Abstract
Background:
Systolic blood pressure (SBP) is an important prognostic indicator for patients with acute heart failure (AHF). However, its changes and the effects in the different phases of the acute management process are not well known.
Methods and Results:
The Tokyo CCU Network prospectively collects on-site information about AHF from emergency medical services (EMS) and the emergency room (ER). The association between in-hospital death and SBP at 2 different time points (on-site SBP [measured by EMS] and in-hospital SBP [measured at the ER; ER-SBP]) was analyzed. From 2010 to 2012, a total of 5,669 patients were registered and stratified into groups according to both their on-site SBP and ER-SBP: >160 mmHg; 100–160 mmHg; and <100 mmHg. In-hospital mortality rates increased when both on-site SBP and ER-SBP were low. After multivariate adjustment, both SBPs were inversely associated with in-hospital death. Notably, the risk for patients with ER-SBP of 100–160 mmHg (intermediate risk) differed according to their on-site SBP; those with on-site SBP <100 or 100–160 mmHg were at higher risk (OR, 7.39; 95% CI, 4.00–13.6 and OR, 2.73; 95% CI, 1.83–4.08, respectively [P<0.001 for both]) than patients with on-site SBP >160 mmHg.
Conclusions:
Monitoring changes in SBP assisted risk stratification of AHF patients, particularly patients with intermediate ER-SBP measurements. (Circ J 2016; 80: 2473–2481)
Acute heart failure (AHF) is frequently encountered by personnel of the emergency medical service (EMS) and in the emergency room (ER), and it is associated with several million hospitalizations worldwide each year, high mortality and morbidity rates, and high costs.1,2
Despite the introduction of effective treatments for HF, 30-day mortality and readmission rates remain high,2
and its management, particularly in the acute phase, remains a challenge.
Systolic blood pressure (SBP) is known to be an important prognostic indicator in patients with AHF.3–8
The recent American College of Cardiology Foundation/American Heart Association (ACCF/AHA) heart failure guideline mentions the clinical application of several risk models, including one related to SBP, and further recommends that patients with clinically significant hypotension (SBP typically <90 mmHg or symptomatic low SBP) and/or worsening renal function during initial therapy might also benefit from invasive hemodynamic measurement.9
SBP is a benchmark for tailoring the treatment for AHF, such as vasodilators, diuretics, and inotropes.10
However, the prognostic implications of changes in SBP as measured during different stages of acute management for AHF are unknown. Typically, during the initial phase of AHF treatment, the patient’s overall condition is evaluated during different phases, and SBP is measured on-site by the EMS staff, on arrival at the ER, and at hospital admission. One study reported changes in SBP during the course of hospitalization,11
but, to date, the value of measuring SBP during acute triage has not been thoroughly investigated.
In the present study, we aimed to evaluate the associations between SBP measured at 2 time points (pre-hospital [on-site by the EMS staff] and in hospital [at the ER]) and the clinical outcomes of patients with AHF. Monitoring alterations in SBP may provide additional information for tailoring the initial treatment strategy during acute triage of these patients. Additionally, assessing the prognostic implications may improve our understanding of AHF.
Methods
Study Design
The design of the Tokyo CCU Network (TCN) database has been previously reported.12,13
Briefly, the TCN database is an ongoing multicenter registry that prospectively collects information from both EMS (Tokyo Metropolitan EMS) and investigators at participating hospitals on emergency admissions to acute cardiac facilities. Its aim is to describe the demographic and clinical characteristics of patients hospitalized with acute cardiovascular diseases. All patients admitted to cardiac care units (CCUs) whose information had been catalogued by the TCN were eligible for participation in the present study. By December 2015, 73 hospitals, serving a population of 1.3 million individuals in the metropolitan Tokyo area, were included in the TCN registry.
Each TCN hospital is accredited by the Metropolitan Tokyo Government and participates in the Tokyo citywide system of acute cardiac care (acute myocardial infarction, unstable angina, arrhythmia, AHF, aortic dissection, and pulmonary embolism). Written or oral informed consent was given in all cases by the patients, if appropriate, and/or by legal representative during their hospital admission according to the institution’s protocol. Both types of informed consent were approved by the TCN Scientific Committee, as the study did not identify individual patients. In addition, the TCN registry conducts an annual epidemiological survey supported by the Tokyo Metropolitan Government. Individual clinical information is recorded into the database by TCN members at each institution, and the final datasets are collected by the TCN Scientific Committee under conditions of anonymity, according to the ethical guidelines on epidemiological surveys released from the Japanese Ministry of Health, Labor, and Welfare. The protocol of the current study was approved by the Institutional Review Board of Keio University School of Medicine and the study was conducted in accordance with the Declaration of Helsinki.
Study Sample
For the present analysis, data for consecutive patients registered in the AHF dataset were extracted from the TCN database; patients presenting with acute coronary syndrome were registered in a separate dataset and were therefore not included in this analysis. We identified 6,232 AHF patients who were transferred via ambulance to a participating hospital between 2010 and 2012. Patients who presented with out-of-hospital cardiac arrest (n=90; 1.4%) and those with missing data for SBP measured in the ER (ER-SBP; n=473; 7.6%) were excluded. Their main clinical characteristics did not differ from those of the patients included in the final analysis, other than for ischemic etiology (30.1% vs. 21.1% in the excluded patients) and prior admissions for HF (31.9% vs. 15.6% in the excluded patients) (Table S1). Finally, data from 5,669 patients were analyzed in the present study (Figure 1).
EMS in Japan and BP Measurement
In Japan, non-private municipal emergency operation systems provide EMS through 800 fire stations via dispatching centers. The paramedics, similar to those in the Western system, provide medical care at an advanced life support level, though they have some limitations (eg, they are unable to use non-invasive positive airway pressure ventilation [NIPPV] or to administer drugs to patients, except for adrenalin in the case of cardiopulmonary arrest).14,15
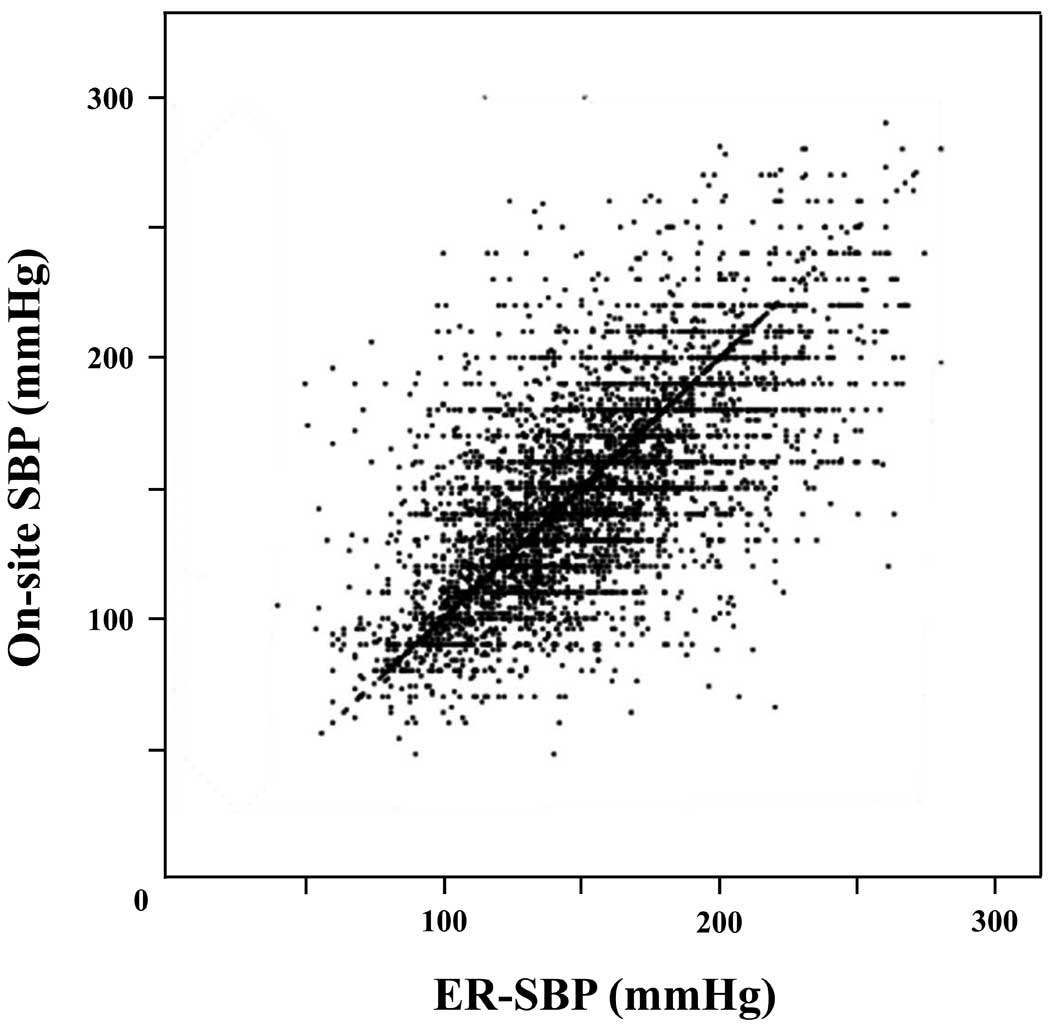
Pre-hospital SBP was measured on-site by the EMS team (on-site SBP), and the in-hospital SBP was measured in the emergency room (ER-SBP) by hospital triage staff. The on-site SBP was defined as SBP measured by EMS on arrival at the scene. ER-SBP was defined as SBP assessed before the initiation of HF therapy. The distribution of SBP in the AHF patients is shown in
Figure 2.
Outcomes and Definitions of Variables
In the present study, the primary outcome was all-cause in-hospital death. All AHF patients were diagnosed using variables estimated on admission using the Framingham criteria.16
The estimated glomerular filtration rate (eGFR) was calculated using serum creatinine levels and the Modification of Diet in Renal Disease study equation.17
Anemia was defined as hemoglobin level <13 g/dl for men and <12 g/dl for women, according to the World Health Organization criteria.18
When N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured instead of BNP, the NT-proBNP value was converted to the BNP values using an equation established in a previous study.19
Statistical Analysis
Results are expressed as mean±standard deviation or median with interquartile range (IQR) for continuous variables and as percentages for categorical variables, as appropriate. Statistical comparisons were performed using analysis of variance or Kruskal-Wallis tests for continuous variables and the Pearson’s chi-squared test for categorical variables. Missing data were rare (<5%) for all variables, except for left ventricular ejection fraction (LVEF) (10.0%) and BNP levels (23.8%). Missing values were imputed as follows: (1) for variables pertaining to comorbidity and medical treatment during hospitalization, missing data were imputed to “no”; (2) for body mass index, missing values were imputed to the sex-specific median; (3) for LVEF, missing values were imputed to the median value of the entire cohort; and (4) for serum creatinine, missing values were imputed to the sex- and prior renal failure-specific medians.
We subdivided the patients by 20-mmHg strata of ER-SBP, and the distribution of in-hospital mortality rates was evaluated to determine whether the relationship was linear (Figure 3A). A similar presentation was prepared for the on-site SBP values (Figure 3B). Based on the patients’ mortality risk in the SBP groups, we categorized the patients into 9 groups by on-site SBP and ER-SBP: (1) low risk (mortality rate <5%), SBP >160 mmHg; (2) intermediate risk (mortality rate 5–15%), SBP 100–160 mmHg; and (3) high risk (mortality rate >15%), SBP <100 mmHg. We then evaluated the unadjusted and adjusted relationships between SBP and in-hospital deaths using logistic regression models. The multivariate model was adjusted for age, sex, prior admissions for HF, chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CVD), atrial fibrillation (AF), anemia, eGFR, and LVEF using a logistic regression model with a backward step-down variable deletion, removing terms with P>0.2. All probability values were 2-tailed, and P<0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS version 19.0 (SPSS Inc, Chicago, IL, USA).

Results
Patients’ Characteristics
The baseline characteristics of the study patients based on ER-SBP are summarized in
Table 1. The patients were predominantly male (56.7%), with an average age of 75.9±12.3 years. Patients with both higher on-site SBP and ER-SBP had higher heart rates and worse respiratory conditions, as suggested by respiratory rates and oxygen saturation. Among the patients with ER-SBP <100 mmHg, prior admissions for HF, CVD, and AF were more frequent than in patients with ER-SBP >160 mmHg (all P<0.001). Laboratory findings revealed lower hemoglobin levels, poorer renal function, and higher C-reactive protein and BNP levels in patients with ER-SBP <100 mmHg than in those with ER-SBP >160 mmHg or 100–160 mmHg. With regard to LVEF, it was likely to be lower in patients with ER-SBP <100 mmHg.
Table 1.
AHF Patients’ Characteristics Based on SBP in the ER (ER-SBP)
| Variable |
All patients
(n=5,669) |
SBP >160 mmHg
(n=2,142) |
SBP 100–160 mmHg
(n=3,090) |
SBP <100 mmHg
(n=437) |
P value |
| Age, year |
75.9±12.3 |
75.3±12.1 |
76.3±12.9 |
73.3±13.4 |
<0.001 |
| Male, % |
56.7 |
58.5 |
55.7 |
55.9 |
0.133 |
| BMI, kg/m2 |
22.3±4.3 |
22.9±4.4 |
22.0±4.3 |
21.1±3.7 |
<0.001 |
| Time from EMS call to ER arrival, min# |
35 (26–47) |
34 (27–46) |
35 (26–46) |
37 (27–50) |
<0.001 |
| EMS call to scene, min# |
8 (6–10) |
8 (6–10) |
8 (6–10) |
8 (6–10) |
0.207 |
| Scene time, min# |
17 (13–23) |
17 (14–23) |
17 (13–22) |
18 (14–24) |
0.182 |
| Transportation time, min# |
10 (7–14) |
9 (7–13) |
10 (7–14) |
11 (7–16) |
<0.001 |
| Vital signs on-site |
| SBP, mmHg |
149±38 |
178±33 |
136±28 |
103±30 |
<0.001 |
| DBP, mmHg |
85±34 |
99±43 |
79±23 |
62±19 |
<0.001 |
| Pulse pressure, mmHg |
65±36 |
79±45 |
58±25 |
43±19 |
<0.001 |
| HR, beats/min |
102±34 |
108±26 |
100±39 |
95±30 |
<0.001 |
| Respiratory rate, /min |
25±8 |
27±7 |
24±8 |
23±6 |
<0.001 |
| Oxygen saturation (SpO2), % |
91 (82–96) |
88 (79–94) |
92 (85–96) |
94 (86–97) |
<0.001 |
| Vital signs at the ER |
| SBP, mmHg |
151±39 |
191±25 |
133±17 |
86±12 |
<0.001 |
| DBP, mmHg |
86±25 |
105±24 |
78±17 |
54±12 |
<0.001 |
| Pulse pressure, mmHg |
65±27 |
87±25 |
55±18 |
33±12 |
<0.001 |
| Heart rate, beats/min |
102±28 |
109±28 |
98±27 |
94±29 |
<0.001 |
| Respiratory rate, /min |
25±8 |
28±8 |
24±8 |
23±8 |
<0.001 |
| Oxygen saturation (SpO2), % |
96 (91–98) |
95 (90–98) |
96 (93–99) |
97 (93–99) |
<0.001 |
| Comorbidities, % |
| Ischemic etiology |
30.1 |
28.5 |
31.4 |
28.0 |
0.055 |
| Prior admissions for HF |
31.9 |
26.4 |
33.0 |
47.7 |
<0.001 |
| Hypertension |
61.9 |
72.5 |
58.2 |
42.1 |
<0.001 |
| Diabetes |
32.4 |
34.4 |
31.7 |
29.4 |
0.048 |
| Dyslipidemia |
23.3 |
24.9 |
23.1 |
18.5 |
0.012 |
| CVD |
10.4 |
9.0 |
10.7 |
13.8 |
0.007 |
| COPD |
6.2 |
5.3 |
6.7 |
6.7 |
0.131 |
| AF |
31.6 |
24.8 |
34.4 |
40.2 |
<0.001 |
| Laboratory findings |
| Hemoglobin, g/dl |
11.9±3.1 |
12.2±3.9 |
11.7±2.5 |
11.3±2.6 |
<0.001 |
| Serum creatinine, g/dl |
1.10 (0.80–1.70) |
1.10 (0.81–1.70) |
1.10 (0.81–1.61) |
1.33 (0.96–2.10) |
<0.001 |
| C-reactive protein, g/dl |
0.70 (0.20–2.40) |
0.50 (0.19–1.58) |
0.83 (0.30–3.00) |
1.30 (0.30–4.30) |
<0.001 |
| BNP, pg/ml† |
768 (386–1,447) |
745 (375–1,364) |
768 (387–1,477) |
899 (440–1,623) |
<0.001 |
| LVEF, % |
43±16 |
45±15 |
42±17 |
37±18 |
<0.001 |
| Treatment for HF in-hospital admission |
| NIPPV, % |
24.1 |
30.3 |
20.8 |
16.8 |
<0.001 |
| Tracheal intubation, % |
10.6 |
9.7 |
9.8 |
21.0 |
<0.001 |
| Dialysis, % |
6.4 |
7.1 |
5.2 |
10.8 |
<0.001 |
| IABP, % |
2.3 |
1.5 |
2.3 |
6.6 |
<0.001 |
| PCPS, % |
0.9 |
0.7 |
0.7 |
2.4 |
0.002 |
| Dobutamine IV, % |
13.9 |
6.7 |
15.6 |
38.0 |
<0.001 |
| Dopamine IV, % |
10.7 |
6.2 |
11.3 |
28.2 |
<0.001 |
| Noradrenalin IV, % |
7.0 |
5.1 |
6.3 |
21.1 |
<0.001 |
| Digoxin IV, % |
11.0 |
7.8 |
12.8 |
14.2 |
<0.001 |
| Furosemide IV, % |
86.9 |
85.8 |
88.6 |
80.4 |
<0.001 |
| Carperitide IV, % |
48.6 |
52.4 |
48.4 |
31.1 |
<0.001 |
| ACEI and/or ARB PO, % |
53.5 |
62.1 |
50.0 |
35.3 |
<0.001 |
| β-blocker PO, % |
42.3 |
45.0 |
40.9 |
38.2 |
0.004 |
| Aldosterone antagonist PO, % |
34.3 |
33.4 |
35.2 |
32.1 |
0.253 |
| Warfarin PO, % |
33.6 |
26.3 |
37.1 |
43.5 |
<0.001 |
| Hospitalization, days |
15 (8–25) |
14 (8–22) |
15 (8–26) |
17 (7–30) |
<0.001 |
Data are reported as % for binary variables and as mean±standard deviation or median (quartile 1 to quartile 3) for continuous variables. #EMS time data were missing for 1,845 patients (32.5%). †Data for 4,321 patients (76.2%) were available. ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AHF, acute heart failure; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DBP, diastolic blood pressure; EMS, emergency medical service; ER, emergency room; HF, heart failure; HR, heart rate; IABP, intra-aortic balloon pumping; IV, intravenous; LVEF, left ventricular ejection fraction; NIPPV, non-invasive positive airway pressure ventilation; PO, per os; PCPS, percutaneous cardiopulmonary support; SBP, systolic blood pressure.
Among patients with ER-SBP >160 mmHg, NIPPV was used more frequently, whereas tracheal intubation was performed more frequently in those with ER-SBP <100 mmHg. In addition, the use of assisted circulation devices, such as intra-aortic balloon pumping and percutaneous cardiopulmonary support, and inotropes occurred frequently in patients with ER-SBP <100 mmHg. No differences were observed in the prescription rate of aldosterone antagonist, but there were significant differences in prescription rates for angiotensin-converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB) and β-blockers (BB); either ACEI/ARB or BB were more frequently prescribed in patients with ER-SBP >160 mmHg (P<0.001 and 0.004, respectively).
SBP and In-Hospital Deaths
Overall, 432 (7.7%) patients died during hospitalization (64 [3.0%] in the ER-SBP >160 mmHg group; 274 [8.9%] in the ER-SBP 100–160 mmHg group; and 94 [21.5%] in the ER-SBP <100 mmHg group), with a lower ER-SBP being significantly associated with increased in-hospital mortality rates in the univariate logistic regression analysis (odds ratio [OR] for each 10-mmHg increment, 0.83; 95% confidence interval [CI], 0.80–0.85; P<0.001). Similarly, on-site SBP was also significantly associated with in-hospital mortality (OR for each 10-mmHg increment, 0.81; 95% CI, 0.78–0.83; P<0.001).
We then evaluated the prognostic value of on-site SBP within the 3 subgroups stratified by ER-SBP (<100, 100–160, and >160 mmHg). Among the patients with intermediate ER-SBP (100–160 mmHg), on-site SBP was significantly and linearly associated with in-hospital death (OR for each 10-mmHg increment, 0.83; 95% CI, 0.79–0.88; P<0.001). On-site SBP also showed a similar association in patients with high ER-SBP (>160 mmHg), but the relationship was not linear, but spline. There was no association between on-site SBP and in-hospital death in the low ER-SBP (<100 mmHg) subgroup.
We further stratified the patients into 9 groups based on the combination of on-site SBP with ER-SBP and evaluated the in-hospital mortality rates (Figure 4). After multivariate adjustment for age, sex, and other significant variables (Table 2), on-site SBP remained associated with in-hospital death, particularly in patients with ER-SBP between 100 and 160 mmHg (Figure 5).

Table 2.
Covariates Other Than SBP in a Logistic Regression Analysis for In-Hospital Death of AHF Patients
| |
Univariate analysis |
Multivariate analysis |
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
| Age (1-year increments) |
1.04 (1.03–1.05) |
<0.001 |
1.04 (1.02–1.05) |
<0.001 |
| Female |
1.20 (0.99–1.46) |
0.069 |
|
|
| Prior admissions for HF |
1.61 (1.32–1.97) |
<0.001 |
1.25 (0.97–1.60) |
0.084 |
| CVD |
1.50 (1.12–2.00) |
0.006 |
1.36 (0.95–1.93) |
0.093 |
| COPD |
2.15 (1.55–2.98) |
<0.001 |
2.01 (1.35–2.99) |
0.001 |
| AF |
1.20 (0.98–1.48) |
0.083 |
|
|
| Anemia |
1.96 (1.57–2.46) |
<0.001 |
1.33 (0.99–1.77) |
0.054 |
| eGFR (1 ml/min/1.73 m2 decrements) |
0.98 (0.98–0.99) |
<0.001 |
0.99 (0.98–1.00) |
0.030 |
| LVEF |
0.99 (0.99–1.00) |
0.070 |
|
|
CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio. Other abbreviations as in Table 1.

Among the patients with ER-SBP between 100 and 160 mmHg, a higher proportion of prior admissions for HF, lower hemoglobin levels, higher BNP levels, and worse renal function was noted for patients with on-site SBP <100 mmHg (Table S2). We also constructed an additional model including BNP level as a covariate, for there was a significant difference in its value among the 3 ER-SBP subgroups. Although there were a modest number of patients without BNP measurement data at the time of admission (23.8%), on-site SBP remained a significant predictor of in-hospital death within the intermediate ER-SBP subgroup (Table S3).
Discussion
In the present AHF dataset, constructed in collaboration with EMS and CCU services in metropolitan Tokyo, both the on-site SBP and the ER-SBP were powerful prognostic indicators for patients with AHF. Measuring the change in SBP while patients were in the acute setting enabled us to precisely discriminate those at high or low risk. Specifically, among patients with ER-SBP between 100 and 160 mmHg, on-site SBP added an additional value for predicting in-hospital death, even after adjustment for known predictors. To our knowledge, this is the first study to investigate the relationship of the change in on-site SBP (assessed on-site by the EMS) and ER-SBP and its association with in-hospital death of patients with AHF.
SBP is a well-known powerful prognostic indicator in patients with HF, to the same extent in both the acute and chronic phases.3–8
SBP is included in several risk stratification models for HF recommended in the ACCF/AHA guideline for the management of heart failure:9
the Acute Decompensated Heart Failure National Registry (ADHERE) risk tree,3
the Get-With-The-Guideline Heart-Failure (GWTG-HF) Model,6
and the Seattle Heart Failure Model (SHFM).7
Ambrosy et al previously demonstrated, from the Efficacy of Vasopressin Antagonism in Heart Failure (EVEREST) trial, that both the SBP after initial HF therapy during hospitalization and the SBP at discharge were independent clinical predictors of morbidity and mortality in patients hospitalized for AHF.11
To date, the clinical significance of the change in SBP during acute management of AHF has been understudied.
Unlike the previous study,11
our study focused less on the absolute change in SBP, as a result of the response to treatment, by nature of the study’s design. In the present study, pre-hospital intervention for AHF patients was oxygenation only; neither drugs nor NIPPV was used. Therefore, our results indicate that the monitoring of changes in SBP itself is important for AHF patients as part of the natural course, rather than during medical intervention in the pre-hospital phase or during hospitalization. The change in SBP from pre-hospital to ER triage can provide new insights into the natural history of early AHF, and acute cardiopulmonary edema. Although the results of the present study demonstrated that the time interval between the EMS call and ER arrival was relatively short (median, 35 min) and subsequently that advanced treatment in the ER begins early in the metropolitan Tokyo area, the on-site SBP is more important in rural regions, where it takes much longer to transfer patients to hospital.
As our results described, several important differences were observed among patients with ER-SBP between 100 and 160 mmHg. These factors are known to be associated with prognosis in patients with HF: prior admissions for HF;20
anemia;7,21,22
and BNP level.23–26
In particular, renal impairment is a powerful prognostic factor in patients with AHF.3
In addition, Mitsnefes et al have reported that patients with chronic renal impairment, particularly those on dialysis, have significantly low contractile reserve.27
In the present analysis, our results indicated that a change in SBP during patient stay in the acute setting was not associated with renal function or other factors, although a change in SBP itself does not reflect cardiac contractile reserve; therefore, SBP change should be considered a useful clinical predictor in patients with AHF.
EMS systems are different in each country or can also vary within regions of the same country, with respect to the expertise of paramedics, availability of diagnostic and therapeutic equipment, and ability to administer drugs. In Japan, paramedics have several limitations, including the inability to use NIPPV and to administer drugs, except for adrenalin in the case of cardiopulmonary arrest.14,15
As our results demonstrated that on-site SBP was associated with the in-hospital death of AHF patients, early treatment initiation, such as fluid infusion, should be considered to improve prognosis, especially among high-risk patients presenting with hypotension and hypoperfusion. Patients in a critical condition may even benefit from administration of pre-hospital drugs during EMS transfer.28
Hemodynamic improvement has been an important target in contemporary AHF therapy, although little evidence exists in this area. Paramedics with sufficient training for HF care and with the ability to provide appropriate management under physicians’ control may be needed to accurately assess the benefit of these interventions.
Study Limitations
The present study using the TCN database was limited by several factors. First, the data were obtained from an observational study, and unmeasured factors may have influenced the clinical outcomes. We had no information regarding ER management. Furthermore, there were substantial numbers of patients who did not have their BNP level measured at the time of hospital admission. It is possible that patients with typical AHF symptoms frequently do not have BNP measured, and this may lead to bias on the predictive value of SBP. Second, this registry is geographically limited to the metropolitan Tokyo area, where there is a dense network of hospitals and EMS.
Conclusions
In our study, both on-site SBP and ER-SBP were independent predictors in patients with AHF; in particular, among patients with ER-SBP between 100 and 160 mmHg, on-site SBP provided an additional value for identifying high-risk patients. Monitoring alterations in SBP aided the stratification of AHF patients by risk.
Acknowledgments
We thank Ms Nobuko Yoshida, and other staff of the Tokyo CCU Network Scientific Committee for their important contributions.
Conflict of Interest
The authors have stated that no such relationships exist and provide the following details: S.K. received lecture fees from Pfizer Japan Inc, and an unrestricted research grant for the Department of Cardiology, Keio University School of Medicine from Bayer Pharmaceutical Co, Ltd. Other authors have no conflicts of interest to disclose. There are no patents, products in development or marketed products to declare.
Funding
This research received no grants from any funding agency in the public, commercial or not-for-profit sectors.
Appendix 1
Participating Hospitals and the Leading Members of the Tokyo CCU Network Scientific Committee
Mitsui Memorial Hospital, Kazuhiro Hara; Nihon University Itabashi Hospital, Tadateru Takayama; Juntendo University Hospital, Hiroyuki Daida; Toho University Ohashi Medical Center, Masato Nakamura; Saiseikai Central Hospital, Shin Nakagawa; Showa University Hospital, Youichi Kobayashi; Sakakibara Heart Institute, Tetsuya Sumiyoshi; Kosei General Hospital, Masao Kawaguchi; St.Luke’s International Hospital, Yutaro Nishi; Tokyo Women’s Medical University Hospital, Nobuhisa Hagiwara; Nishiarai Heart Center Hospital, Katsumi Saito; Teikyo University Hospital, Takaaki Isshiki; The Cardiovascular Institute, Akira Koike; Toranomon Hospital, Haruo Mitani; Toho University Omori Medical Center, Nobuya Koyama; Surugadai Nihon University Hospital, Ken Nagao; Tokyo Medical University Hachioji Medical Center, Hiroshi Kobayashi; Kyorin University Hospital, Hideaki Yoshino; Tokyo Metropolitan Geriatric Hospital, Kazumasa Harada; Tokyo Metropolitan Hiroo Hospital, Harumizu Sakurada; Nippon Medical School Hospital, Keiji Tanaka; Tokyo Women’s Medical University Medical Center East, Kuniaki Otsuka; Bokutoh Metropolitan General Hospital, Toru Iwama; Tobu Chiiki Hospital, Takashi Tamura; Musashino Red Cross Hospital, Tohru Obayashi; Showa General Hospital, Yuji Kira; Tokyo Metropolitan Tama Medical Center, Tetsuro Ueda; Disaster Medical Center, Yasuhiro Satoh; Ome Municipal General Hospital, Shigeo Shimizu; Edogawa Hospital, Yoji Oohira; Tokyo Rinkai Hospital, Toru Kohno; Jikei University Katsushika Medical Center, Shingo Seki; IMS Katsushika Heart Center, Shigehiko Yoshida; Ayase Heart Hospital, Imun Tei; Hakujikai Memorial Hospital, Kunio Tanaka; The Jikei University Hospital, Michihiro Yoshimura; Senpo Tokyo Takanawa Hospital, Masato Yamamoto; NTT Medical Center Tokyo, Masao Yamazaki; Ikegami General Hospital, Takao Machimura; The University of Tokyo Hospital, Issei Komuro; Tokyo Metropolitan Police Hospital, Tetsuro Shirai; Kohseichuo General Hospital, Akio Hirai; Mishuku Hospital, Akimi Uehata; Keio University Hospital, Keiichi Fukuda; Tokyo Medical University Hospital, Akira Yamashina; National Center for Global Health and Medicine, Hisao Hara; Kawakita General Hospital, Yoichi Sugimura; Itabashi Chuo Medical Center, Hiroshi Ota; Toshima Hospital, Takashi Shibui; Nishitokyo Central General Hospital, Hiroyuki Suesada; Higashiyamato Hospital, Masao Kuwada; Tokai University Hachioji-hospital, Yoshinori Kobayashi; Nippon Medical School Tama-Nagayama Hospital, Hirotsugu Atarashi; Tama Nambu Chiiki Hospital, Tatenori Suzuki; Tokyo Medical and Dental University, Mitsuaki Isobe; Japanese Red Cross Medical Center, Hiroshi Ikenouchi; Kanto Central Hospital, Akira Nozaki; National Hospital Organization Tokyo Medical Center, Yukihiko Momiyama; Social Insurance Central General Hospital, Makoto Noda; Tokyo Kosei Nenkin Hospital, Seiji Ayabe; Nihon University Nerima Hikarigaoka hospital, Seiji Fukushima; Jikei University Daisan Hospital, Takahiro Shibata; Tokyo-kita Social Insurance Hospital, Yoshio Tsuruya; Kasai Cardiology & Neurosurgery Hospital, Akihiro Hata; Tokyo Heart Center, Joji Hosokawa; Ogikubo Hospital, Yasuhiro Ishii; Tama-Hokubu Medical Center, Satoshi Murasaki (lead author, Morimasa Takayama [mtakaya@shi.heart.or.jp]).
Appendix 2
Affiliations of Each Author Member of the Tokyo CCU Network Scientific Committee
• Kazumasa Harada, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan
• Takamichi Miyamoto, Musashino Red Cross Hospital, Tokyo, Japan
• Shuzou Tanimoto, Mitsui Memorial Hospital, Tokyo, Japan
• Kiyoshi Iida, Nihon University Surugadai Hospital, Tokyo, Japan
• Tetsuro Sakai, Showa University school of Medicine, Tokyo, Japan
• Tetsuro Miyazaki, Juntendo University Graduate School of Medicine, Tokyo, Japan
• Mayuko Yagawa, Sakakibara Heart Institute, Tokyo, Japan
• Kenichi Matsushita, Kyorin University School of Medicine, Tokyo, Japan
• Shuta Furihata, Omori Red Cross Hospital, Tokyo, Japan
• Naoki Sato, Nippon Medical School Musashi-Kosugi Hospital, Kanagawa, Japan
• Keiichi Fukuda, Keio University School of Medicine, Tokyo, Japan
• Takeshi Yamamoto, Nippon Medical School Hospital, Tokyo, Japan
• Ken Nagao, Nihon University Surugadai Hospital, Tokyo, Japan
• Morimasa Takayama, Sakakibara Heart Institute, Tokyo, Japan
Supplementary Files
Supplementary File 1
Table S1.
Characteristics of included and excluded patients in a study of AHF
Table S2.
Characteristics of the AHF patients with ER-SBP ranging from 100 to 160 mmHg according to on-site SBP
Table S3.
Covariates of logistic regression model including BNP in a study of AHF
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0837
References
- 1.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics – 2015 update: A report from the American Heart Association. Circulation 2015; 131: e29–e322, doi:10.1161/CIR.0000000000000152.
- 2.
Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA 2010; 303: 2141–2147.
- 3.
Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005; 293: 572–580.
- 4.
Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217–2226.
- 5.
Zannad F, Mebazaa A, Juilliere Y, Cohen-Solal A, Guize L, Alla F, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail 2006; 8: 697–705.
- 6.
Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes 2010; 3: 25–32.
- 7.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006; 113: 1424–1433.
- 8.
Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, Tadaki S, et al. Usefulness of combined risk stratification with heart rate and systolic blood pressure in the management of chronic heart failure: A report from the CHART-2 study. Circ J 2013; 77: 2954–2962.
- 9.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239, doi:10.1016/j.jacc.2013.05.019.
- 10.
Mebazaa A, Gheorghiade M, Pina IL, Harjola VP, Hollenberg SM, Follath F, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36: S129–S139.
- 11.
Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subacius H, Konstam MA, et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: Insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J 2013; 165: 216–225.
- 12.
Tokyo CCU Network Scientific Committee. Latest management and outcomes of major pulmonary embolism in the cardiovascular disease early transport system: Tokyo CCU Network. Circ J 2010; 74: 289–293.
- 13.
Shiraishi Y, Kohsaka S, Harada K, Sakai T, Takagi A, Miyamoto T, et al. Time interval from symptom onset to hospital care in patients with acute heart failure: A report from the Tokyo Cardiac Care Unit Network Emergency Medical Service Database. PLoS One 2015; 10: e0142017, doi:10.1371/journal.pone.0142017.
- 14.
Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012; 307: 1161–1168.
- 15.
Hasegawa K, Hiraide A, Chang Y, Brown DF. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA 2013; 309: 257–266.
- 16.
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993; 88: 107–115.
- 17.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470.
- 18.
Nutritional anaemias: Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405: 5–37.
- 19.
Alibay Y, Schmitt C, Beauchet A, Dubourg O, Alexandre JA, Boileau C, et al. Non-radioimmunometric NT-ProBNP and BNP assays: Impact of diluent, age, gender, BMI. Ann Biol Clin 2005; 63: 43–49.
- 20.
Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am Heart J 2008; 155: 339–347.
- 21.
Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Takeshita A, Yokoshiki H, et al. Anemia is an independent predictot of long-term adverse outcomes in patients hospitalized with heart failure in Japan: A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1901–1908.
- 22.
Yamaguchi T, Sakata Y, Takada T, Nochioka K, Miura M, Tadaki S, et al. Prognostic impact of anemia with chronic heart failure: With special reference to clinical background: Report from the CHART-2 study. Circ J 2015; 79: 1984–1993.
- 23.
Richards AM, Doughty R, Nicholls MG, Macmahon S, Ikram H, Sharpe N, et al. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction: Australia-New Zealand Heart Failure Group. Circulation 1999; 99: 786–792.
- 24.
Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1587–1593.
- 25.
Stanek B, Frey B, Hulsmann M, Berger R, Sturm B, Strametz-Juranek J, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol 2001; 38: 436–442.
- 26.
Chen CY, Yoshida A, Asakura M, Hasegawa T, Takahama H, Amaki M, et al. Serum blood urea nitrogen and plasma brain natriuretic peptide and low diastolic blood pressure predict cardiovascular morbidity and mortality following discharge in acute decompensated heart failure patients. Circ J 2012; 76: 2372–2379.
- 27.
Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation 2003; 107: 864–868.
- 28.
Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med 1992; 21: 669–674.