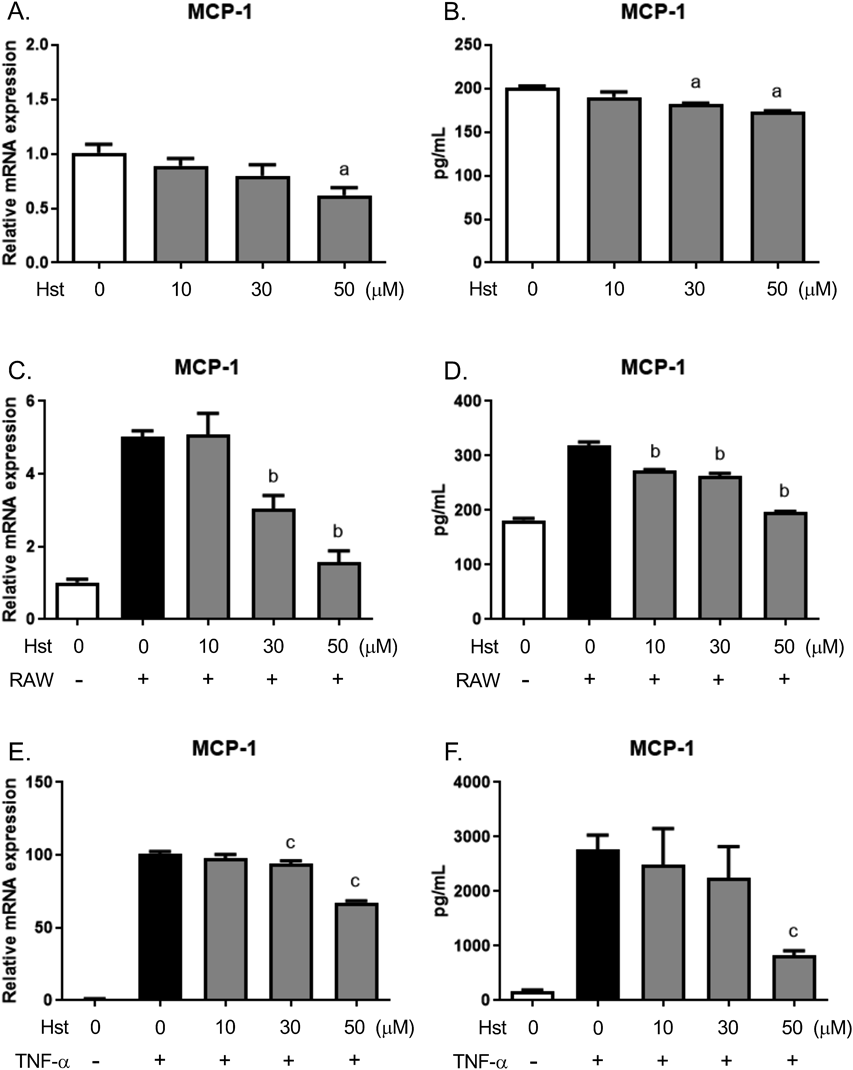
(A) Real-time PCR analysis of MCP-1 expression in 3T3-L1 adipocytes treated with hesperetin for 3 h. (B) MCP-1 concentration in the culture media of 3T3-L1 adipocytes treated with hesperetin for 6 h, measured by ELISA. (C) MCP-1 mRNA expression in co-cultures of 3T3-L1 adipocytes and RAW264 cells, measured using RT-PCR. 3T3-L1 adipocytes were pre-treated with hesperetin for 30 min, and then co-cultured with RAW264 cells for 3 h. (D) The MCP-1 concentration in the media of 3T3-L1 adipocytes co-cultured with RAW264 cells for 6 h. (E) MCP-1 expression in 3T3-L1 adipocytes treated with hesperetin and/or TNF-α, measured using RT-PCR. 3T3-L1 adipocytes were pre-treated with hesperetin for 30 min, and then treated with TNF-α (10 ng/mL) for 3 h. (F) MCP-1 concentration in the culture media of 3T3-L1 adipocytes treated with hesperetin and/or TNF-α for 6 h, measured by ELISA. a p < 0.05 vs. vehicle control. b p < 0.05 vs. vehicle-treated co-culture. c p < 0.05 vs. TNF-α-treatment alone. Hst, hesperetin; RAW, RAW264 cells.
Because previous studies have shown that GH has various pharmacological effects, including hypolipidemic and hypouricemic effects,7–10) its potential for use as a supplement or drug is of great interest. In the present study, to further characterize its potential usefulness for disease treatment or prevention, we explored the effects of GH on the HFD-induced hyperglycemia and macrophage infiltration into adipose tissue in mice. We found that long-term (11-week) GH consumption ameliorated both the hyperglycemia and macrophage infiltration into adipose tissue induced by HFD-feeding (Figs. 2, 3). Furthermore, although short-term (2-week) GH consumption did not affect the blood glucose concentration of the mice, it did significantly reduce the macrophage infiltration and MCP-1 expression in the adipose tissue of mice in the early stages of the development of obesity (Figs. 4–6). Moreover, hesperetin, a GH aglycone, inhibited the induction of MCP-1 expression in 3T3-L1 adipocytes, 3T3-L1 adipocytes co-cultured with RAW 264 macrophages, and TNF-α-treated 3T3-L1 adipocytes (Fig. 7). These results suggest that daily GH intake has a preventive effect against HFD-induced hyperglycemia by inhibiting macrophage infiltration and MCP-1 expression in adipose tissue.
We also found that the long-term intake of 0.125% GH reduced the mean daily water intake by the mice (Fig. 2B). Water intake is affected, among other things, by the urine output and the degree of water reabsorption through aquaporin in the kidney. Previous studies have shown that several flavonoids and polyphenols, such as rutin and butein, have nephroprotective effects in rat renal injury models and increase aquaporin expression in kidney tissue.20,21) Currently, it is unknown whether GH, hesperidin, or hesperetin affect aquaporin expression in the kidney. However, previous studies have shown that hesperidin and hesperetin have protective effects against renal injury in rats by reducing oxidative stress and inflammation.22,23) Further studies are required to determine the effects of hesperidin and hesperetin on aquaporin expression and renal function, to identify the exact mechanisms by which GH reduces water intake.
Short-term GH intake did not decrease the HFD-induced body weight increase (Fig. 4A). However, the 0.125% GH intake tended to decrease the HFD-induced increase in fat pad weight (Fig. 5A). In contrast, long-term GH intake did not affect the HFD-induced increase in fat pad weight (Fig. 3A). Thus, the 2-week intake of HFD and GH may not be long enough to show clear results because of individual variation. Unfortunately, we did not measure the weight of other organs in the present study. However, it has been reported that GH did not affect the liver weight in HFD-induced obese KK mice.24) These results suggest that GH regulates the tissue or cell function without quantitative changes.
HFD increases the expression of various chemokines in adipose tissue and induces immune cell infiltration. These changes commence as early as a few days following the start of HFD feeding.12) We found that HFD feeding increased the expression of MCP-1 and MCP-3 in the adipose tissue of mice in the early stages of the development of obesity and that GH tended to ameliorate the HFD-induced MCP-3 expression (Fig. 6). MCP-3 has chemotactic effects on macrophages and neutrophils.16,25,26) Therefore, GH may have an anti-inflammatory effect in adipose tissue by regulating the functions of a broad range of immune cells.
We also showed that hesperetin, an GH aglycone, reduced the basal expression of MCP-1 in adipocytes and the MCP-1 expression in adipocytes that were subjected to an inflammatory stimulus (Fig. 7). However, GH administration did not completely suppress macrophage infiltration into the adipose tissue of mice in the early stages of obesity (Fig. 5B). These results suggest that GH reduces the high MCP-1 expression that is associated with the excess macrophage infiltration and inflammation that characterizes obesity, and thereby prevents further exacerbation of macrophage infiltration and inflammation.
Orally administrated GH is hydrolyzed by glucosidases to form a hesperetin aglycone before its absorption.4–6) A previous study has shown that GH was not detected in the sera of rats administered GH, whereas hesperetin was detected.3) Additionally, hesperetin is mainly present in the blood circulation in the form of glucuronide conjugates.6) After a single oral administration of GH (1 mmol/kg) to rats, the mean peak concentration of hesperetin-glucuronide was 6.3 µM.3) In the present study, we showed that 30 or 50 µM hesperetin significantly suppressed MCP-1 expression in vitro (Fig. 7). There is a discrepancy between the blood concentrations of hesperetin and the concentrations that affect cells. However, we also showed that a repeated intake of GH suppressed MCP-1 expression and macrophage infiltration in mouse adipose tissue (Figs. 3, 5, 6). There are reports that can explain these discrepancies. Quercetin, a typical flavonoid in vegetables, is metabolized after oral intake into glucuronide conjugates. Quercetin–glucuronide has been shown to accumulate at inflammation sites such as atherosclerotic lesions, followed by deconjugation into active aglycone by macrophage-derived β-glucuronidase.27,28) Thus, inflamed local tissues and cells may be exposed to higher concentrations of hesperetin than those found in the blood. Further studies using hesperetin–glucuronide will reveal the molecular actions of hesperetin in vivo and in vitro, and contribute to a better understanding of the anti-diabetic effect of GH.
The results of this study suggest that daily intake of GH has an anti-diabetic effect by inhibiting macrophage infiltration and MCP-1 expression in obese adipose tissue. Thus, we have provided evidence for the usefulness of GH against obesity-related diseases.
We are grateful to Ms. Yukiko Shimoda and the members of our laboratory (Department of Biochemistry, Kyushu University of Health and Welfare) for their kind support and helpful suggestions. This work was supported in part by a Grant-in-Aid for Scientific Research (Grant No. JP15K18949 to H. Yoshida) from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.