Abstract
Background:
There is no robust evidence of pharmacological interventions to improve mortality in heart failure (HF) patients with preserved left ventricular ejection fraction (LVEF) (HFpEF). In this subanalysis study of the SUPPORT Trial, we addressed the influence of LVEF on the effects of olmesartan in HF.
Methods and Results:
Among 1,147 patients enrolled in the SUPPORT Trial, we examined 429 patients with reduced LVEF (HFrEF, LVEF <50%) and 709 with HFpEF (LVEF ≥50%). During a median follow-up of 4.4 years, 21.9% and 12.5% patients died in the HFrEF and HFpEF groups, respectively. In HFrEF patients, the addition of olmesartan to the combination of angiotensin-converting enzyme inhibitor (ACEI) and β-blocker (BB) was associated with increased incidence of death (hazard ratio (HR) 2.26, P=0.002) and worsening renal function (HR 2.01, P=0.01), whereas its addition to ACEI or BB alone was not. In contrast, in HFpEF patients, the addition of olmesartan to BB alone was significantly associated with reduced mortality (HR 0.32, P=0.03), whereas with ACEIs alone or in combination with BB and ACEI was not. The linear mixed-effect model showed that in HFpEF, the urinary albumin/creatinine ratio was unaltered when BB were combined with olmesartan, but significantly increased when not combined with olmesartan (P=0.01).
Conclusions:
LVEF substantially influences the effects of additive use of olmesartan, with beneficial effects noted when combined with BB in hypertensive HFpEF patients. (Circ J 2016; 80: 2155–2164)
Recent studies report that the prevalence of heart failure (HF) with preserved left ventricular ejection fraction (LVEF) (HFpEF) has been increasing worldwide.1–3
Although guidelines recommend the use of β-blockers (BB) and renin-angiotensin system inhibitors (RASI), such as angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor blockers (ARB), and aldosterone receptor antagonists, to improve mortality and morbidity in HF patients with reduced EF (HFrEF), the guidelines merely recommend the use of diuretics to relieve symptoms and for adequate blood pressure control in HFpEF patients,4,5
as there is no robust evidence of pharmacological interventions to improve mortality in HFpEF patients.6,7
In current HF management, combinations of evidence-based medications are commonly applied. However, it remains unclear whether the combination of RASI and BB is generally beneficial in HF patients, even those with HFrEF.8,9
In the post-hoc analysis of the Valsartan Heart Failure Trial (Val-HeFT), triple combination therapy with valsartan, ACEI and BB was significantly associated with increased adverse effects on mortality and morbidity.8
In contrast, the prospective Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM)-Added Trial demonstrated that the addition of an ARB, candesartan, to ACEI was beneficial in patients with symptomatic CHF regardless of BB use.9
We recently conducted the SUPPORT (Supplemental Benefit of Angiotensin Receptor Blocker in Hypertensive Patients with Stable Heart Failure Using Olmesartan) trial, demonstrating that the addition of olmesartan to ACEI and/or BB did not improve clinical outcomes but worsened renal function in hypertensive CHF.10,11
The SUPPORT trial further demonstrated that the triple combination of olmesartan, ACEI and BB was associated with increased incidence of all-cause death, whereas the dual combination of olmesartan and BB was associated with reduced mortality.11
However, it remains to be elucidated whether LVEF influences the effects of additive use of ARB in the management of HF. This is clinically important from the viewpoint that the prevalence of HFpEF is increasing worldwide1–3
and no therapeutic strategy has been established.4
In the present study, we thus examined whether LVEF influences the effects of additive use of olmesartan in the management of CHF in the SUPPORT trial.
Methods
The SUPPORT Trial
The details of the SUPPORT trial have been described previously (NCT00417222).10,11
Briefly, it was a prospective, randomized, open-label blinded endpoint (PROBE) study,10,11
conducted according to the ethical principles of the Declaration of Helsinki and approved by the ethics committees of the 17 participating institutions in the Tohoku District of Japan (Appendix S1). The inclusion criteria of the present study were designed to enroll symptomatic CHF patients with hypertension aged 20–79 years who were treated with ACEI and/or BB.10,11
The exclusion criteria were designed to exclude patients with substantive confounding medical conditions or an inability to meaningfully participate in the SUPPORT trial.10,11
Finally, a total of 1,147 symptomatic CHF patients with a previous history of hypertension who gave written informed consent for the trial were assigned to either the olmesartan or the control group in a 1:1 ratio, through stratification by participating institute, sex and age between October 2006 and March 2010. The patients were followed until March 31, 2013. Olmesartan was initiated at a dose of 5–10 mg/day, and attending physicians were encouraged to up-titrate it to 40 mg/day whenever possible in the olmesartan group, but no ARB use was allowed in the control group. The diagnosis of CHF was made based on the Framingham criteria12
by an attending physician(s) at each hospital. All physicians were encouraged to control the blood pressure of the patients in each group according to the recommendations of the JNC7.13
Study Design
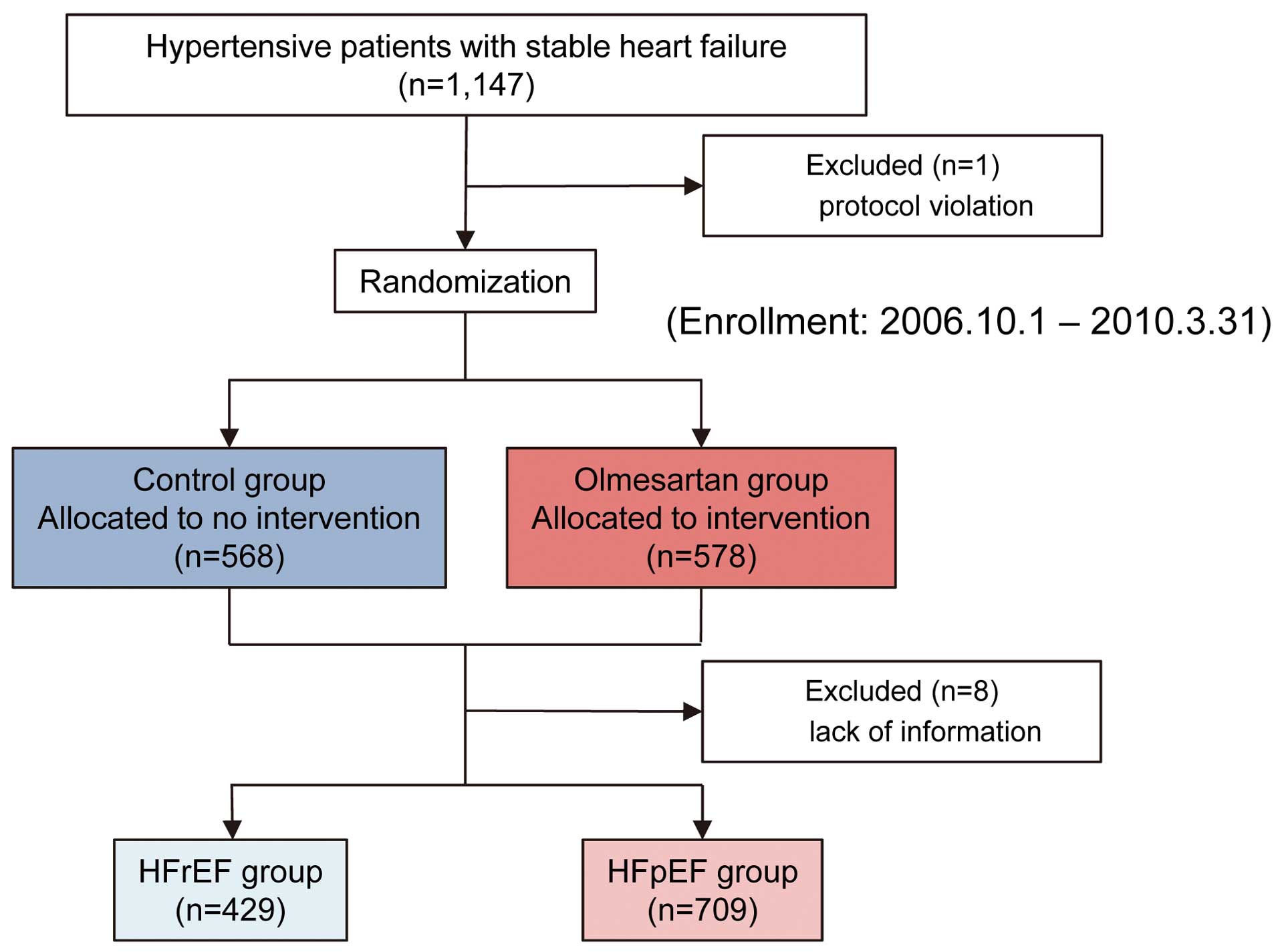
From among 1,147 patients in the SUPPORT trial, we enrolled 1,138 consecutive patients with stage C/D hypertensive CHF in the present study, after excluding 1 patient for protocol violation and 8 who did not have LVEF data (Figure 1). We divided them into HFpEF and HFrEF based on LVEF levels measured by echocardiography at the time of enrollment at each hospital. In the present study, patients with LVEF ≥50% were classified as HFpEF, and those with LVEF <50% as HFrEF.4
The primary endpoint of the present study was all-cause death and the secondary endpoint was worsening renal function (WRF).10,11
WRF was defined as an increase in serum creatinine level >2-fold from the baseline at any time point during the follow-up period.14
To evaluate WRF, we further evaluated changes in the urinary albumin to creatinine ratio (UACR)15
during the follow-up period. Urine samples were collected in outpatient clinics or before discharge, and were transferred to the central laboratory (SRL, Inc, Tokyo, Japan) to calculate the UACR.
The primary and secondary endpoints were analyzed based on the time to the first occurrence, according to the intention-to-treat principle, including all patients lost to follow-up and censored at the day of the last contact. Survival curves were estimated using the Kaplan-Meier method and compared with a 2-sided log-rank test. The effects of olmesartan were examined using Cox proportional hazards models. Subgroup analyses were performed according to baseline medications and other clinical parameters. Continuous variables are presented as mean±standard deviation except for B-type natriuretic peptide (BNP). BNP levels are presented as median and interquartile range. Categorical variables are presented as number and percentage. Group comparisons were made with the Mann-Whitney test for continuous variables, and the chi-squared test without continuity correction for categorical variables. For statistical analysis of longitudinal change in the logarithm of (UACR+1), a linear mixed-effect model (LMEM)16
was utilized. The LMEM was fitted with the random intercept for each patient and the trend in duration as the fixed effect, using the nlme package of R. All statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM, Somers, NY, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna. http://www.R-project.org/). Two-sided probability values <0.05 and P values for interaction <0.1 were considered to be statistically significant.
Results
Patients Characteristics
In the HFrEF patients (mean LVEF, 38%), baseline characteristics were almost comparable between the olmesartan and control groups except for history of admission for HF and serum hemoglobin and albumin levels (Table 1). The mean age was 64 years and 82% were male. The prevalence of ischemic heart disease (IHD) was 48%. ACEIs and BB were prescribed in 84% and 84%, respectively, and 68% were treated with both drugs.
Table 1.
Baseline Characteristics of the Symptomatic CHF Patients With Hypertension
| |
HFrEF |
HFpEF |
Control
(n=218) |
Olmesartan
(n=211) |
P value |
Control
(n=346) |
Olmesartan
(n=363) |
P value |
| Age, years |
64.9±10.8 |
64.6±10.7 |
0.792 |
65.9±9.7 |
66.5±10.1 |
0.454 |
| Males, % |
178 (81.7%) |
172 (81.5%) |
0.971 |
246 (71.1%) |
255 (70.2%) |
0.805 |
| Body weight, kg |
64.1±13.1 |
63.6±12.4 |
0.696 |
64.1±12.9 |
63.0±12.9 |
0.259 |
| Body mass index, kg/m2 |
24.3±3.9 |
23.9±3.8 |
0.342 |
24.8±4.2 |
24.4±4.2 |
0.265 |
| NYHA functional class |
|
|
0.341 |
|
|
0.891 |
| II |
187 (88.6%) |
202 (92.7%) |
|
323 (93.4%) |
342 (94.2%) |
|
| III |
23 (10.9%) |
15 (6.9%) |
|
22 (6.4%) |
20 (5.5%) |
|
| History of HF admission |
133 (61.0%) |
151 (71.6%) |
0.021 |
153 (44.1%) |
167 (52.2%) |
0.609 |
| IHD |
103 (47.2%) |
104 (49.3%) |
0.700 |
156 (45.1%) |
177 (48.8%) |
0.329 |
| Diabetes mellitus |
102 (46.8%) |
113 (53.6%) |
0.177 |
186 (53.9%) |
169 (46.6%) |
0.060 |
| Hemodynamics and LV function |
| Systolic blood pressure, mmHg |
122.4±18.5 |
123.7±19.0 |
0.488 |
130.1±17.1 |
131.5±17.1 |
0.274 |
| Diastolic blood pressure, mmHg |
71.7±11.2 |
73.8±13.5 |
0.090 |
75.2±11.7 |
75.3±11.4 |
0.935 |
| Heart rate, beats/min |
71.4±13.7 |
72.1±14.7 |
0.626 |
71.4±14.9 |
70.6±13.2 |
0.434 |
| LVDd, mm |
60.3±7.7 |
59.7±8.4 |
0.483 |
50.0±6.8 |
49.6±6.8 |
0.385 |
| LVEF, % |
38.7±8.1 |
38.6±8.4 |
0.898 |
63.1±8.6 |
63.8±8.8 |
0.272 |
| Laboratory findings |
| Hemoglobin, g/dl |
13.7±2.0 |
14.1±1.7 |
0.024 |
13.8±1.8 |
13.7±1.7 |
0.573 |
| Blood urea nitrogen, mg/dl |
19.3±7.6 |
19.5±8.9 |
0.793 |
17.2±6.4 |
17.6±6.4 |
0.436 |
| Creatinine, mg/dl |
1.0±0.4 |
1.0±0.4 |
0.946 |
0.9±0.3 |
0.9±0.3 |
0.953 |
| Serum sodium, mEq/L |
141±2.6 |
141±2.8 |
0.140 |
141±2.5 |
142±2.2 |
0.042 |
| Albumin, g/dl |
4.1±0.5 |
4.2±0.4 |
0.006 |
4.2±0.4 |
4.2±0.4 |
0.577 |
| LDL-C, mg/dl |
108.3±30.1 |
107.3±30.6 |
0.735 |
106.6±30.1 |
108.9±31.1 |
0.358 |
| eGFR, ml/min/1.73 m2 |
63.2±21.9 |
62.1±19.2 |
0.588 |
66.0±19.3 |
65.3±18.1 |
0.620 |
| BNP, pg/ml |
122.0
(56.6, 237.8) |
117.0
(55.5, 260.0) |
0.750 |
58.7
(27.5, 139.0) |
71.1
(30.2, 148.0) |
0.280 |
| Medication at baseline |
| BB |
172 (81.5%) |
188 (86.2%) |
0.183 |
227 (65.4%) |
230 (63.4%) |
0.567 |
| ACEI |
175 (82.9%) |
184 (84.4%) |
0.681 |
274 (79.0%) |
290 (79.9%) |
0.760 |
| Diuretic |
154 (70.6%) |
161 (76.3%) |
0.191 |
166 (48.0%) |
166 (45.7%) |
0.598 |
| Loop diuretic |
142 (65.1%) |
142 (67.3%) |
0.683 |
152 (43.9%) |
149 (41.0%) |
0.448 |
| Spironolactone |
76 (34.9%) |
85 (40.3%) |
0.273 |
76 (22.0%) |
67 (18.5%) |
0.262 |
| Calcium-channel blocker |
65 (29.8%) |
55 (26.1%) |
0.392 |
144 (41.6%) |
166 (45.7%) |
0.289 |
| Statin |
112 (51.4%) |
111 (52.6%) |
0.847 |
160 (46.2%) |
175 (48.2%) |
0.652 |
ACEI, angiotensin-converting enzyme inhibitor; BB, β-blocker; BNP, B-type natriuretic peptide; CHF, chronic heart failure; eGFR, estimated glomerular filtration rate; HFpEF, HF patients with preserved LVEF; HFrEF, HF patients with reduced LVEF; IHD, ischemic heart disease; LDL-C, low-density lipoprotein cholesterol; LVDd, left ventricular diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
In the HFpEF patients (mean LVEF, 63%), baseline characteristics were comparable between the olmesartan and control groups except for serum sodium level (Table 1). The mean age was 66 years and 71% were male. The prevalence of IHD was 47%. ACEIs and BB were prescribed in 80% and 64%, respectively, and 45% were treated with both drugs.
Additive Effects of Olmesartan on Mortality in HFrEF and HFpEF Patients
All-cause death occurred in 39 and 55 patients in the control and olmesartan groups, respectively, of HFrEF patients, and in 46 and 43 patients in the control and olmesartan groups, respectively of HFpEF patients (P for interaction, 0.07) (Figure 2A). In the patients treated with both ACEI and BB, the addition of olmesartan was significantly associated with increased incidence of all-cause death in HFrEF, but not in HFpEF patients (P for interaction, 0.02) (Figure 2B). Importantly, in HFrEF patients, the addition of olmesartan to the combination of ACEI and BB was associated with increased mortality regardless of the presence or absence of IHD (P for interaction, 0.835), and it tended to be associated with decreased mortality in HFpEF patients with IHD, with a significant interaction vs. HFpEF patients without IHD (P for interaction, 0.057) (Table S1). In the patients treated with ACEI but not BB, the addition of olmesartan was not associated with reduced incidence of all-cause death in either HFrEF or HFpEF patients (P for interaction vs. HFpEF, 0.22) (Figure 2C), regardless of the presence or absence of IHD (P for interaction, 0.527 and 0.173 for HFrEF and HFpEF patients, respectively) (Table S1).

In contrast, in the patients treated with BB but not an ACEI, the addition of olmesartan was significantly associated with reduced incidence of all-cause death in HFpEF patients, but not in HFrEF patients (P for interaction, 0.52) (Figure 2D). Of note, however, the combination of olmesartan and BB tended to be associated with decreased mortality in HFrEF patients with IHD, with a significant interaction vs. those without IHD (P for interaction, 0.091), whereas the effect of combination use of olmesartan and BB was comparable between HFpEF patients with and without IHD (P for interaction, 0.531) (Table S1).
Additive Effect of Olmesartan on WRF in HFrEF and HFpEF Patients
WRF occurred in 31 patients (14.2%) in the control group and in 42 (19.9%) in the olmesartan group of HFrEF patients (P=0.09), and in 30 (8.6%) patients in the control group and 54 (14.9%) in the olmesartan group of HFpEF patients (P=0.01) (P for interaction, 0.70) (Figure 3A). In the patients treated with both ACEI and BB, the addition of olmesartan tended to be associated with increased WRF in HFpEF patients (P=0.09), and was significantly associated with increased incidence of WRF in HFrEF patients (P=0.01) (P for interaction, 0.55) (Figure 3B). In patients treated with an ACEI but not BB, the addition of olmesartan was associated with increased WRF in HFpEF patients (P=0.02), but not in HFrEF patients (P=0.63) (P for interaction, 0.09) (Figure 3C). Interestingly, in the patients treated with BB but without an ACEI, the addition of olmesartan was not associated with increased incidence of WRF in either HFpEF (P=0.92) or HFrEF (P=0.61) patients (P for interaction, 0.69) (Figure 3D).

UACR was increased in HFrEF patients during the follow-up period regardless of the addition of olmesartan, but was unaltered in HFpEF patients treated with the addition of olmesartan (Figure 4A). In the patients treated with both ACEI and BB, UACR was increased in HFrEF patients regardless of the addition of olmesartan, but not in HFpEF patients (Figure 4B). In the patients treated with ACEI but without BB, UACR was unaltered in both HFrEF and HFpEF patients regardless of the addition of olmesartan (Figure 4C). In contrast, in the patients treated with BB but without ACEI, UACR was increased in both HFrEF and HFpEF patients when olmesartan was not added, but it was unaltered in both groups when olmesartan was added (Figure 4D).
Table 2
shows the rearrangement of drug combinations in a descending manner in terms of the slope of UACR changes during the follow-up period. In HFrEF patients, not only the single use of BB, but also the dual combination use of ACEI and BB and the triple combination of olmesartan, ACEI and BB were significantly associated with an increase in the UACR, whereas the dual combination of olmesartan and BB was not. On the other hand, in HFpEF patients, none of the drug combinations, except the single use of BB, was significantly associated with UACR increase.

Table 2.
Trends in UACR During Follow-up
| Olmesartan |
ACEI |
BB |
Slope |
P value |
| HFrEF |
| − |
− |
+ |
0.117 |
0.015 |
| + |
+ |
+ |
0.093 |
0.001 |
| − |
+ |
+ |
0.060 |
0.016 |
| − |
+ |
− |
0.043 |
0.387 |
| + |
− |
+ |
0.022 |
0.626 |
| + |
+ |
− |
−0.050 |
0.332 |
| HFpEF |
| − |
− |
+ |
0.080 |
0.014 |
| − |
+ |
+ |
0.034 |
0.142 |
| − |
+ |
− |
0.026 |
0.294 |
| + |
− |
+ |
0.022 |
0.475 |
| + |
+ |
− |
0.011 |
0.625 |
| + |
+ |
+ |
0.010 |
0.680 |
Drug combinations are arranged in descending order in terms of the slope value in both HFrEF and HFpEF patients. UACR, urine albumin to creatinine ratio. Other abbreviations as in Table 1.
Discussion
In the present substudy of the SUPPORT trial, we examined whether additive treatment with an ARB, olmesartan, reduced the mortality and morbidity of CHF patients with a history of hypertension treated with ACEI and/or BB with special reference to LVEF. In HFpEF patients, the addition of olmesartan to BB was associated with improved mortality rate without developing WRF. On the other hand, the triple combination of olmesartan, ACEI and BB was associated with increased incidence of death and WRF in HFrEF patients. These results may provide us with important information on the use of ARBs in the management of CHF.
Combination Use of RASI and BB in Hypertensive HFpEF Patients
In the present study, we demonstrated that the addition of olmesartan to BB therapy was associated with reduced mortality rate without development of WRF in HFpEF patients, but to the combination of ACEI and BB or to ACEI alone it was not. To the best of our knowledge, this is the first study to demonstrate a clinical benefit of drug combination in HFpEF patients. HFpEF patients, as compared with HFrEF patients, are characterized by their older age and higher prevalence of female sex and hypertension.4
Among them, hypertension has been implicated in a central role in the pathogenesis of HFpEF.17
Thus, management of blood pressure is crucial in HFpEF patients, especially in those with hypertension. Indeed, clinical guidelines simply recommend adequate control of systolic and diastolic blood pressures in HFpEF patients without specifying any type of antihypertensive medication.4,5
In the present study, however, control of blood pressure during the follow-up period were comparable among the subgroups based on the combination of medications (Figure S1). Thus, factor(s) other than blood pressure control could explain why the combined use of olmesartan and BB was associated with improved mortality rates in HFpEF patients in the present study.
However, it should be noted that the present result that a combination of olmesartan and BB was associated with reduced mortality in HFpEF patients was not consistent with the J-DHF, which found no beneficial effect of carvedilol in patients with diastolic HF regardless of treatment with ACEI or ARB.18
This discrepancy could be explained by differences in the baseline characteristics of the patients in the J-DHF and the SUPPORT trial, such as age (72.0 vs. 65.7 years), prevalence of males (58.4% vs. 75.0%) and BNP levels (227 vs. 143 pg/ml), in addition to differences in study design such as the definition of HF (diastolic dysfunction vs. HFpEF), and the BB used (carvedilol vs. any BB) and ARB (any ARB vs. olmesartan). However, it should be noted that the baseline characteristics of the present patients, characterized by relatively younger age, higher prevalence of males and stable HF status with low BNP levels, were similar to those of the HFpEF patients in the CHARM-Preserved study, which demonstrated a benefit of candesartan for HFpEF.7
Furthermore, a subanalysis of the I-PRESERVE Study demonstrated that irbesartan was effective in patients with lower NT-proBNP levels but not in those with higher NT-proBNP levels.19
These lines of evidence suggest that ARBs are beneficial for relatively younger patient populations and/or in the early stage of HFpEF, although the underlying mechanism is unclear.
It has been reported that olmesartan has beneficial effects on glucose metabolism, insulin resistance, and lipid metabolism,20
and that olmesartan significantly reduced vascular inflammation in patients with essential hypertension.21
Because the inflammatory state, including endothelial dysfunction and increased oxidative stress, is considered as one of the central pathophysiological aspects of HFpEF,22
these anti-inflammatory and anti-metabolic effects of olmesartan could be beneficial for HFpEF patients. On the other hand, it has been also reported that BB have beneficial effects in hypertensive HFpEF patients because they improve hypertension, and LV filling and thus reduce heart rate and myocardial oxygen demand.23
However, although a meta-analysis showed that RASI decreased HF hospitalizations24
and a propensity score-matched cohort study showed that use of RASI was associated with improved mortality rates,25
previous RCTs failed to show beneficial effects of RASI to improve the mortality or morbidity of HFpEF patients.6,7
Similarly, although a meta-analysis26
and a propensity score-matched cohort study23
suggested improved outcomes in HFpEF patients treated with BB, previous RCTs18,27
failed to find a benefit of BB for improved outcomes. These lines of evidence are the reasons why the current guidelines do not recommend routine use of RASI or BB for the control of blood pressure in HFpEF patients.4,5
Thus, it is conceivable that the beneficial effects of olmesartan and BB alone are not strong enough alone to counteract these other factors, but when combined, olmesartan and BB may have synergistic effects that show beneficial cardioprotective actions in HFpEF patients.
Combination Use of RASI and BB in Hypertensive HFrEF Patients
In the present study, more than 80% of HFrEF patients were treated with both ACEI and BB before randomization. However, the addition of olmesartan was significantly associated with poor prognosis and WRF in these patients. Indeed, the benefit of the triple combination of ACEI, ARB and BB remains controversial.8,9
The Val-HeFT trial showed that valsartan use was associated with increased adverse effects on mortality and morbidity in the subgroup receiving both an ACEI and BB,8
a similar finding in the HFrEF patients of the present study. On the other hand, the CHARM-Added trial showed that the addition of an ARB, candesartan, was beneficial in symptomatic CHF patients being treated with an ACEI and BB.9
Although the precise mechanism of the discrepancy in effectiveness of the triple combination therapy between the Val-HeFT and the CHARM-added trials is unclear, it could be explained by differences in the patients’ backgrounds; the majority of the patients in the Val-HeFT trial were in NYHA class II (62%), whereas those in the CHARM-added Trial were in NYHA class III (73%), although LVEF levels were comparable between the 2 trials (27% vs. 28%, respectively). In our SUPPORT trial, the majority of the HFrEF patients (91%) were in NYHA class II. Thus, the effect of the triple combination of ARB, ACEI and BB may differ according to the severity of HF in HFrEF patients, and routine application of the triple combination should be avoided in hypertensive patients with HFrEF. In the present study, the combination of olmesartan and BB was associated with improved mortality rates in HFpEF patients, but not in HFrEF patients. However, this discrepancy between HFpEF and HFrEF patients could be explained by the relatively small number of patients with BB alone at baseline in the HFrEF group, as the addition of olmesartan tended to be associated with reduced mortality in HFrEF patients without a significant interaction vs. HFpEF patients. Thus, the present results may not deny the beneficial effects of combined use of BB and olmesartan in HFrEF patients. In particular, the combination of olmesartan and BB tended to be associated with better prognosis in HFrEF patients with IHD compared with those without it. It is conceivable that the difference in the prognostic effect of the dual combination of olmesartan and BB between the HFrEF patients with and without IHD could be explained, at least in part, by the beneficial effect of olmesartan in reducing coronary atheroma progression.28
Renal Protective Effects of Olmesartan and BB
In the present study, although the addition of olmesartan to the combination of ACEI and BB was associated with an increase in WRF in HFrEF patients and tended to be so in HFpEF patients, that to BB alone was not associated with increased WRF in either HFrEF or HFpEF patients. The RAS is considered essential for preserving renal function and glomerular filtration.29
Renal perfusion pressure is a major determinant of glomerular hydraulic filtration pressure.29
The kidney responds to a decrease in blood supply by increasing renin and angiotensin in order to maintain its function and glomerular filtration within the normal range, which is known as the nephrocentric reaction.29
In CHF patients, renal perfusion usually reduces along with a decrease in cardiac output, followed by activation of the RAS to maintain renal function. Thus, excessive blockade of the RAS by the combination of olmesartan and ACEI without BB in HFpEF patients might have resulted in decreased renal perfusion and subsequent WRF in the present study.
We recently reported that increased albuminuria, when evaluated by urine dipstick test, predicted the mortality risk of HFpEF patients regardless of glomerular filtration ratio levels.30
In the present study, albuminuria, a marker of glomerular damage,15
was not increased during the follow-up period in HFpEF patients when BB were combined with olmesartan, ACEI or both, but was significantly increased when not combined with RAS inhibitors. In contrast, in the HFrEF patients, use of BB was generally associated with an increase in albuminuria except for combined use with olmesartan but not with ACEI. Because albuminuria is caused by activation of the RAS and/or sympathetic nervous system, as well as by inflammation,15,30
combined use of RASI and BB may be ideal to reduce albuminuria. Thus, from the viewpoint of renal protection, the present results suggest that the combination of olmesartan and BB could be beneficial in hypertensive CHF patients.
Study Limitations
Several limitations should be mentioned. First, because the SUPPORT trial was conducted in an open-label fashion, caution is warranted when interpreting the present results. Second, it should be noted that the patients enrolled in the SUPPORT trial had relatively well-controlled blood pressure and were only mildly symptomatic before randomization as compared with previous HF trials. Third, we did not take into consideration information about the dose of olmesartan, ACEIs or BB. Fourth, no detailed information on diastolic dysfunction other than LVEF was available. Fifth, because the present populations of both HFrEF and HFpEF were small, further studies with a large sample size are needed to confirm the present findings. Sixth, the possible influence of the Great East Japan Earthquake in 2011 in the Tohoku area should be considered,31
because it occurred after the randomization and during the follow-up period of the present study. However, because the results remained unaltered even after exclusion of results from hospitals located in the area with severe damage (data not shown), the influence of the earthquake may be minimal.
Conclusions
The present subanalysis of the SUPPORT trial suggests that the combination of olmesartan and BB is beneficial for hypertensive patients with HFpEF, whereas the triple combination therapy of olmesartan, ACEI and BB is harmful for those with HFrEF.
Acknowledgments
We thank all the members of the Tohoku Heart Failure Society and the staff of the Departments of Cardiovascular Medicine and Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, for their valuable contributions. This study was supported in part by grants-in-aid from the Ministry of Health, Labour, and Welfare and from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Disclosures
The Department of Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by the unrestricted research grants from Daiichi Sankyo Co, Ltd (Tokyo, Japan), Bayer Yakuhin, Ltd (Osaka, Japan), Kyowa Hakko Kirin Co, Ltd (Tokyo, Japan), Kowa Pharmaceutical Co, Ltd (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), Dainippon Sumitomo Pharma, Co, Ltd (Osaka, Japan), and Nippon Boehringer Ingelheim Co, Ltd (Tokyo, Japan). H.S. has received lecture fees from Bayer Yakuhin, Ltd (Osaka, Japan), Daiichi Sankyo Co, Ltd (Tokyo, Japan) and Novartis Pharma K.K. (Tokyo, Japan).
Supplementary Files
Supplementary File 1
Appendix S1.
SUPPORT Trial Investigators
Table S1.
Effect of olmesartan on all-cause death according to the presence or absence of IHD
Figure S1.
Time course in blood pressure values presented as mean±standard deviation.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0577
References
- 1.
Ushigome R, Sakata Y, Nochioka K, Miyata S, Miura M, Tadaki S, et al. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan: Report From the CHART Studies. Circ J 2015; 79: 2396–2407.
- 2.
Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892.
- 3.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75.
- 4.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847 [erratum in: Eur Heart J 2013; 34: 158].
- 5.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [Writing Committee Members]. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240–e327, doi:10.1161/CIR.0b013e31829e8776.
- 6.
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467.
- 7.
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM Preserved Trial. Lancet 2003; 362: 777–781.
- 8.
Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675.
- 9.
McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003; 362: 767–771.
- 10.
Sakata Y, Nochioka K, Miura M, Takada T, Tadaki S, Miyata S, et al. Supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial: Rationale and design. J Cardiol 2013; 62: 31–36.
- 11.
Sakata Y, Shiba N, Takahashi J, Miyata S, Nochioka K, Miura M, et al. Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure: The supplemental benefit of an angiotensin receptor blocker in hypertensive patients with stable heart failure using olmesartan (SUPPORT) trial. Eur Heart J 2015; 36: 915–923.
- 12.
McKee PA, Castelli WP, McNamara PM, Kannel WB. Natural history of congestive heart failure: The Framingham Study. N Engl J Med 1971; 285: 1441–1446.
- 13.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003; 289: 2560–2572.
- 14.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869.
- 15.
Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, Tadaki S, et al. Prognostic impact of subclinical microalbuminuria in patients with chronic heart failure. Circ J 2014; 78: 2890–2898.
- 16.
Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Berlin: Springer-Verlag Inc., 2000.
- 17.
Agarwal V, Briasoulis A, Messerli FH. Effects of renin-angiotensin system blockade on mortality and hospitalization in heart failure with preserved ejection fraction. Heart Fail Rev 2013; 18: 429–437.
- 18.
Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013; 15: 110–118.
- 19.
Kristensen SL, Jhund PS, Køber L, McKelvie RS, Zile MR, Anand IS, et al. Relative importance of history of heart failure hospitalization and N-terminal pro-B-type natriuretic peptide level as predictors of outcomes in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2015; 3: 478–486.
- 20.
Arao T, Okada Y, Mori H, Nishida K, Tanaka Y. Antihypertensive and metabolic effects of high-dose olmesartan and telmisartan in type 2 diabetes patients with hypertension. Endocr J 2012; 59: 1051–1056.
- 21.
Fliser D, Buchholz K, Haller H; EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) Investigators. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 2004; 110: 1103–1107.
- 22.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271.
- 23.
Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014; 312: 2008–2018.
- 24.
Zhang Q, Chen Y, Liu Q, Shan Q. Effects of renin-angiotensin-aldosterone system inhibitors on mortality, hospitalization, and diastolic function in patients with HFpEF: A meta-analysis of 13 randomized controlled trials. Herz 2016; 41: 76–86.
- 25.
Lund LH, Benson L, Dahlström U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117.
- 26.
Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta-blockers on heart failure with preserved ejection fraction: A meta-analysis. PLoS One 2014; 9: e90555, doi:10.1371/journal.pone.0090555.
- 27.
Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: Findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol 2009; 53: 184–192.
- 28.
Hirohata A, Yamamoto K, Miyoshi T, Hatanaka K, Hirohata S, Yamawaki H, et al. Impact of olmesartan on progression of coronary atherosclerosis a serial volumetric intravascular ultrasound analysis from the OLIVUS (impact of OLmesarten on progression of coronary atherosclerosis: Evaluation by IntraVascular UltraSound) trial. J Am Coll Cardiol 2010; 55: 976–982.
- 29.
Danser AH, van den Meiracker AH. Heart failure: New data do not SUPPORT triple RAAS blockade. Nat Rev Nephrol 2015; 11: 260–262.
- 30.
Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, et al. Urinary albumin excretion in heart failure with preserved ejection fraction: An interim analysis of the CHART 2 study. Eur J Heart Fail 2012; 14: 367–376.
- 31.
Aoki T, Fukumoto Y, Yasuda S, Sakata Y, Ito K, Takahashi J, et al. The Great East Japan Earthquake Disaster and cardiovascular diseases. Eur Heart J 2012; 33: 2796–2803.





