2016 Volume 80 Issue 12 Pages 2496-2505
2016 Volume 80 Issue 12 Pages 2496-2505
Background: In heart failure patients, chronic hyperactivation of sympathetic signaling is known to exacerbate cardiac dysfunction. In this study, the cardioprotective effect of vidarabine, an anti-herpes virus agent, which we identified as a cardiac adenylyl cyclase inhibitor, in dogs with pacing-induced dilated cardiomyopathy (DCM) was evaluated. In addition, the adverse effects of vidarabine on basal cardiac function was compared to those of the β-blocker, carvedilol.
Methods and Results: Vidarabine and carvedilol attenuated the development of pacing-induced systolic dysfunction significantly and with equal effectiveness. Both agents also inhibited the development of cardiac apoptosis and fibrosis and reduced the Na+-Ca2+ exchanger-1 protein level in the heart. Importantly, carvedilol significantly enlarged the left ventricle and atrium; vidarabine, in contrast, did not. Vidarabine-treated dogs maintained cardiac response to β-AR stimulation better than carvedilol-treated dogs did.
Conclusions: Vidarabine may protect against pacing-induced DCM with less suppression of basal cardiac function than carvedilol in a dog model. (Circ J 2016; 80: 2496–2505)
Beta-adrenergic receptor (β-AR)-mediated signaling is hyperactivated in heart failure (HF) patients.1 Although the sympathetic nervous system is quite important in the maintenance of blood perfusion in various organs during acute HF, long-term hyperactivation of β-AR signaling exacerbates the development of left ventricular dysfunction and arrhythmia, which results in further deterioration of cardiac function.2 Consistently, β-AR blocking agents (β-blockers) have been demonstrated to prevent the development of cardiac dysfunction and improve prognosis of HF patients.3,4 They have one major adverse property, however; a negative inotropic effect, which can exacerbate HF. For this reason, it is recommended that treatment with β-blockers for patients with severe systolic dysfunction (eg, dilated cardiomyopathy; DCM) is initiated at low doses and increased by gradual increments under careful observation. Even when these recommendations are followed, some patients are intolerant to β-blockers.1,5 Thus, there is a great need for the development of a novel anti-sympathetic drug that does not suppress basal cardiac function and thus can be administered safely to patients with severe HF.
β-Adrenergic receptor stimulation first induces adenylyl cyclase (AC) activation, then increases intracellular cAMP concentration. The AC is composed of several isoforms,6 each of which is expressed in a tissue-specific manner. AC type 5 (AC5) is one of the dominant isoforms in the heart.7–9 Interestingly, our previous reports have suggested that AC5 does not contribute to maintaining basal cardiac function, though it does play a major role in exacerbating cardiac dysfunction due to its pathological activation of β-AR signaling. AC5 deficiency in mice caused no significant change in baseline hemodynamic parameters compared with the wild-type mice.10 In contrast, AC5 deficiency was protective against the development of HF induced by transverse aortic banding and chronic isoproterenol infusion.11,12 The protein expression levels of AC5 are upregulated in the failing heart.12,13 These findings indicate that AC5 accelerates the development of HF without affecting basal cardiac functions. Thus, we presumed that pharmacological inhibition of AC5 would offer protection against the development of HF without suppressing basal cardiac function, a desirable quality for HF therapy.
Previously, we discovered that vidarabine, 9-β-D-arabinofuranosyladenine, which is approved by the Food and Drug Administration (FDA) as an anti-herpes virus agent,14 inhibits cardiac AC activity.15 Vidarabine has higher selectivity for AC5 activity than for type 2 or 3 AC, both of which are more abundantly expressed in non-cardiac tissue.15 We reported that vidarabine improves cardiac function and survival time in a mouse HF model induced by myocardial infarction and chronic isoproterenol infusion.16 In addition, in healthy mice, acute treatment with vidarabine did not affect basal cardiac function.16 Similarly, vidarabine did not affect cardio-hemodynamic or electrophysiological variables in anesthetized healthy dogs.17
To further evaluate the usefulness of vidarabine for the treatment of severe HF, we used a canine model of pacing-induced DCM, which has been well-characterized in previous studies.18–20 Chronic rapid ventricular pacing causes a progressive and reliable model of DCM in dogs, pigs and sheep. This model shows a progression of functional, structural, and neurohormonal changes, similar to that associated with DCM in humans.19 In the present study, we assessed the effectiveness of vidarabine against this canine model of pacing-induced DCM. We also compared the therapeutic and adverse effects of vidarabine with those of carvedilol, one of the most commonly used β-blockers, by evaluating the degree to which cardiac responses to sympathetic activation were preserved during each type of therapy.
Eighteen female beagles underwent surgical implantation of a pacing wire on the right ventricle, as described previously.21 The study was approved by the Animal Care and Use Committee of the College of Bioresource Science, Nihon University and performed by the Department of Veterinary Medicine, College of Bioresource Science of Nihon University, Japan.
Experimental ProtocolFigure 1 shows the schematic diagrams of our experimental procedure. All dogs underwent baseline hemodynamic recordings before the initiation of pacing (Day 0). Subsequently, HF was induced by rapid right ventricular pacing at a rate of 250 beats/min for 3 weeks. And then, after tachycardia pacing was decreased to 240 beats/min to limit mortality,22 vidarabine (24 mg·kg–1·day–1 i.v., n=6) or carvedilol (1 mg·kg–1·day–1 p.o., n=6) was administered for 7 days. Treatment was continued for another 3 days during which tachycardia pacing was suspended. Finally (Day 31), dogs were euthanized under general anesthesia, and their hearts were removed and sectioned for histological and biochemical examination.

Schematic diagrams of the experimental procedure. Tachycardia pacing was performed for 21 days to induce heart failure (HF) (Day 21). After the development of HF, vidarabine or carvedilol treatment was initiated and continued for 7 days with tachycardia pacing (Day 28). Thereafter, drug administration was continued for another 3 days without tachycardia pacing (recovery period) (Day 31). Hemodynamic studies were performed on Days 0, 4, 7, 11, 14, 18, 21, 28, and 31. In addition, an isoproterenol challenge test and blood measurements were performed on Day 31.
We collected hemodynamic data at several time points, as shown in Figure 1. Cardiac function was evaluated by transthoracic echocardiography with the animals in a conscious state. The left ventricular internal diameter at diastole (LVIDd) and systole, the interventricular septal thickness at diastole, the left ventricular posterior wall thickness at diastole, the left ventricular fractional shortening (LVFS), and the ejection fraction were obtained through M mode echocardiogram. Left atrium diameter (LAD) was divided by the aortic root diameter taken from the same frame to obtain an LAD index. The cardiac output (CO) was calculated according to the pulsed Doppler echocardiogram method.23 The blood pressure was measured by the oscillometric method. Concomitantly, glomerular filtration rate (GFR) using urinary inulin clearance was measured for evaluation of renal function.24 All hemodynamic measurements were performed 30 min after cessation of pacing.
Histological AnalysesLeft atrium and ventricle samples were used for histological analysis. Apoptosis in the tissue sections was examined by terminal dUTP nick end-labeling (TUNEL) staining. Interstitial fibrosis was evaluated by Sirius red staining.
Western Blot AnalysisWestern blot analysis was performed, as previously described.25 In brief, homogenized tissue samples were separated on a 6% to 15% SDS-polyacrylamide gel and blotted onto polyvinylidene fluoride (PVDF) membranes. The membranes were then reacted with primary antibodies. The expression level of each protein was quantified by laser densitometry.
Cardiac Response to Isoproterenol InfusionAn isoproterenol challenge test was performed 10 days after the administration of the treatment agent (Day 31) using the method reported by Nikolaidis et al, with minor modifications.18 Isoproterenol was administered intravenously at a dosage of 0.02, 0.04 or 0.08 μg·kg–1·min–1 for 10 min. At the end of the dosage period, cardiac function was evaluated in a steady state.
Blood MeasurementsBlood measurements were performed 10 days after administration of the treatment agent (Day 31). Plasma concentrations of alanine aminotransferase, aspartate aminotransferase, urea nitrogen, and creatinine were measured.
Statistical AnalysisAll data are expressed as the mean±SEM. The differences in each parameter were analyzed by 1-way or 2-way analysis of variance, followed by post-hoc testing (Tukey-Kramer test). A value of P<0.05 was considered statistically significant.
Please refer to the Supplementary section for more detailed Methods information.
Chronic tachycardia pacing results in the progression of HF with a severe decrease in systolic function and enlargement of the left ventricle, as seen in human DCM.18 In the present study, 3 weeks of tachycardia pacing led to severe declines in LVFS, CO, and mean arterial pressure, and remarkable increases in the LVIDd and LAD indices, as Figure 2 shows.

Changes in parameters of left ventricular function and systemic hemodynamics during development of pacing-induced dilated cardiomyopathy in dogs. (A) Heart rate. (B) Mean arterial pressure (MAP). (C) Glomerular filtration rate (GFR). (D) Left ventricular internal diameter at diastole (LVIDd). (E) Left atrium diameter (LAD) index. (F) Left ventricular fractional shortening (LVFS). (G) Cardiac output (CO). Results are presented as mean±SEM. *P<0.05, **P<0.01 compared with Day 0. n=18. Statistics were analyzed using a 1-way analysis of variance followed by post-hoc testing.
At this point in the experiment (Day 21), we initiated treatment with either vidarabine or carvedilol, and evaluated cardiac function at the 2 time points. We first assessed cardiac function after 7 days of concomitant treatment and tachycardia pacing (Day 28).26 Next, to assess whether the beneficial effects of the treatments were maintained during recovery from HF, we evaluated cardiac function 3 days after cessation of tachycardia pacing (Day 31). After cessation of tachycardia pacing, the tachycardia-induced DCM model shows recovery from cardiac dysfunction and chamber dilation.27,28 The hemodynamic characteristics of this recovery period are thought to resemble those achieved through appropriate medical therapy in patients with chronic HF.27
As shown in Figure 3 and Table S1, compared with placebo, vidarabine was associated with a significantly (P<0.01) greater degree of LVFS and a lower LAD index on Day 28; these significant (P<0.01) differences were preserved on Day 31. In addition, CO increased gradually after the initiation of vidarabine treatment and was significantly (P<0.01) higher after vidarabine treatment (Day 31) than it had been before (Day 21). Likewise, carvedilol was associated with a significantly (P<0.01) greater degree of LVFS on Day 28 compared with placebo, and this effect was preserved on Day 31. Yet carvedilol significantly (P<0.01) increased the LVIDd and LAD indices on Days 28 and 31 compared with their values before treatment (Day 21). In addition, carvedilol treatment led to a significantly (P<0.01) greater LAD index on Day 28 and significantly (P<0.05) greater LVIDd and LAD indices on Day 31 compared with the placebo- and vidarabine-treated groups. In the placebo-treated group, GFR, which is an index of renal function, tended to decline at Day 28. Under vidarabine or carvedilol treatment, however, GFR remained significantly (P<0.05) higher at the same time point. These results indicate that either vidarabine or carvedilol treatment can prevent the decline of systolic function during HF, enabling the maintenance of a significantly higher level of systolic function during the recovery period, in a canine pacing-induced DCM model. Interestingly, while carvedilol treatment enlarged the left ventricle and atrium, vidarabine did not induce such cardiac remodeling. In addition, vidarabine significantly increased CO, while carvedilol did not cause a significant change in this parameter.

Effects of vidarabine or carvedilol treatment on the parameters of left ventricular function and systemic hemodynamics. (A) Heart rate. (B) Mean arterial pressure (MAP). (C) Glomerular filtration rate (GFR). (D) Left ventricular internal diameter at diastole (LVIDd). (E) Left atrium diameter (LAD) index. (F) Left ventricular fractional shortening (LVFS). (G) Cardiac output (CO). Results are presented as mean±SEM. *P<0.05, **P<0.01. †P<0.01 compared with Day 21. n=6 in each group. The difference in inter- and intragroup was analyzed using 2-way analysis of variance followed by post-hoc testing.
We examined whether vidarabine or carvedilol treatment suppressed apoptosis and fibrosis in the left ventricle and atrium tissues in the canine pacing-induced DCM model. Myocardial apoptosis was evaluated by TUNEL staining, and cardiac interstitial fibrosis was evaluated by Sirius red staining. Both vidarabine and carvedilol treatment significantly (P<0.01) prevented myocardial apoptosis in both the left ventricle (Figure 4A) and the atrium (Figure 4B). To examine the changes in the molecules that are involved in apoptosis signaling, we examined the protein level of Bax, which is a promoter of apoptosis.25 A previous report has shown that chronic tachycardia pacing induces cardiomyocyte apoptosis, with an increasing level of Bax protein expression.29 In this study, Bax protein expression significantly (P<0.01) decreased in the left ventricle of vidarabine- and carvedilol-treated dogs compared with the placebo-treated group (Figure 4C). In addition, vidarabine and carvedilol treatment significantly (P<0.01) suppressed cardiac fibrosis in the left ventricle (Figure 5A) and atrium (Figure 5B) compared with the placebo-treated group.

Effects of vidarabine or carvedilol treatment on myocardial apoptosis in the left ventricle and atrium. The percentage of terminal dUTP nick end-labeling (TUNEL)-positive cells in the left ventricle (A) and left atrium (B). (C) The expression level of the Bax protein in the left ventricle. Results are presented as mean±SEM. **P<0.01. n=6 in each group. The differences between the groups were analyzed using 1-way analysis of variance followed by post-hoc testing.

Effects of vidarabine or carvedilol treatment on cardiac fibrosis in the left ventricle and atrium. Percentage of fibrosis area in the left ventricle (A) and atrium (B). Left panel shows representative left ventricular (A) and atrial (B) sections stained with Sirius red (×100, Scale bars: 100 μm). Results are presented as mean±SEM. **P<0.01. n=6 in each group. The differences between the groups were analyzed using 1-way analysis of variance followed by post-hoc testing.
Cardiac function is regulated by the intracellular Ca2+ concentration, which is modulated by the Ca2+-handling proteins located in the sarcoplasmic reticulum and the sarcolemmal membrane, including sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLN), Na+-Ca2+ exchanger-1 (NCX1), and ryanodine receptor-2 (RYR2).30 Changes in the expression levels of these proteins in failing myocytes have been reported to result in abnormal intracellular Ca2+ homeostasis and cardiac dysfunction.31,32 We then examined the effect of vidarabine treatment on expression of calcium-handling proteins, as shown in Figure 6. Although the protein levels of SERCA2a, PLN and RYR2 were not different among the treatment groups, the protein expression of NCX1 was significantly lower in the vidarabine- and carvedilol-treated groups compared with the placebo-treated group (P<0.01). These results indicate that the suppression of NCX1 expression by vidarabine or carvedilol might be one of the mechanisms underlying the cardioprotective effect of these agents against pacing-induced DCM.

Effects of vidarabine or carvedilol treatment on the expression levels of calcium handling proteins in the left ventricle. Protein expression levels of NCX1 (A), SERCA2a (B), PLN (C), and RYR2 (D) were determined by Western blot analysis. NCX1, Na+-Ca2+ exchanger-1; PLN, phospholamban; RYR2, ryanodine receptor-2; SERCA2a, sarcoplasmic reticulum Ca2+-ATPase. Results are presented as mean±SEM. **P<0.01. n=6 in each group. The differences between the groups were analyzed using 1-way analysis of variance followed by post-hoc testing.
To evaluate the adverse effects of these agents on cardiac function, we examined the suppressive effect of vidarabine and carvedilol on positive chronotropic and inotropic responses to isoproterenol infusion. Although the positive chronotropic response to isoproterenol infusion was significantly (P<0.05) attenuated by both vidarabine and carvedilol compared with the placebo (Figure 7A), it remained significantly (P<0.01) higher in vidarabine-treated dogs than in carvedilol-treated dogs (Figure 7A). Similarly, during a 0.08 μg·kg–1·min–1 isoproterenol infusion, the inotropic response was significantly (P<0.01) attenuated in the carvedilol-treated group, but not in the vidarabine-treated group, compared with the placebo-treated group (Figure 7B).
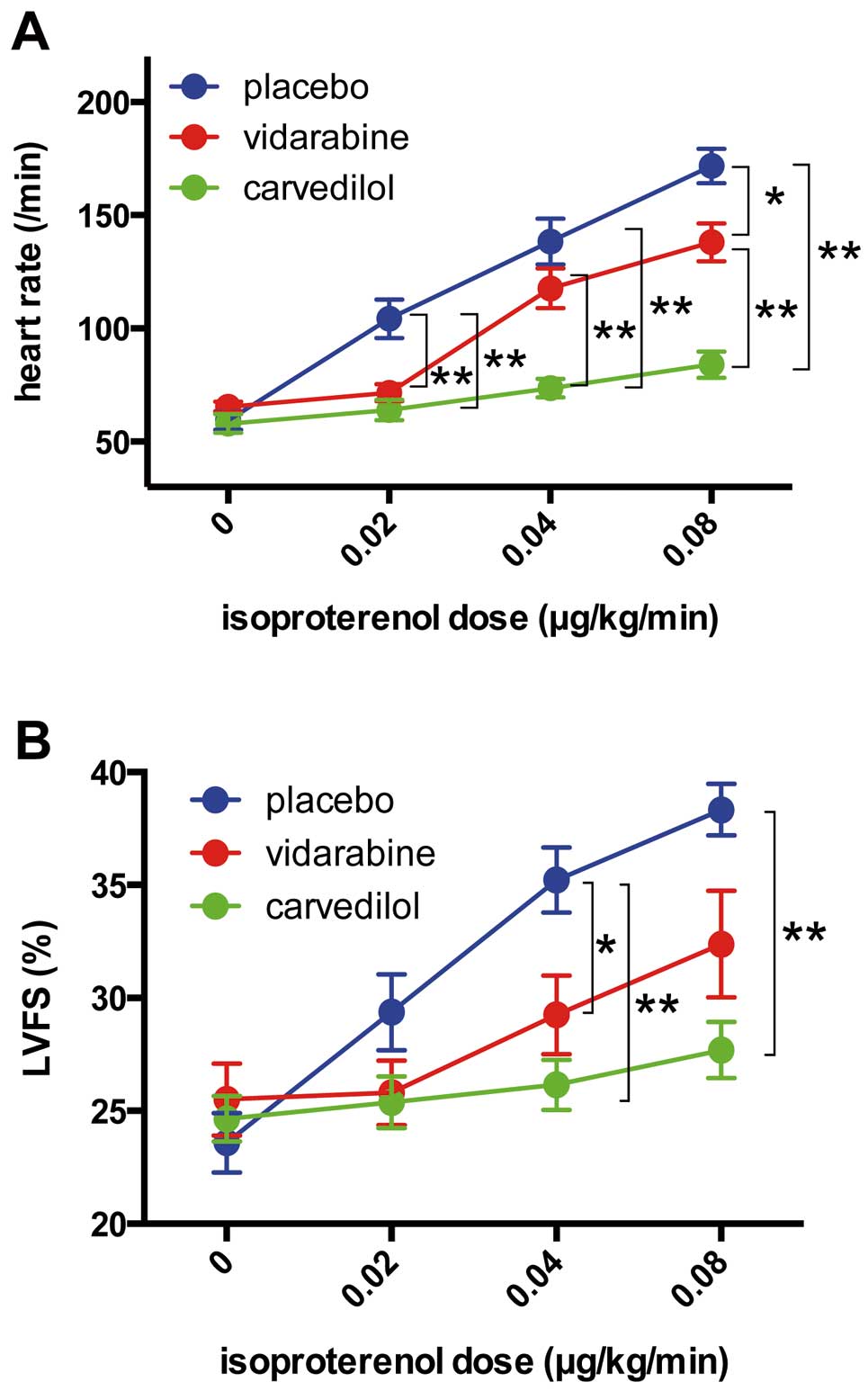
Effects of vidarabine or carvedilol treatment on the chronotropic and inotropic responses to acute β adrenergic stimulation in dogs with pacing-induced dilated cardiomyopathy (DCM). Heart rate (A) and LVFS (B) response to acute β adrenergic stimulation induced by isoproterenol infusion after 10 days of treatment with vidarabine or carvedilol in pacing-induced DCM dogs. LVFS, left ventricular fractional shortening. Results are presented as mean±SEM. *P<0.05, **P<0.01. n=6 in each group. The differences between the groups were analyzed using 1-way analysis of variance for each dose of isoproterenol.
We evaluated the effect of vidarabine on plasma parameters related to liver and kidney dysfunction. As shown in Table S2, there were no differences in the plasma concentrations of alanine aminotransferase, aspartate aminotransferase, urea nitrogen, and creatinine between the vidarabine and placebo groups after 10 days infusion of vidarabine (24 mg·kg–1·day–1) (Day 31).
In this study, we demonstrated that vidarabine prevented the progression of cardiac systolic dysfunction and suppressed myocardial apoptosis and cardiac interstitial fibrosis in a canine pacing-induced DCM model. Previously, we reported that vidarabine prevented the development of HF in a mouse model with myocardial infarction and chronic isoproterenol infusion.16 In this study, as a next step, we evaluated the effects of vidarabine in a large-animal DCM model. In addition, we compared the therapeutic and adverse effects of vidarabine on cardiac function with those of carvedilol, the first-line drug for DCM.33 Our results indicated that vidarabine and carvedilol protect the heart with equal effectiveness against the development of pacing-induced DCM. Importantly, however, vidarabine caused less adverse effects on cardiac function compared with carvedilol.
Previous reports have shown that the expression levels of calcium-handling proteins are altered in dogs with pacing-induced DCM.34,35 The protein expression of NCX1 is increased after the development of HF,31,32,34 and this upregulation is related to impairment of cardiac contractile function.36 In addition, β-blocker treatment suppresses cardiac upregulation in a canine HF model.32 Based on these findings, normalization of the expression levels of calcium-handling proteins by β-blockers has been recognized as one mechanism underlying the beneficial effect of β-blockers in HF therapy.32,35 In this study, vidarabine and carvedilol attenuated the expression of NCX1, which plays a crucial role in the regulation of intracellular Ca2+ concentration, with equal effectiveness.30 The downregulation of NCX1 expression by vidarabine and carvedilol might be one of the mechanisms underlying the cardioprotective effects of these agents against pacing-induced DCM.
We previously reported that vidarabine treatment suppressed cardiac AC activity in mice.16 We evaluated forskolin-stimulated AC activity in the left ventricle of pacing-induced DCM dogs (Day 31). The AC activity in the heart of vidarabine-treated dogs was approximately 30% lower than that in placebo-treated dogs, although the differences did not achieve statistical significance (P=0.10; data not shown). Previous reports showed that cardiac AC activity was decreased in HF model animals.12,18 As the cardiac dysfunction was improved in vidarabine-treated dogs (Figure 3), the difference of HF severity between placebo- and vidarabine-treated groups might attenuate the difference of cardiac AC activity between these groups.
Interestingly, although carvedilol caused enlargement of the left ventricle and atrium, presumably as a result of the exacerbation of cardiac dysfunction,37–39 vidarabine did not cause this type of cardiac remodeling. In addition, the cardiac response to isoproterenol was preserved at a significantly higher level after treatment with vidarabine compared with carvedilol. In the previous study, β-blockade therapy resulted in cardiac dilation due to its negative inotropic effect in patients with chronic HF.37–39 In addition, Bozkurt et al reported that the slope of the velocity of circumferential fiber shortening to left ventricular end-systolic wall stress, a load-independent index of left ventricular contractility, worsened after the initiation of carvedilol despite improvement in left ventricular ejection fraction,40 suggesting that carvedilol therapy exerts a negative inotropic effect during the initiation period, and therefore that carvedilol may cause cardiac enlargement after the initiation of high-dose therapy (1 mg·kg–1·day–1). Vidarabine therapy (24 mg·kg–1·day–1), in contrast, although it is sufficient to attenuate cardiac dysfunction, apoptosis and fibrosis to a degree similar to that of carvedilol therapy, did not cause cardiac enlargement. Additionally, the vidarabine-treated group exhibited higher levels of cardiac response to sympathetic stimulation than the carvedilol-treated group did. Although long-term observation is needed to evaluate whether the maintenance of this response is beneficial or harmful for prognosis, it can be said that, during the period of this study, vidarabine treatment was as useful as carvedilol treatment in protecting against pacing-induced DCM with less adverse effects on cardiac function.
In this study, although both vidarabine and carvedilol treatment improved the LVFS, only vidarabine treatment significantly increased the CO (Figures 3F,G). A previous report showed that mitral regurgitation (MR) may develop in the evolution of HF in the pacing-induced DCM model.41 The severity of MR assessed by the MR jet area/left atrium area ratio was significantly greater in carvedilol-treated dogs compared with vidarabine-treated dogs on Day 28 (P<0.01; data not shown). Based on these findings, we speculated that greater MR is one of the reasons for the absence of the CO increase in carvedilol-treated dogs.
While recommending β-blockers for patients with DCM, the American Heart Association (AHA) guidelines warn healthcare professionals about their adverse effects, including negative inotropic effect, bradycardia and hypotension.33 To avoid the worsening of HF by adverse effects of β-blockers on cardiac function, it is recommended that therapy for patients with severe systolic dysfunction is initiated at low doses and gradually increased under careful monitoring for adverse signs.1,33 Because carvedilol yields improvement of cardiac function in a dose-related manner,42 however, this complicated initiation protocol increases the time required to obtain the beneficial effects of carvedilol.
In this study, we administered vidarabine at a dose of 24 mg·kg–1·day–1 and carvedilol at a dose of 1.0 mg·kg–1·day–1. This dose of carvedilol is equivalent to a final maintenance dose, which is recognized as the optimal dose for obtaining the maximum beneficial effect of carvedilol.3,4 The dose of vidarabine used in the present study, meanwhile, is 1.6-fold the maximum daily dose for herpes viral infection.14 A recent study found that no significant effects on hemodynamic and electrophysiological parameters were caused by 10 min of intravenous administration of vidarabine (100 mg/kg).17 Our results demonstrate that vidarabine (24 mg·kg–1·day–1) and carvedilol (1.0 mg·kg–1·day–1) prevented pacing-induced systolic dysfunction and development of apoptosis and fibrosis with equal effectiveness. In addition, vidarabine did not cause cardiac enlargement. These results indicate that vidarabine (24 mg·kg–1·day–1) can be initiated safely and that complicated initiation protocols, such as that recommended for β-blockers, may not be necessary, even for patients with severe cardiac dysfunction.
Vidarabine is FDA-approved and has been used as an anti-herpes virus drug for many decades.14 There have been no reports, however, about its possible adverse effects on liver and kidney function in HF patients. In our canine model with pacing-induced DCM, enzymes related to liver and kidney function, including alanine aminotransferase, aspartate aminotransferase, urea nitrogen and creatinine,43 were not significantly changed 10 days after the initiation of vidarabine treatment (24 mg·kg–1·day–1). In addition, GFR, the index of kidney function, was also preserved at its baseline level in the vidarabine- and carvedilol-treated groups, while that in the placebo-treated group decreased during progression of HF. Although the effect of long-term vidarabine administration needs to be examined, the present study results suggest the short-term safety of vidarabine (24 mg·kg–1·day–1) continuous therapy in a canine pacing-induced DCM model with regard to liver and kidney function.
Study LimitationsIn this study, we observed the effects of 7 and 10 days’ treatment of each agent (vidarabine and carvedilol). These periods might be too short to fully examine the beneficial effect of both agents.3,4,40 In addition, in this study, carvedilol therapy was initiated at a relatively high dose (1.0 mg·kg–1·day–1) and caused cardiac enlargement, indicating that the high-dose initiation of carvedilol therapy may cause an adverse effect in this HF model. Relatively low-dose initiation followed by a stepwise increase might prevent the adverse effect. Importantly, in contrast, high-dose initiation of vidarabine therapy did not cause such an adverse effect and showed similar preventive effects compared with carvedilol therapy against the development of HF in this model.
In order to evaluate cardiac function, we used the LVFS in this study. However, LVFS is known as a load-dependent index. Our results showed no significant differences in mean arterial pressure and heart rate between the 3 groups. In contrast, it is noteworthy that carvedilol-treated dogs showed an increased LVIDd on Day 28 and 31 compared with Day 21 (Figure 3D), which was not seen in the other groups. In addition, as mentioned previously, significantly more severe MR was observed in carvedilol-treated dogs on Day 28 compared with vidarabine-treated dogs. These findings indicate that the left ventricular preload may be increased in the carvedilol-treated dogs. Therefore, it is possible that we have overestimated the cardiac function of the carvedilol-treated group. Importantly, even if we consider this point, we may be able to conclude that the preventive effect of vidarabine treatment against the pacing-induced cardiac systolic dysfunction was at least equal to that of carvedilol treatment.
In conclusion, vidarabine prevented pacing-induced systolic dysfunction and development of apoptosis and fibrosis to a degree similar to that achieved by carvedilol in a canine pacing-induced DCM model. Importantly, vidarabine did not cause cardiac enlargement. In addition, the response of cardiac function to sympathetic activation was maintained at a higher level in the vidarabine-treated group than in the carvedilol-treated group. These findings indicate that, compared with carvedilol, vidarabine may be a safer, yet equally cardioprotective anti-sympathetic agent against the development of HF in patients with severe cardiac dysfunction.
The authors thank Naoko Doi for her excellent technical assistance.
This study was supported, in part, by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (16K08501 to T.F., 16H05300, 16K15205 to Y.I.); the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant (22136009 to Y.I.); the Japan Agency for Medical Research and Development (AMED) (66890005, 66890011, 66890001, 66890023 to Y.I.) and a Grant for Strategic Research Promotion of Yokohama City University (T.F.).
All authors declare no conflicts of interest.
Supplementary File 1
Methods
Table S1. Effects of vidarabine or carvedilol treatment on the parameters of left ventricular function and systemic hemodynamics
Table S2. Plasma parameters in dogs 10 days after treatment
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0736