2021 年 4 巻 1 号 p. 12-16
2021 年 4 巻 1 号 p. 12-16
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are effective drugs against non-small cell lung cancer (NSCLC) cells harboring common EGFR mutations such as in-frame exon 19 deletions (Del19) and the exon 21 L858R point mutations (L858R). However, currently used EGFR-TKIs are less effective against cells showing uncommon EGFR mutations. Because there is less information available on the sensitivities of the uncommon EGFR mutations, it is important to evaluate the effect of EGFR-TKIs on uncommon mutations. In this study, we sought to establish H1299 NSCLC cell lines stably expressing mutated EGFRs having Del18, G719S, or L861Q that are uncommon mutations as well as Del19 or L858R common mutations, and evaluated whether these cell lines could be applied as a prediction system for the therapeutic effects of EGFR-TKIs. In fact, the levels of phosphorylated EGFR in these cell lines were assessed after treatment with various EGFR-TKIs (4 approved and 4 unapproved drugs). Gefitinib, erlotinib, afatinib, and osimertinib, approved drugs, were effective against Del19, L858R, and L861Q mutations. However, these EGFR-TKIs were less effective against G719S and Del18 mutations. The unapproved drugs neratinib and poziotinib were effective against Del19, L858R, Del18, and L861Q mutations. Interestingly, canertinib and sapitinib had effects against Del19, Del18, and L861Q mutations and no effect against L858R mutation. These results indicate that the established cell lines are suitable for assessing the effects of the EGFR-TKIs on EGFR mutations, including uncommon mutations, and that some of the EGFR-TKIs used are also effective against uncommon mutations.
Activating mutations in the epidermal growth factor receptor (EGFR) gene occur in approximately 40% of the patients with lung adenocarcinoma, among the Japanese population.1) The most common EGFR mutations are in‐frame exon 19 deletions (Del19) and the exon 21 L858R point mutations, which constitute approximately 90% of all EGFR mutations.2) Other EGFR mutations are named uncommon mutations, and include exon 18 point mutations in position G719 (G719A, -C or -S; 3% of EGFR mutations), in-frame exon 18 deletions (Del18), and exon 21 L861Q mutations (approximately 0.9% of EGFR mutations).
Several minor studies show that treatment with EGFR-tyrosine kinase inhibitors (TKIs) is ineffective in patients with non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations, compared with patients harboring common EGFR mutations.3-5) To date, both the experimental and clinical efficacies of various types of EGFR‐TKIs for the inhibition of phosphorylated EGFR encoded by common EGFR mutations have been reported; however, there is less information available on the sensitivities of the uncommon EGFR mutations.
In addition, information is difficult to obtain due to the low frequency of uncommon mutations, and the exclusion of these mutations in many clinical trials.6-8) Unlike common mutations, cell lines bearing a single uncommon mutation that are established for research are unavailable, whereas several cell lines harboring Del19 or L858R mutations are available for use, such as PC-9 and H3255. Furthermore, to assess the efficacy of EGFR-TKIs in studies using commercially available cell lines, we need to consider the genetic background of the cell lines. Therefore, in order to evaluate the effect of EGFR-TKIs on uncommon mutations, it is desirable to construct an experimental system comprising cells that show the same genetic background.
In this study, we focused on EGFR Del19 and L858R, which are common mutations, and Del18, G719S, and L861Q, which are uncommon mutations, and created an in vitro model, to determine the therapeutic window of EGFR-TKIs. In addition, we investigated the in vitro sensitivities of cells carrying these mutations, to various EGFR-TKIs (4 approved drugs; gefitinib, erlotinib, afatinib, osimertinib and 4 unapproved drugs in Japan; neratinib, poziotinib, canertinib, sapitinib).
Gefitinib, erlotinib, afatinib, osimertinib, neratinib, poziotinib, canertinib, and sapitinib were purchased from Selleck Chemicals (Houston, TX, USA). Puromycin was purchased from Wako Pure Chemical Industries (Osaka, Japan).
Plasmid ConstructionThe EGFR coding region was subcloned into a pIRESpuro vector (Clontech, Mountain View, CA, USA). The protein expression vectors used for the mutagenesis of EGFR (L858R, Del19, G719A, Del18, and L861Q) were constructed by using inverse PCR using the respective primers (Table 1).
| FW EGFR EcoRV | ATCACCATGCGACCCTCCGGGACGGCCGGGG |
| Rev EGFR NotI | GCGCGGCCGCTCATGCTCCAATAAATTCACT |
| FW EGFR L858R | GGGCCAAACTGCTGGGTG |
| Rev EGFR L858R | GCCCAAAATCTGTGATCT |
| FW EGFR Del19 | ACATCTCCGAAAGCCAACAAGGAAATCCTC |
| Rev EGFR Del19 | CTTGATAGCGACGGGAATTTTAACT |
| FW EGFR G719S | AGCTCCGGTGCGTTCGGCA |
| Rev EGFR G719S | CAGCACTTTGATCTTTTTG |
| FW EGFR Del18 | TGAATTCAAAAAGATCAAAGT |
| Rev EGFR Del18 | TCCTTCAAGATCCTCAAGAG |
| FW EGFR L861Q | AGCTGGGTGCGGAAGAGAAAGAATAC |
| Rev EGFR L861Q | GTTTGGCCAGCCCAAAATCTGTGATC |
The human embryonic kidney cell line HEK293, was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wako Pure Chemical Industries) containing 10% fetal bovine serum (FBS) and penicillin-streptomycin, in a humidified atmosphere containing 5% CO2 at 37°C. Using the PEI Max reagent (Polysciences Inc., Warrington, PA, USA), the cells were transfected with the appropriate expression vector. After 48 h of incubation, the cells were harvested for performing western blotting.
The human NSCLC cell line H1299, was cultured in DMEM containing 5% FBS and penicillin-streptomycin in a humidified 5% CO2 incubator at 37°C. Using the TransFectin transfection reagent (Bio-Rad, Hercules, CA, USA), the cells were transfected with the appropriate expression vector.
Establishment of Stable EGFR-mutant-overexpressing H1299 CellsIndividual stable EGFR-mutant-overexpressing H1299 cells were selected based on the observed puromycin resistance in limiting dilution assays, from H1299 cells transfected with the EGFR-mutant expression vectors (pIRESpuro containing L858R, Del19, G719S, Del18, and L861Q).
Cell ImagingCells were seeded at 15,000 cells/well in 24-well plates. On days 1 and 3, the cell morphologies were observed using an Axio Observer Z1 microscope (Carl Zeiss, Germany).
MTS Cell Proliferation AssayCells were seeded at 1500 cells/well in 96-well plates. On days 1, 3, and 5, the cell viability was evaluated using a CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Western Blot AnalysisCells were harvested and lysed in sodium dodecyl sulphate (SDS) sample buffer containing 125 mM Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 10% dithiothreitol, and 0.01% bromophenol blue. The proteins in whole-cell lysates were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto a polyvinylidene fluoride membrane (Immobilon; Millipore, Burlington, MA, USA). Immunoblotting was performed using antibodies against EGFR (phospho Y1068) (ab40815) (1:4,000 dilution; Abcam, Cambridge, MA, USA), EGFR (phospho Y1173) (AF1095) (1:2,000 dilution; R&D Systems, Minneapolis, MN, USA), EGFR (phospho Y845) (AF3394) (1:2,000 dilution; R&D systems), EGF-R (MI-12-1) (1:4,000 dilution; MBL, Nagoya, Japan), phospho-extracellular signal-regulated protein kinase (ERK) 1/2 (#9101) (1:2,000 dilution; Cell Signaling Technology, Beverly, MA, USA), ERK1/2 (#9102) (1:2,000 dilution; Cell Signaling Technology), and α-tubulin (PM054) (1:10,000 dilution; MBL), as primary antibodies. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (7074P2) (IgG; 1:4000 dilution; Cell Signaling Technology) and horseradish peroxidase-conjugated anti-mouse IgG (PM009-7) (1:4000 dilution; MBL) were used as secondary antibodies. The band intensity was detected using a LuminoGraph II Chemiluminescent Imaging System (ATTO, Tokyo, Japan).
We transiently transfected expression vectors containing the mutant genes into HEK293 cells, to evaluate the selected common and uncommon EGFR mutants. A transient overexpression of phosphorylated EGFR (pY-1068) was observed in HEK293 cells (Fig. 1). Further, ERK1/2 phosphorylation was increased in mutant-EGFR-transfected cells (L858R, Del19, G719S, Del18, and L861Q). These results suggest that these common and uncommon mutations of EGFR are constitutively expressed mutations.

Expression of EGFR Mutants in HEK293 Cells
HEK293 cells were transiently transfected with expression vectors containing mutant EGFR genes (0.5 µg/well). After 48 h, the cells were harvested in SDS sample buffer. The proteins in whole-cell lysates were separated using SDS-PAGE, and detected by immunoblotting using antibodies against EGFR (phospho Y1068), total-EGFR, phosphorylated ERK1/2, total-ERK1/2, and α-tubulin as the loading control.
To evaluate the effectiveness of EGFR-TKI activity on human lung cancer cells, we established permanently transfected cells containing mutated EGFRs using an H1299 NSCLC cell line expressing wild type EGFR. The phosphorylated-EGFR and total-EGFR protein levels in transfected H1299 cells were higher than those in the parent H1299 cells (Fig. 2). Therefore, the subclones, with similar expression levels of the total EGFR; L858R clone 2, Del19 clone 2, G719S clone 2, Del18 clone 1, and L861Q clone 1, were used for further experiments.
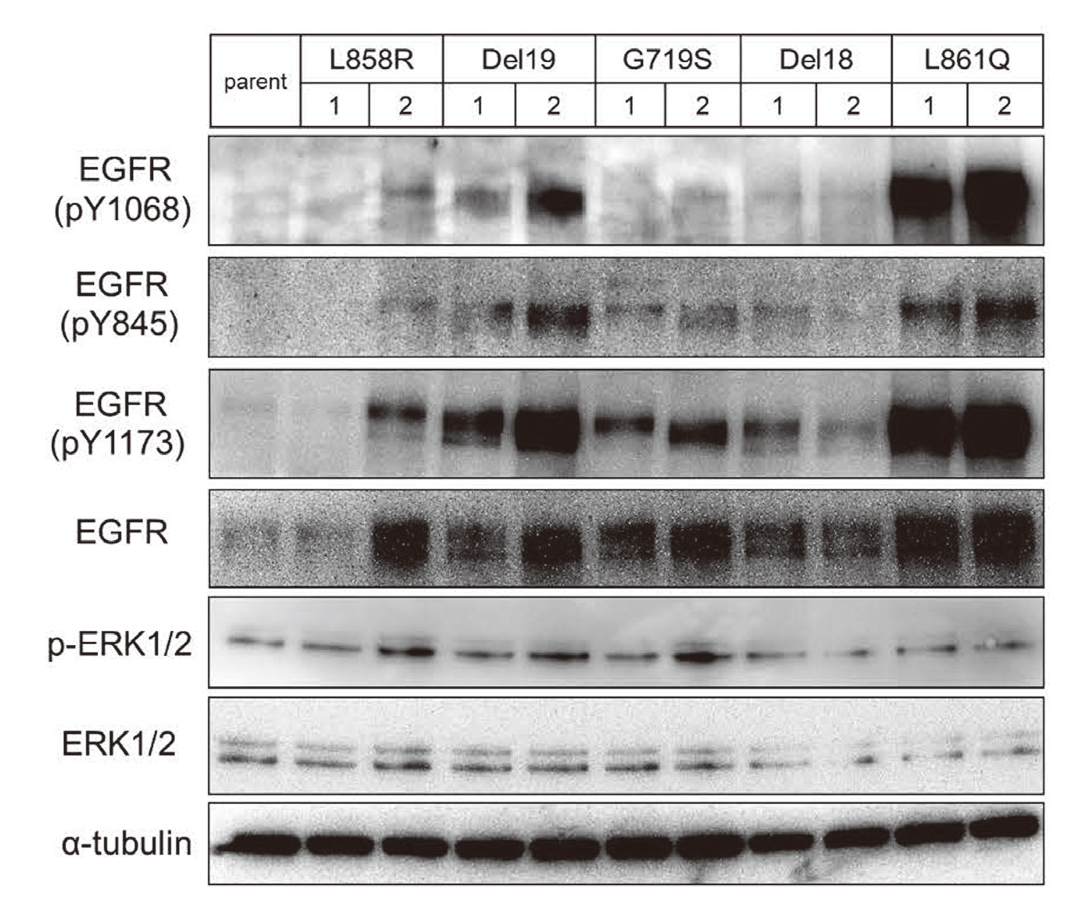
Establishment of Stably Mutant-EGFR-Expressing H1299 Cells
Parent and transfected H1299 cells were harvested in SDS sample buffer. The proteins in whole-cell lysates were separated using SDS-PAGE, and detected by immunoblotting using antibodies against phosphorylated-EGFR, total-EGFR, phosphorylated ERK1/2, total-ERK1/2, and α-tubulin as the loading control. The numbers represent the clone numbers.
The cell morphologies of the parent and transfected H1299 cells were observed on days 1, 3, and 5 (Fig. 3A). The relative cell proliferation ratio of the transfected cells, compared with that of the parent H1299 cells, was determined using the MTS assay (Fig. 3B). No remarkable differences were observed in cell morphologies and growth rates between the parent and the transfected H1299 cells.

Characterization of Mutant-EGFR-Expressing Transfectants of H1299 Cells
(A) Parent and transfected H1299 cells were seeded at 15,000 cells/well into 24-well plates. Cell morphologies were observed at Day 1, 3, and 5. (B) The relative cell proliferation ratio, compared to parent H1299 cells, was determined using the MTS assay.
To evaluate the effect of different EGFR-TKIs on these gene mutations, the phosphorylation levels of EGFR were investigated after the cells were exposed to clinically available (Fig. 4A) and clinically unavailable TKIs (Fig. 4B). The phosphorylation of EGFR was almost inhibited by clinically available EGFR-TKIs in Del19-, L858R-, and L861Q-containing cells. Additionally, neratinib and poziotinib effectively inhibited the phosphorylation of EGFR in Del18-containing cells. Canertinib and sapitinib were also effective in EGFR Del19-, Del18-, and L861Q-containing cells. In G719S-containing cells, EGFR phosphorylation was not inhibited by all TKIs tested.

Sensitivities of the Transfected Cells to Various EGFR-TKIs
Transfected cells were cultured in the growth medium. The next day, the cells were treated with (A) clinically available and (B) clinically unavailable TKIs for 24 h. The cells were then harvested in SDS sample buffer. The proteins in whole-cell lysates were separated using SDS-PAGE, detected by immunoblotting using antibodies against phosphorylated-EGFR and total-EGFR. The blot shows one of two independent experiments.
In this study, we created an in vitro model to evaluate the sensitivity of lung cancer cells showing uncommon EGFR gene mutations, to EGFR-TKIs. To verify these results in a system that is more relevant to NSCLC, we established stable mutant-EGFR-transfected cells using an H1299 NSCLC cell line. The H1299 cell line does not have any mutations in EGFR gene, so it was selected in the present study. Under regular culture conditions, all EGFR mutants displayed a higher level of tyrosine phosphorylation in established H1299 cells (Fig. 2). These stable cell lines have same genetic backgrounds, except for EGFR mutations. Therefore, it is beneficial to assess the EGFR-TKI susceptibility for each gene mutation.
First, gefitinib, erlotinib, afatinib, and osimertinib were investigated, which are approved in Japan. These EGFR-TKIs were effective against cells containing EGFR Del19, L858R mutations. Gefitinib and erlotinib were less effective against cells containing the EGFR G719S and Del18 uncommon mutations. These results are consistent with data from a preclinical study using an in vitro model,9) and with the data obtained from human clinical trials.10) In a small number of clinical trials, it is reported that afatinib and osimertinib were effective against uncommon EGFR mutations, including G719X, L861Q, and S768I mutations.5,11) In our study, they did not show promising efficacy against G719S (Fig. 4A). This mechanism is not clear. Additional studies to evaluate the efficacy against G719S are warranted.
Next, we examined the sensitivity of the EGFR-TKIs unapproved in Japan, which included neratinib, poziotinib, canertinib, and sapitinib. Neratinib and poziotinib were effective against EGFR Del19, L858R, Del18, and L861Q. Interestingly, canertinib and sapitinib appeared to be ineffective against cells containing EGFR L858R, although they were effective against EGFR Del19, Del18, and L861Q. The underlying mechanism for this difference is not clear.
Neratinib is an irreversible inhibitor of the human EGFRs HER1, HER2, and HER4. Neratinib has been approved by The United States Food and Drug Administration and The European Medicines Agency for treating HER2-positive breast cancer. Neratinib was found to be effective against EGFR G719X in a phase II trial of NSCLC.12) However, the development of neratinib for the treatment of patients with lung cancer was discontinued, because it was not effective for EGFR Del19. One of the primary mechanisms underlying the varying sensitivities of lung cancers harboring each EGFR mutation, to TKIs has been considered to be the different affinities between kinases and TKIs.13) Davis et al. present comprehensive data on the dissociation constants between several types of EGFR kinases and TKIs.14) It has been reported that the affinity with gefitinib was 2.0- to 3.7-fold higher in Del19 than that in G719C/S, whereas the affinity with neratinib was 2.5- to 6.2-fold lower in Del19 than that in G719C/S. However, the results of our study suggest that it may be a new treatment option for inhibiting Del18 and L861Q phosphorylation in the treatment of lung cancer.
Additionally, poziotinib and canertinib are irreversible inhibitors of the human EGFRs HER1, HER2, and HER4. Similarly, sapitinib is an irreversible inhibitor of the human EGFRs HER1, HER2, and HER3. Poziotinib is expected to be effective against NSCLC with mutations in EGFR or HER2 exon 20 mutations.15) Canertinib and sapitinib are not currently used in clinical treatment. There are a few reports on these drugs. It has been reported that canertinib has marginal efficacy, with a response rate of 2% to 4% in each arm, but failed to meet its primary statistical endpoint in a phase II clinical trial.16) These observations suggest that canertinib has a lower efficacy than that of gefitinib or erlotinib in similar patient populations. However, the study did not consider the type of the EGFR mutation. Our study suggests that canertinib use may be a treatment option against Del18 and L861Q. To the best of our knowledge, this is the first study to investigate the in vitro efficacy of canertinib and sapitinib on several EGFR mutations. To confirm these findings, further basic and clinical research is needed.
In summary, we created an in vitro model to evaluate the sensitivity of lung cancer cells to different EGFR-TKIs. It is suggested that some of the unapproved drugs may be useful in the development of treatment strategies for mutant-EGFR-positive NSCLC cases. This model will provide a preclinical rationale for the proper selection of EGFR-TKIs against clinically relevant EGFR mutations.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare no conflict of interest.