2016 Volume 80 Issue 4 Pages 998-1007
2016 Volume 80 Issue 4 Pages 998-1007
Background: Although stem cells have been regarded as a promising therapeutic option, the marginal therapeutic effects of stem cells are limitations that must be overcome for the development of effective cell therapy. This study sought to identify the angio-vasculogenic properties of endothelial differentiated mesenchymal stem cells (MSCs) and to determine whether these cells are effective for vascular repair.
Methods and Results: Adipose MSCs were cultured for 10 days under endothelial cell (EC) culture conditions. These endothelial cell differentiated adipose MSCs (EA) and undifferentiated adipose MSCs (UA) were characterized via angiogenesis and adhesion assays. These cells were transplanted into a hindlimb ischemia (HLI) model to determine therapeutic effects and their underlying mechanisms. EA displayed low adhesion and angiogenic properties in vitro compared with UA. When implanted into mouse HLI models, EA exhibited the decreased recovery of blood perfusion in limb ischemia than uncultured UA. Histology data showed that injected EA exhibited lower retention, angiogenic cytokine levels, and neovascularization in vivo than did UA. Short-term differentiated EA display less cell engraftment and angio-vasculogenic potential, and are less effective for peripheral vascular repair than UA.
Conclusions: EC differentiation of MSCs may not present an effective strategy for the promotion of therapeutic neovascularization. (Circ J 2016; 80: 998–1007)
In recent years, cell-based therapy using stem cells has emerged as a novel therapeutic option for the treatment of various diseases. Mesenchymal stem cells (MSCs) derived from various tissues are attractive for autologous cell therapy due to their multipotency, easy and repeatable tissue-of-origin access, ease of isolation, and high yield.1,2
Numerous studies have demonstrated that MSCs possess the potential for vascular cell differentiation.3,4 In fact, growth factor signaling such as fibroblast growth factor (FGF) and epigenic modification mediates endothelial differentiation of adipose stem cells.5,6 In addition, recent studies have reported that transplanted MSCs are resident in blood vessels, pericytic area, and play an important role in vascular stabilization.4,7,8
Undifferentiated MSCs secrete various angiogenic growth factors for tissue repair.3 However, the marginal therapeutic effects of transplanted stem or progenitor cells are one of the major limitations to be overcome in cell therapy.9–11 Over the past 10 years, our previous work has tried to find the best stem cell lineage for angiogenesis/vasculogenesis and to enhance therapeutic potential in ischemic peripheral vascular or heart disease.12 To promote regenerative potential, we have studied gene overexpression, cell differentiation, and priming, and their therapeutic mechanisms.3,4,13
In this study, we characterized the angiogenic potential of endothelial-induced human adipose tissue-derived MSCs and their therapeutic capacities.
Human umbilical vein endothelial cells (HUVECs) were purchased from the ATCC (Manassas, VA, USA). Human adipose MSCs and red fluorescent protein (RFP) expressing MSCs were purchased from Cyagen Biosciences (Santa Clara, CA, USA). Undifferentiated adipose MSCs (UA) were cultured at 37℃ under 5% CO2 in culture medium [a-MEM, 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 mg/ml of streptomycin]. All experiments involved three to five passages of cells.
Endothelial DifferentiationEndothelial cell differentiated adipose MSCs (EA) were cultured in endothelial basal medium-2 (Lonza Walkersville, MD, USA). This medium was supplemented with endothelial growth medium-2 (EGM-2). Single Quots containing human vascular endothelial growth factor (VEGF)-A, human FGF-B, human epidermal growth factor (EGF), human insulin-like growth factor (IGF)-1, 2% FBS and ascorbic acid for ~5–10 days.
Flow CytometryCells were suspended in phosphate buffered saline (PBS) containing 1% BSA. Cells were incubated for 20 min with FITC- or phycoerythrin-conjugated monoclonal antibodies specific for CD31, CD34, CD44, CD73, CD90, CD45, and CD166 (BD Pharmingen, San Diego, CA, USA). Proper isotype-identical IgGs were used as controls. Cells were first stained and then analyzed with a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Real-Time Polymerase Chain Reaction (PCR) AnalysesQuantitative real-time (qRT)-PCR assays were performed, as previously described.12 Briefly, total RNA was isolated from cells using RNA-stat (Iso-Tex Diagnostics, Friendswood, TX, USA) and a previously reported method.14 Extracted RNA was subsequently reverse transcribed using Taqman Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The synthesized cDNA was subjected to qRT-PCR or reverse transcription (RT)-PCR using human/mouse-specific primers and probes. RNA levels were quantitatively assessed using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Relative mRNA expression normalized to GAPDH expression was calculated.
qRT-PCR and RT-PCR PrimersThe primers used for qRT-PCR were human VEGF-A (Hs99999070_m1), angiopoietin (Ang)-1 (Hs00181613_m1), FGF-2 (Hs00266645_m1), interleukin (IL)-1β, (Hs00174097_m1); IGF-1 (Hs01547657_m1); stromal cell-derived factor (SDF)-1a (Hs00171022_m1), platelet-derived growth factor (PDGF)-b, Hs00966526-m1; IL-8, Hs00174103-m1, and GAPDH (Hs99999905_m1). The primers used for qRT-PCR were Mouse VEGF-A (Mm01204733_m1), Ang-1 (Mm00456503_m1), and GAPDH (Mm99999915_g1). The following paired RT-PCR primers were used: 5’-cctgttttggg ggcagttat/gacagt tgctggtctgggtg-3’ for kinase insert domain receptor (KDR) (266 bp), 5’-ccaaagagtctccatgccct/ttaaag ttgccacacagccc-3’ for von Willebrand factor (vWF) (189 bp) and 5’-tggggcagcccagaacatca/gccgcctgctt cacacctt-3’ for GAPDH (198 bp). All primer/probe sets were purchased from Applied Biosystems.
Matrigel Tube Formation AssayCulture media (CM) were collected as previously described, with modifications.15 Cells (1×106 cells each) were seeded into T-75 cell culture flasks and grown in low-glucose DMEM (Gibco, Grand Island, NY, USA) containing 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco) for 48 h until the cells reached an approximate confluence of 80%. Each sample was then centrifuged at 1,000 g for 10 min, and the supernatants were collected. To measure the tube formation potential, HUVECs at a concentration of 1×104 cells/well were embedded in low-glucose DMEM (Gibco) containing 1% FBS (control) and each CM derived from the UA and EA-D10 in basement membrane matrix gel (Matrigel, BD)-coated 2-well glass slides (NUNC). After 6 h of incubation, representative fields were randomly photographed using fluorescence microscopy, and the tube length and branching point from each sample were measured as previously report.16
Enzyme-Linked Immunosorbent Assay (ELISA)Media were replaced with serum-free medium, and cell cultured supernatants were collected after 24 h. In vitro cytokine secretion levels were assessed by using an ELISA kit (Abcam, Cambridge, MA, USA) and previous reports.17
Immunoblot AnalysisWestern blot assays were performed as described previously, with modifications.17,18 Briefly, cells were resuspended in lysis buffer and kept on ice with occasional tapping for 20 min. After centrifugation, the supernatant was harvested as a protein extract. Equal amount of proteins were fractionated by electrophoresis on 8% or 10% acrylamide gels and transferred onto polyvinylidene fluoride (Millipore, Billerica, MA, USA) membranes, followed by blotting with antibodies against anti-human endothelial NO synthase (Abcam, Cambridge, UK), intercellular adhesion molecule-1, vascular adhesion molecule (VCAM)-1, E-selectin (Calbiochem, San Diego, CA, USA), or β-actin (Sigma-Aldrich, St. Louis, MO, USA). Expression levels of proteins were determined with a LAS-3000 SYSTEM (Fuji Photo Film, Tokyo, Japan).
Transplantation of Cells in the Ischemic Hindlimb Animal ModelExperimental protocols were approved by the Catholic Kwandong University Institutional Animal Care and Use Committee, and all procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011). Male NOD/severe combined immunodeficiency (SCID) mice (NOD.CB17-Prkdcscid/J strain; The Jackson Laboratory, Bar Harbor, ME, USA) that were 8–10 weeks of age were used. Animal experiments were performed as described previously.12 Mice were anesthetized with isoflurane (induction: 450 ml air, 4.5% isoflurane; maintenance: 200 ml air, 2.0% isoflurane; Baxter International, Inc, Deerfield, IL, USA), and the depth of anesthesia was monitored by respiratory rate and lack of withdrawal reflex upon toe pinching.4 Hindlimb ischemia was induced by ligation of the right femoral artery. A solution of 1×106 RFP-expressing MSCs was prepared in PBS and then intramuscularly injected into the ischemic hindlimb area after surgery (n=8 for each group). Buprenorphine (0.1 mg/kg) was injected subcutaneously at the end of the surgery and every 5–10 h until they recovered. Euthanasia was performed by intravenous injection of thiopental sodium (40 mg/kg). We used a laser Doppler perfusion image (LDPI) analyzer (Moor Instruments, Axminster, UK) to measure serial blood flow in the hindlimb.19
Histological AnalysisAll histological experiments were performed as described previously.14,20 The adductor muscles of hindlimbs were harvested, fixed in 4% paraformaldehyde for 4 h, and incubated overnight in a 15% sucrose solution. The tissues were embedded in OCT compound (Sakura Finetek USA, Torrance, CA, USA), snap frozen in liquid nitrogen, and sectioned in thickness increments of 10–20 μm. For capillary density measurement, 5 frozen sections from each group of ischemic tissue from the adductor muscles were stained with biotinylated isolectin B4 primary antibody (anti-ILB4, 1:250; Vector Laboratory Inc, Burlingame, CA, USA) and CD31 (1:200; Abcam) followed by streptavidin Alexa Fluor 488 secondary antibody (1:400; Invitrogen, Carlsbad, CA, USA) and anti-Rabbit Alexa Fluor 488 (1:400; Abcam). Five fields from 5 tissue sections were randomly selected, and the number of capillaries was counted in each field. The tissue sections were hybridized according to the manufacturer’s protocol. Photographs were taken using fluorescent-inverted microscopy or confocal microscopy.
Quantification of Cell Engraftment in Ischemic HindlimbsCell engraftment in the ischemic hindlimbs was quantified, as per previous reports.4,20 Briefly, RFP expressing MSCs were injected into ischemic hindlimbs of mice. After 4 weeks, the hindlimb tissues were harvested, and tissue sections were embedded and sectioned. Six fields from 5 tissue sections were randomly selected, and the number of Dil-positive cells was counted in each field.
Statistical AnalysisAll data are presented as mean±SD. We performed statistical analyses using a Student’s t-test for comparisons of the 2 groups, and ANOVA with Bonferroni’s multiple comparison test using SPSS v11.0. Data with P<0.05 were considered statistically significant.
To induce the EC differentiation, human adipose MSCs were cultured in EGM-2, which is EC basal media-2 with 2% FBS and an EC-specific-cytokine cocktail (SingleQuots; Lonza), for 10 days. Figure 1A shows the morphologies of UA and EA. UA show a spindle shape. However, some of EA were aggregated for 10 days. This morphological change is same as previously reported our data.4 Our previous study clearly demonstrated that EC differentiated MSCs using EGM-2 for 10 days are expressing EC markers, CD146, indicating the EC differentiation.

Cell characteristics of endothelial cell (EC) differentiated mesenchymal stem cells (MSCs). (A) Cell morphology of human umbilical vein ECs (HUVEC) (HUV), EC undifferentiated adipose MSCs (UA) and EC differentiated adipose MSCs (EA). (B) Immunocytochemical results. Fluorescent microscopic images showed that EC differentiated MSCs are positive for von Willebrand factor (vWF) and kinase insert domain receptor (KDR), which are EC-specific markers. (C) Characterization of UA and EA were analyzed by flow cytometry. Isotype control (black), specific antigen (red). (D) Reverse transcriptase (RT)-Polymerase chain reaction (PCR) results demonstrated that EC differentiated MSCs underwent a change in expression of EC-specific genes.
To confirm the EC differentiation, we performed an immunocytochemistry assay. After differentiation into ECs on day 10, EC differentiated cells expressed KDR, and vWF, which are EC-specific markers, suggesting that MSCs partially differentiated into ECs (Figure 1B). In addition, we characterized EA. EA still exhibited MSC markers and partially expressed CD31, a representative endothelial marker, indicating partial differentiation into EC or endothelial progenitor cells (Figure 1C).
Next, to evaluate the EC differentiation, we also measured gene expression using RT-PCR. Ten days after EC differentiation of cells, the levels of vWF and KDR expression were elevated (Figure 1D).
Taken together, we defined these EC induced MSCs for 10 days as EA and compared them with UA.
UA Shows High Angiogenic Gene ExpressionTo examine the angiogenic capacity of human adipose MSCs, we analyzed them by qRT-PCR using 3 donors of human adipose MSCs. Surprisingly, the UA expressed significantly higher levels of the representative pro-angiogenic genes, VEGF-A, Ang-1, FGF-2, IL-1β, and PDGF-b, than did the EC differentiated cell lines, EA-D5 and EA-D10, or HUVECs (Figure 2A). Chemokines, such as lymphocyte chemoattractant CXC-chemokine SDF-1 (SDF-1 or CXCL12), that play an important role in mediating homing of stem cells and neovascularization by binding to CXCR4, were also significantly upregulated.21 Furthermore, the cardiomyogenic and anti-apoptotic factor, IGF-1, was highly upregulated.

Angiogenic factor expression profiles. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to examine multiple angiogenic gene expression levels in human umbilical vein endothelial cells (HUVECs), undifferentiated mesenchymal stem cells (MSCs) (D0), and EC differentiated MSCs (D5 and D10). Various angiogenic genes were upregulated in EC undifferentiated adipose MSCs (UA) compared with EC differentiated adipose MSCs (EA) and HUVECs (HUV). All individual values were normalized to GAPDH expression (n=4 per group). **P<0.01 D0 vs. D5; †P<0.01 D0 vs. D5. (B) Enzyme-linked immunosorbent assay (ELISA) for vascular endothelial growth factor (VEGF)-A, fibroblast growth factor (FGF)-2, insulin-like growth factor (IGF)-1, epidermal growth factor (EGF) and stromal cell-derived factor (SDF)-1 in the supernatant from HUV, UA and D10-EA (n=4 per group). *P<0.05, **P<0.01 vs. D10-EA.
Next, we quantified the protein levels of the growth factors released in the cell culture supernatant using a serum-free culture method. The ELISA result also detected significantly higher levels of VEGF-A, FGF-2, EGF, IGF-1, SDF-1, and IL-1β in UA supernatant compared to EA (D10) supernatant (Figure 2B).
Secreted Factors Derived From UA Improve Endothelial Network FormationTo evaluate the angiogenic properties of secreted factors derived from cells, a Matrigel tube formation assay was performed. Low-glucose DMEM (Gibco) containing 1% FBS (control) medium and each CM derived from UA and EA-D10 was seeded with 1×104 HUVECs/well in basement membrane matrix gel-coated two-well glass slides. After 6 h of incubation, 8 fields from each sample were randomly photographed using fluorescence microscopy, and the tube length and branching point from each sample were measured by image analysis (Image J, Windows version; National Institutes of Health, USA). The results showed that UA-CM induced significantly higher tube lengths (1.9-fold) and branching points (2.3-fold) than did EA-CM or the control group (Figure 3). However, we did not observe the clear tube formation after treatment of the culture medium of the cells in this study. We just observed the line and branch formation, indicating unlikely vasculogenic property EA and/or UA as a study limitation. Taken together, these data suggest that secreted factors from UA have better angiogenic potential than EA.

Tube formation potential. Tube lengths and the number of branching points of human umbilical vein endothelial cells (HUVECs) were measured 10 h after seeding in culture medium from EC undifferentiated adipose mesenchymal stem cells (MSCs) (UA) or EC differentiated adipose MSCs (EA) in Matrigel-coated plates. n=5 per group.
As adhesion capacity is an indicator of cell survival and cell engraftment in an ischemic environment, we performed cell adhesion assays using extracellular matrix protein collagen I. The number of adherent cells was significantly higher in UA than in EA (P<0.05) (Figure 4A). In line with the results of the adhesion assay, the expression levels of adhesion protein VCAM-1 and E-selectin were also significantly higher in UA than in EA or HUVECs (all P<0.05) (Figure 4B).
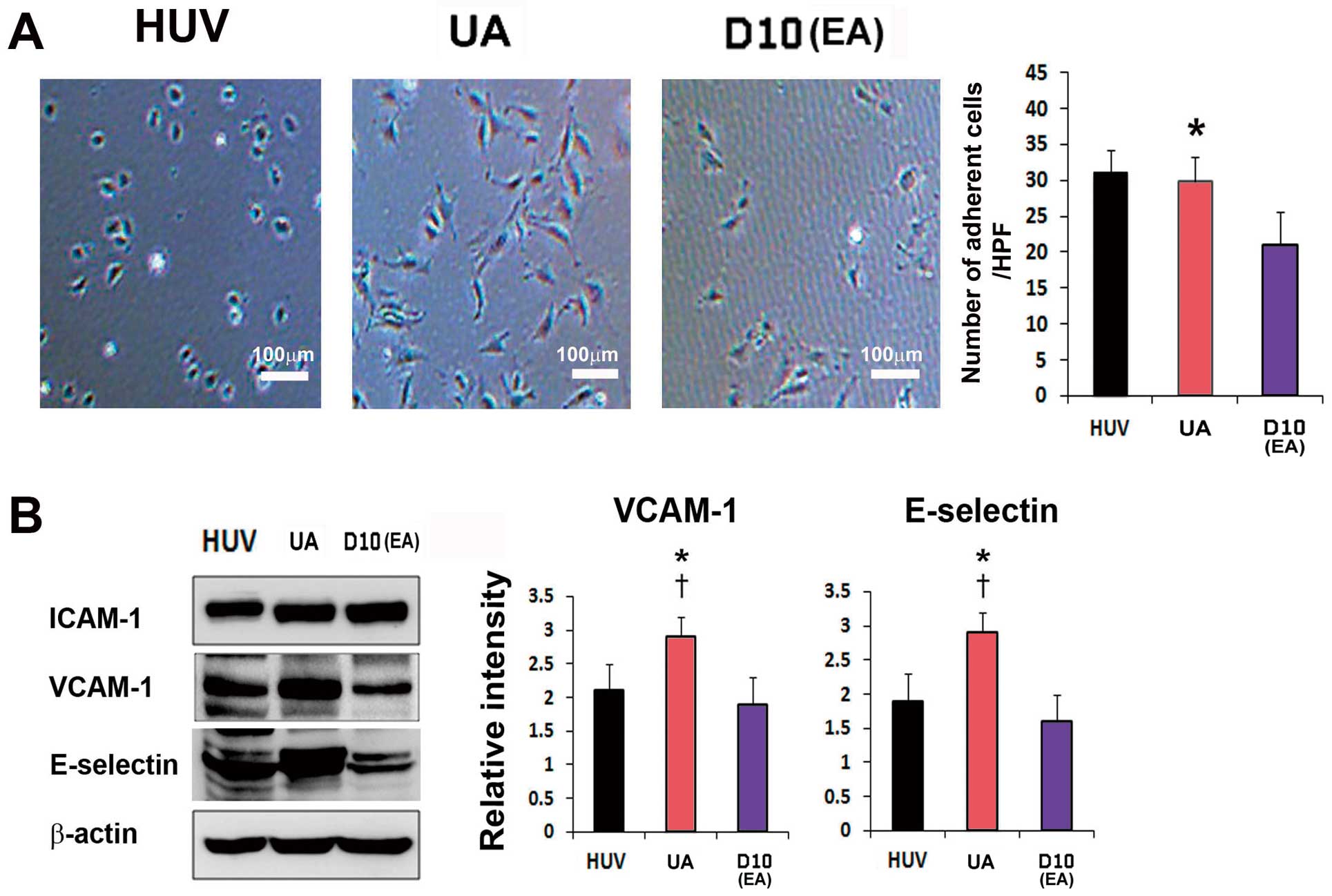
Analysis of adhesion properties. (A) Cell adhesion assays revealed higher adhesion of EC undifferentiated adipose mesenchymal stem cells (MSCs) (UA) via extracellular matrix proteins (type I collagen) compared with EC differentiated adipose MSCs (EA). n=5 per group. (B) Immunoblots showing that the levels of adhesion proteins (vascular adhesion molecule (VCAM)-1, E-selectin) correlate with adhesion assays. n=5 per group. *P<0.05 D0 vs. D5; †P<0.01 D0 vs. D5.
After identifying the angiogenic characteristics of cells in vitro, we evaluated the therapeutic potential of cells to repair hindlimb ischemia in vivo. LDPI analysis revealed that the recovery of blood perfusion was significantly higher in UA-injected limbs vs. EA- or PBS-injected limbs at days 14 and 28 (Figures 5A,B).

Transplantation of EC differentiated adipose mesenchymal stem cells (MSCs) (EA) confers less effective recovery than EC undifferentiated adipose MSCs (UA) from ischemia and induces neovascularization in vivo. (A) Laser Doppler perfusion images demonstrated the recovery of blood flow in ischemic hindlimbs. (B) Quantitative analysis revealed improved blood perfusion in the UA-injected group compared with the EA- and phosphate buffered saline (PBS)-injected groups up to 4 weeks after cell transplantation. Blue represents low perfusion and red represents high perfusion. n=6 per group. **P<0.01 UA vs. EA; *P<0.05 UA vs. EA; PBS; †P<0.05 UA vs. EA.
The vascular and capillary density in the ischemic hindlimb adductor muscle after cell injection was measured using 2 endothelial markers, isolectin B4 (ILB4) and CD31. Analysis results revealed that the UA-injected group induced significantly higher capillary density than did EA– group or the PBS group (P<0.01) (Figure 6A).

Transplantation of EC differentiated adipose mesenchymal stem cells (MSCs) (EA) shows low neovascularization and angiogenic gene expression in hindlimb tissues. (A) Isolectin B4 (ILB4) and CD31 staining in the adductor muscle of ischemic hindlimbs and quantitative analysis of capillary density (n=6 each; *P<0.05, **P<0.01). (B) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of ischemic hindlimb tissues injected with UC or endothelial cells (EC). EC transplantation resulted in a higher expression of representative angiogenic factors. n=5 per group.
Next, to determine the effects of injected cells on cytokine expression in ischemic hindlimbs, mice were sacrificed and hindlimb tissues were collected. The expression levels of Ang-1, VEGF-A, and FGF-2 were significantly increased in the UA-injected limbs compared with the EA- or PBS-injected limbs (Figure 6B). These data suggest that UA transplantation induces the upregulation of multiple biological factors associated with neovascularization.
Increased Engraftment of UA and Contribution to VasculogenesisThe engraftment/survival potential of the injected cells was evaluated in a hindlimb ischemia model using NOD/SCID mice. A total of 1×106 RFP expressing MSCs of each group was directly injected into areas of the ischemic limb. Immunohistochemistry results demonstrated that UA underwent significantly higher engraftment (29.6±13.5) than EA (14.1±7.3) (Figure 7A). To confirm the immunohistochemistry data and quantify the rate of cell engraftment, we performed FACS analysis. We performed flow cytometric analysis using enzymatically digested limbs after systemic injection of ILB4, as reported.14 This result demonstrated that the UA group showed a 2.3-fold higher engraftment rate than the EA group (Figure 7B).

Endothelial cell differentiated adipose mesenchymal stem cells (MSCs) (EA) shows lower engraftment than EC undifferentiated adipose MSCs (UA) in the ischemic hindlimb. Histological analyses of engrafted cells in hindlimb sections 4 weeks after UA or EA (red fluorescent protein (RFP) red fluorescence) injection. ILB4 (green); DAPI (blue). (A) EA had lower retention than UA. n=5 each. *P<0.05. (B) Hindlimb tissues were collected 4 weeks after transplantation with RFP expressing MSCs and digested with an enzyme cocktail. Flow cytometric analysis showed that EA had lower engraftment capacity than UA. n=5 each. *P<0.05. (C) Confocal microscopic examination using 3-dimensional z-stacked orthogonal and multipanel images showed that injected UA (Upper panel) or EA (Lower panel) colocalized with ILB4 expression (white arrows). (D) EA colocalized with ILB4 expression in the vascular-like structure. Arrows indicate colocalizing cells. RFP (red); ILB4 (green); DAPI (blue).
The controversy remains regarding the (trans) differentiation potential of MSCs in vasculature.22 Thus, we further sought to evaluate the potential of the contribution of injected MSCs to vasculogenesis. To track the injected cells, we intramuscularly injected RFP expressing MSCs into the ischemic hindlimbs of NOD/SCID mice. Histologic analysis revealed that most of the injected UA were concentrated in the pericytic or perivascular areas, and a small population of UA expressed an EC-specific marker, ILB4, at 4 weeks (Figure 7C). Intriguingly, we observed that few of ILB4-expressing UA colocalized with vasculature-like structure (Figure 7D). These data suggest that UA may give rise to ECs, indicating their vasculogenic potential.
In the present study, we demonstrated for the first time the angiogenic properties of vascular cell-induced MSCs and their therapeutic capacity in an ischemic vascular disease model. The major findings of this study are the following: (1) EA exhibit lower angiogenic gene expression than UA; (2) EA display highly reduced matrix tube formation and adhesion capacity relative to UA; (3) transplanted EA confer less recovery of blood perfusion than do UA in hindlimb ischemia; and (4) EA exhibit lower engraftment capacity than do UA in ischemic limbs.
One of the recent concerns or controversies in the field of stem cell therapy is that of marginal therapeutic effects of stem cells.11 The failure of transplanted cells to thrive has been explained by 5 mechanisms: ischemia, lack of vasculature in the environment, anoikis, apoptosis resulting from a lack of intercellular contact, and inflammation-related factors.23 These pathways lead to poor engraftment and low survival rates of transplanted cells and may influence therapeutic outcome. To overcome these limitations, we recently attempted to find highly angiogenic stem/progenitor cells and establish a differentiation protocol.13,19 One essential question is whether EC differentiation of MSCs is effective in improving therapeutic outcomes in the treatment of ischemic disease. What changes of angiogenic characteristics do MSCs exhibit? Our previous study demonstrated that a specific cytokine cocktail promoted angiogenic properties in mononuclear cells derived from cord blood.19 In addition, a pro-survival cocktail improved stem cell survival in infarcted hearts.23 Thus, we hypothesized that EC differentiation, using specific cytokines, might enhance the angiogenic or cell survival potency of MSCs. To differentiate ECs, we used conventional EC differentiation medium, EGM-2 BulletKit, which contains various EC differentiation growth factors, namely VEGF-A, FGF, IGF, and EGF. Our previous study showed that this EGM-2 BulletKit effectively induced MSCs into EC-like cells.4 Contrary to our expectation, EC differentiation of MSC reduced the expression of various angiogenic genes. Adhesion potential is one of the important characteristics of stem cells, and is associated with cell engraftment and survival in tissues. In this study, EC differentiation reduced the expression of adhesion proteins, as was the case with angiogenic gene expression. These data indicate that EC differentiation using cytokine cocktails is not suitable for the promotion of angiogenic and engraftment potential in MSCs.
The gene and protein expression data were further confirmed via in vitro EC tube formation and adhesion assays. Matrix tube formation potential is a common assay used to test angiogenic capability. As with the angiogenic gene expression data, CM derived from EA exhibited decreased EC network formation relative to UM-derived CM. Adhesion capacity is important in cell therapy because it is an indicator of cell survival and engraftment. In the present study, the results of adhesion assays were also consistent with the data on VCAM-1 and E-selectin protein expression. The adhesion capacity of EA was lower than that of UA. From these data, we presume that the angiogenic properties of EC differentiated MSCs are similar to the angiogenic characteristics of ECs.
In vivo cell injection experiments further confirmed the in vitro data. DM transplantation induced higher recovery of blood perfusion during ischemic tissue repair. The therapeutic mechanisms responsible for the accelerated ischemic tissue repair remain to be elucidated. Presently, paracrine activities of stem/progenitor cells have been regarded as the major therapeutic mechanism. In this study, we found that the expression levels of the angiogenic factors, Ang-1, FGF-2, and VEGF-A were significantly decreased in ischemic hindlimb tissues after EA injection compared with UA injection, suggesting the main cause of the low therapeutic effects of VM. It has been reported that Ang-1 increases the survival of ECs, interacts with other angiogenic factors to enhance neovascularization, and prevents apoptotic death.24,25 Both FGF-2 and VEGF-A are also well-known representative pro-angiogenic molecules.26 These decreased levels of angiogenic factors in tissues may result in limited recovery of blood flow and low capillary density.
Another therapeutic mechanism, transdifferentiation of transplanted cells, has been suggested. Numerous studies have investigated whether injected stem cells incorporate into vasculature and differentiate into ECs. This remains controversial.22 To test this, we carefully observed ischemic limb tissues after cell injection. Interestingly, we found that a few transplanted UA incorporated into blood vessels and expressed an endothelial specific protein (ILB4), as determined by three-dimensional reconstruction using confocal microscopy, suggesting transdifferentiation into ECs.
In the present study, our findings may seem controversial, as recent studies revealed that other stem or progenitor cell populations differentiated into ECs demonstrated endothelial functionality in vitro and promoted vessel growth-enhancing blood flow recovery in animal models of myocardial infarction and peripheral arterial disease.13,14,27,28 These data demonstrated that embryonic stem cells, induced pluripotent stem cells, mononuclear cells derived ECs showed beneficial therapeutic effects. Therefore, we speculate that the angiogenic and their therapeutic properties of endothelial or differentiated cells from different tissues are diverse.
In conclusion, EC transdifferentiation of MSCs does not enhance their angiogenic potential, and local implantation of EC differentiated MSCs is not a suitable strategy for the promotion of therapeutic neovascularization. Thus, the therapeutic potential of differentiated cells from various kinds of stem or progenitor cells should be re-considered. These data are important to understand the characteristics of MSCs in differentiation, and the clinical applications thereof. We speculate that other stimulation strategies, such as other combinations of growth factor cocktails, micro RNAs, or small molecules, might promote the angiogenic capacity and therapeutic neovascularization of MSCs.
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (No. NRF-2013R1A1A2059998), (No. NRF-2015M3A9E6029558), and a research fund from the Catholic Kwandong University International St. Mary’s Hospital (CKURF-20150059) and by the Dong-A University research fund.
None to declare.