2017 Volume 81 Issue 10 Pages 1454-1462
2017 Volume 81 Issue 10 Pages 1454-1462
Background: Differences in the predictive value of daytime systolic blood pressure (SBP) and night-time SBP by ambulatory blood pressure monitoring on renal outcomes have not been fully investigated in chronic kidney disease (CKD) patients. This study compared the prognostic value between daytime and night-time SBP on renal outcomes in CKD.
Methods and Results: This prospective observational study included 421 patients. The composite renal endpoint was endstage renal disease (ESRD) or death. Cox models were used to determine associations of daytime and night-time SBP with renal outcomes. There were 150 renal events (ESRD, 130; death, 20). Multivariable Cox analyses demonstrated that hazard ratios (HRs) [95% confidence interval (CI)] for composite renal outcomes of every 10-mmHg increase in daytime and night-time SBP levels were 1.13 (1.02–1.26) (P=0.02) and 1.15 (1.05–1.27) (P<0.01), respectively. In addition, compared with the 1st daytime or night-time SBP quartile, HRs (95% CI) for outcomes in the 2nd, 3rd, and 4th quartiles were: daytime SBP, 1.25 (0.70–2.25), 1.09 (0.61–1.94), and 1.58 (0.88–2.85; P=0.13) (P for trend=0.16); night-time SBP, 1.09 (0.61–1.96), 1.31 (0.76–2.28), and 1.82 (1.00–3.30; P=0.049) (P for trend=0.03), respectively.
Conclusions: Night-time SBP appeared superior to daytime SBP for predicting renal outcomes in this population of patients.
Patients with chronic kidney disease (CKD) are reported to have an increased risk for cardiovascular events and death, and the risk increases with advancing CKD stage.1 CKD is recognized as a major public health issue and high blood pressure (BP) is a key pathogenetic factor that contributes to kidney disease progression. Accordingly, treatment for hypertension has become the most important intervention in the management of CKD patients.2 In this context, BP monitoring is important to assess the risk for cardiovascular and renal events and death.
Ambulatory blood pressure monitoring (ABPM) is useful for accurately diagnosing hypertension, and also provides important information such as dipping status and BP variability associated with target-organ damage, which cannot be captured with clinic BP readings.3 Components derived from ABPM have been reported to serve as predictors for cardiovascular events and death in the general population4,5 and in hypertensive subjects.6–11 It has also been demonstrated that ABPM significantly better predicts all-cause and cardiovascular deaths in hypertensive patients than does clinic BP.6,9 ABPM is reported to be a better tool for predicting cardiovascular risk, CKD progression, endstage renal disease (ESRD) and death compared with office-based BP in patients with CKD.12
Some studies have addressed the association of components of ABPM with outcomes in patients with CKD, and it has been noted that ABPM is superior to clinic BP for predicting cardiovascular events and poor renal outcomes.13–15 Among components derived from ABPM, night-time BP provides more prognostic information regarding cardiovascular events and death than daytime BP in hypertensive patients,6,8–10 whereas night-time BP was found to be superior to daytime BP for predicting incident CKD in the general population.16 Night-time systolic BP (SBP), in particular, is reported to be a better predictor for cardiovascular events in hypertensive patients.8,10
Few studies have compared daytime and night-time SBP with renal outcomes in CKD patients. Redon et al reported a greater risk for progression to ESRD in patients with night-time SBP higher than 130 mmHg in nondiabetic patients at CKD stages 3 and 4.17 Another study reported that night-time SBP levels enabled more accurate prediction of renal and cardiovascular risk than daytime SBP levels in patients with CKD stages 2–5.14 Nevertheless, whether night-time SBP is superior to daytime SBP has not been fully investigated in the general CKD patient population.
The aim of this study was to determine whether daytime or night-time SBP was independently associated with renal outcomes, and to compare the predictive values of these 2 SBP measurements on renal outcomes in patients with CKD stages 1–5.
Figure 1 shows a flow chart of the patient enrollment process: 630 consecutive Japanese patients with CKD were admitted to the National Hospital Organization Kyushu Medical Center for evaluation of, and education about, CKD between June 2009 and May 2016. Patients with any malignancy (n=53), acute exacerbation of CKD (n=15), estimated glomerular filtration rate (eGFR) <8 mL/min/1.73 m2 at baseline (n=33), or no available data for ABPM (n=51) were excluded. The remaining 478 patients were enrolled in this prospective observational study. All patients were discharged from hospital alive and not on dialysis and thereafter were followed up at the hospital. Of these 478 patients, 57 who were followed for within 6 months after discharge were also excluded. Finally, 421 patients were analyzed. Data were collected through until December 2016. All patients gave written informed consent to the protocol, which was approved by the Ethics Committee of the National Hospital Organization Kyushu Medical Center (UMIN: #000017519).

Flow chart of the enrollment process. ABPM, ambulatory blood pressure monitoring; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
The composite renal endpoint was ESRD or death, whichever occurred first. ESRD was defined as requiring maintenance hemodialysis or peritoneal dialysis, and time to ESRD was the duration from baseline to the day of the first dialysis session. Death was defined as occurring before reaching ESRD. Information about deaths was obtained from the study hospital’s records for inpatients who died, from the respective institutions for patients who died while in other hospitals, and from a patient’s family for patients who died at home. Patients who were lost to follow-up (n=67) or who started maintenance dialysis therapy for acute exacerbation of kidney function caused by congestive heart failure or infectious diseases (n=5) during the more than 6-month follow-up period were censored. The median follow-up period for these 72 censored patients was 19.5 months (full range, 6.2–66.4 months). Because these censored patients were able to be followed for some time, they were included in the survival analysis, and the time to censoring was defined as the follow-up period without composite renal events.
Clinical and Biochemical AssessmentsBlood samples were obtained from each patient early in the morning after an overnight fast. Daily proteinuria was measured. Serum creatinine (SCr), hemoglobin, serum albumin, and serum phosphorus concentrations were determined. eGFR (mL/min/1.73 m2) was calculated using the new Japanese equation: eGFR=194×SCr−1.094×age−0.287×0.739 (if female).18
All enrolled patients were interviewed and clinically examined at the time of hospital admission. Their medical histories and outpatient records were evaluated in detail. Demographic information (age and sex), medication history, and atherosclerotic risk factors (hypertension, history of smoking, dyslipidemia, and diabetes mellitus) at the time of hospital admission were recorded. Hypertension was defined as SBP ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg, or the current use of antihypertensive drugs. Dyslipidemia was defined as plasma triglycerides ≥150 mg/dL, plasma low-density lipoprotein cholesterol ≥140 mg/dL, plasma high-density lipoprotein cholesterol <40 mg/dL, or the use of lipid-lowering drugs based on a history of dyslipidemia. Diabetes mellitus was defined as previous or current plasma fasting glucose ≥126 mg/dL or the use of hypoglycemic agents. Ischemic heart disease was defined as a history of angina, myocardial infarction, coronary angioplasty, or coronary artery bypass surgery. Cigarette smoking was evaluated as current or past. Body mass index was calculated as weight in kg divided by height in m2.
Measurement of ABPMDuring hospitalization, 24-h noninvasive ABPM was performed using an ES-H531 device (Terumo, Co., Ltd., Tokyo, Japan) from June 2009 to November 2011, and a FM-800 device (Fukuda Denshi, Co., Ltd., Tokyo, Japan) was used from December 2011 onward. Waking and sleeping periods were defined as: daytime (7:00 to 22:00 hours); and night-time (22:00 to 7:00 hours). A cuff-oscillometric method was used. The measurement interval was set at 30 min for daytime and 60 min for night-time for the ES-H531 device, and 30 min for the whole time for the FM-800 device. Patients who obtained less than 80% of either awake or asleep valid BP readings were excluded. Mean 24-h and daytime and night-time BPs were calculated. Nocturnal decline was defined by calculating the percentage of decline in night-time SBP using the following formula: [1-night-time SBP/daytime SBP ratio]×100. According to the percentage of nocturnal decline, patients were classified as either extreme dippers (SBP decline ≥20%), dippers (SBP >10% and <20%), non-dippers (SBP decline between 0% and 10%), and risers (increase in SBP during night-time).7,11
Statistical AnalysisContinuous data are expressed as mean±SD or median (interquartile range), depending on their distribution. Participants were divided into quartiles of each component of ABPM. Categorical data were compared across quartiles of daytime or night-time SBP using the chi-squared test, with the Fisher’s exact test used for groups containing fewer than 5 individuals in any given cell. ANOVA was used to compare continuous variables that were approximately normally distributed, and the Kruskal-Wallis test was used to compare skewed continuous variables. Significance of differences between groups was examined using the Wilcoxon rank sum test for nonparametric data.
A Cox proportional hazards model was used to determine whether each component of ABPM was associated with renal outcomes. Cox models were also used to assess the association of higher quartiles of each variable with renal outcomes, with the lowest quartile serving as the reference category. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each variable. Restricted cubic spline analyses were performed to qualitatively evaluate any nonlinear relationship between renal outcomes and daytime or night-time SBP, adjusted for age, sex, diabetes mellitus, smoking, dyslipidemia, ischemic heart disease, use of immunosuppressants, use of renin-angiotensin-aldosterone system inhibitors, use of antihypertensive drugs only in the daytime (in the morning and/or afternoon), only at night (in the evening and/or at bedtime) or use in both the daytime and at night, body mass index, daily proteinuria, hemoglobin, eGFR, serum phosphorus, and serum albumin. We placed 4 knots at the 5th, 35th, 65th, and 95th percentiles of daytime or night-time SBP levels, and median values of daytime and night-time SBP in the reference group (1st quartile) were selected as references for spline plots: 110 mmHg for daytime SBP and 104 mmHg for night-time SBP. Survival curves were estimated using the Kaplan-Meier method and evaluated using the log-rank test. All statistical analyses were performed using STATA ver. 14 (Stata Corp., College Station, TX, USA). P<0.05 was considered to indicate statistical significance.
Median age of the 421 patients (281 men and 140 women) was 69.4 years (full range, 20–91 years). Median eGFR for all participants was 26.3 mL/min/1.73 m2 (full range, 8.1–120.3 mL/min/1.73 m2). Of the 421 patients, 68 (16.2%), 117 (27.8%), 142 (33.7%), and 94 (22.3%) were categorized as CKD stage 1–2, 3, 4, and 5, respectively. The primary causes of renal disease were chronic glomerulonephritis (30.0%, 126 patients), hypertensive nephrosclerosis (29.0%, 122 patients), diabetic nephropathy (25.4%, 107 patients), other defined causes (13.3%, 56 patients), and unknown (2.4%, 10 patients). The prevalence of diabetes mellitus, hypertension, smoking, dyslipidemia, and ischemic heart disease was 177 (42%), 351 (83%), 226 (54%), 302 (72%), and 76 (18%), respectively. Median values of daytime and night-time SBP for all participants were 131 (interquartile range, 119–143) mmHg and 127 (interquartile range, 113–141) mmHg, respectively. The prevalence of each dipping status was: dipper, 79 (19%); non-dipper, 202 (48%); extreme dipper, 6 (1%); and riser, 134 (32%), and the prevalence of patients with non-dipping (non-dippers+risers) was high (80%). The number of patients treated with antihypertensive drugs was 342 (81%); use of antihypertensive drugs only in the daytime, 170; only at night, 10; and in both the daytime and at night, 162. Renin-angiotensin-aldosterone system inhibitors, calcium-channel blockers, α-blockers, β-blockers, and diuretics were administered in 278, 274, 42, 75, and 138 patients, respectively.
Table 1 and Table 2 show the clinical parameters according to quartiles of daytime and night-time SBP, respectively. As both daytime and night-time SBP levels increased, the prevalence of male sex, diabetes mellitus, and hypertension significantly increased. With both higher daytime and night-time SBP levels, values for daily proteinuria and serum phosphorus significantly increased, while significant decreases in values for hemoglobin, eGFR, and serum albumin were observed. Higher night-time SBP levels, but not daytime SBP levels, correlated with an increase in the night-to-day ratio of SBP and a higher prevalence of non-dipping. The median value of night-time SBP was higher in patients with non-dipping compared with those with dipping (130 mmHg vs. 110 mmHg, P<0.01), whereas there was no significant difference in median value of daytime SBP between the 2 groups (non-dipping, 132 mmHg; dipping, 128 mmHg). In addition, in 336 patients with non-dipping, the full range of night-time SBP levels was 91–194 mmHg, and the proportions of each quartile of night-time SBP were 18% (1st quartile), 27% (2nd quartile), 26% (3rd quartile) and 29% (4th quartile).
| Variable | Daytime SBP level | P value | |||
|---|---|---|---|---|---|
| 94–119 mmHg (n=107) |
120–131 mmHg (n=108) |
132–143 mmHg (n=106) |
144–188 mmHg (n=100) |
||
| Age (years) | 65.3 (48.8–74.9) | 67.8 (60.5–75.8) | 72.5 (61.1–78.7) | 71.5 (62.2–79.3) | <0.01 |
| Male, n (%) | 56 (52) | 80 (74) | 71 (67) | 74 (74) | <0.01 |
| Diabetes mellitus, n (%) | 30 (28) | 42 (39) | 45 (42) | 60 (60) | <0.01 |
| Hypertension, n (%) | 68 (64) | 88 (81) | 96 (91) | 99 (99) | <0.01 |
| Smoking, n (%) | 48 (45) | 57 (53) | 59 (56) | 62 (62) | 0.10 |
| Dyslipidemia, n (%) | 71 (66) | 76 (70) | 81 (76) | 74 (74) | 0.39 |
| Ischemic heart disease, n (%) | 11 (10) | 21 (19) | 23 (22) | 21 (21) | 0.11 |
| Body mass index (kg/m2) | 22.7 (20.4–25.7) | 22.7 (20.5–25.5) | 22.2 (20.5–25.6) | 22.2 (20.9–24.7) | 0.99 |
| Daily proteinuria (g/day) | 0.43 (0.14–1.09) | 0.90 (0.23–1.75) | 1.43 (0.45–3.24) | 2.29 (0.82–5.46) | <0.01 |
| Hemoglobin (g/dL) | 11.7 (10.3–13.1) | 11.0 (10.0–12.9) | 10.2 (9.0–12.1) | 9.6 (8.5–11.1) | <0.01 |
| eGFR (mL/min/1.73 m2) | 40.1 (25.8–63.9) | 27.1 (17.2–45.8) | 23.7 (15.5–35.5) | 17.6 (12.8–30.8) | <0.01 |
| Serum albumin (g/dL) | 3.7 (3.4–3.9) | 3.6 (3.2–3.8) | 3.4 (3.0–3.7) | 3.1 (2.5–3.6) | <0.01 |
| Serum phosphorus (mg/dL) | 3.6 (3.1–4.0) | 3.5 (3.2–4.0) | 3.8 (3.4–4.2) | 3.8 (3.4–4.2) | <0.01 |
| 24-h SBP (mmHg) | 109 (103–115) | 124 (120–128) | 136 (133–140) | 156 (148–163) | <0.01 |
| 24-h DBP (mmHg) | 66 (62–71) | 73 (67–79) | 78 (73–84) | 85 (76–91) | <0.01 |
| Daytime SBP (mmHg) | 110 (106–115) | 126 (123–129) | 137 (134–140) | 158 (150–164) | <0.01 |
| Daytime DBP (mmHg) | 68±7 | 74±9 | 80±9 | 85±12 | <0.01 |
| Night-time SBP (mmHg) | 106 (96–115) | 121 (113–129) | 134 (127–142) | 152 (140–164) | <0.01 |
| Night-time DBP (mmHg) | 64 (59–70) | 70 (63–76) | 76 (69–81) | 80 (72–88) | <0.01 |
| Night-to-day ratio of SBP | 0.96 (0.91–1.03) | 0.96 (0.91–1.00) | 0.97 (0.93–1.03) | 0.95 (0.90–1.02) | 0.38 |
| Dipper, n (%) | 21 (20) | 23 (21) | 13 (12) | 22 (22) | 0.25 |
| Non-dipper, n (%) | 51 (48) | 56 (52) | 54 (51) | 41 (41) | 0.40 |
| Extreme dipper, n (%) | 2 (2) | 2 (2) | 0 (0) | 2 (2) | 0.56 |
| Riser, n (%) | 33 (31) | 27 (25) | 39 (37) | 35 (35) | 0.26 |
| Non-dipping, n (%)a | 84 (79) | 83 (77) | 93 (88) | 76 (76) | 0.13 |
| Antihypertensive drugs, n (%) | 71 (66) | 83 (77) | 92 (87) | 96 (96) | <0.01 |
| Antihypertensive drugs only in the daytime, n (%) |
47 (44) | 41 (38) | 45 (42) | 37 (37) | 0.69 |
| Antihypertensive drugs only at night, n (%) |
3 (3) | 3 (3) | 2 (2) | 2 (2) | 0.95 |
| Antihypertensive drugs in both the daytime and at night, n (%) |
21 (20) | 39 (36) | 45 (42) | 57 (57) | <0.01 |
| RAAS inhibitors, n (%) | 60 (56) | 69 (64) | 70 (66) | 79 (79) | <0.01 |
| CCB, n (%) | 42 (39) | 63 (58) | 84 (79) | 85 (85) | <0.01 |
| α-blocker, n (%) | 2 (2) | 11 (10) | 10 (9) | 19 (19) | <0.01 |
| β-blocker, n (%) | 13 (12) | 20 (19) | 22 (21) | 20 (20) | 0.34 |
| Diuretics, n (%) | 28 (26) | 30 (28) | 33 (31) | 47 (47) | <0.01 |
Values are expressed as mean±SD, number (%) or median (interquartile range). aNon-dipping, non-dipper+riser. ABPM, ambulatory blood pressure monitoring; CCB, calcium-channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure.
| Variable | Night-time SBP level | P value | |||
|---|---|---|---|---|---|
| 84–113 mmHg (n=110) |
114–127 mmHg (n=104) |
128–141 mmHg (n=103) |
142–194 mmHg (n=104) |
||
| Age (years) | 64.5 (47.7–74.4) | 70.2 (61.0–75.9) | 69.3 (60.4–78.6) | 72.6 (62.3–79.8) | <0.01 |
| Male, n (%) | 60 (55) | 69 (66) | 78 (76) | 74 (71) | <0.01 |
| Diabetes mellitus, n (%) | 24 (22) | 39 (38) | 43 (42) | 71 (68) | <0.01 |
| Hypertension, n (%) | 66 (60) | 86 (83) | 97 (94) | 102 (98) | <0.01 |
| Smoking, n (%) | 42 (38) | 58 (56) | 57 (55) | 69 (66) | <0.01 |
| Dyslipidemia, n (%) | 72 (65) | 75 (72) | 85 (83) | 70 (67) | 0.03 |
| Ischemic heart disease, n (%) | 9 (8) | 20 (19) | 27 (26) | 20 (19) | <0.01 |
| Body mass index (kg/m2) | 22.6 (20.2–25.4) | 22.8 (21.1–25.9) | 22.2 (20.3–25.8) | 22.2 (20.2–24.6) | 0.29 |
| Daily proteinuria (g/day) | 0.44 (0.15–1.10) | 0.78 (0.20–2.03) | 1.15 (0.45–2.86) | 2.54 (0.94–4.99) | <0.01 |
| Hemoglobin (g/dL) | 11.7 (10.4–13.4) | 11.2 (9.9–12.8) | 10.2 (8.9–12.3) | 9.6 (8.8–11.0) | <0.01 |
| eGFR (mL/min/1.73 m2) | 38.7 (21.8–63.9) | 32.1 (17.1–56.0) | 22.1 (14.7–34.3) | 19.7 (13.0–30.5) | <0.01 |
| Serum albumin (g/dL) | 3.7 (3.4–3.9) | 3.6 (3.3–3.8) | 3.5 (3.1–3.7) | 3.0 (2.4–3.4) | <0.01 |
| Serum phosphorus (mg/dL) | 3.5 (3.1–4.0) | 3.6 (3.3–4.0) | 3.9 (3.4–4.2) | 3.7 (3.4–4.2) | <0.01 |
| 24-h SBP (mmHg) | 110 (103–116) | 124 (120–128) | 137 (133–142) | 155 (144–163) | <0.01 |
| 24-h DBP (mmHg) | 66 (61–72) | 74 (67–79) | 79 (71–84) | 82 (75–89) | <0.01 |
| Daytime SBP (mmHg) | 112 (107–121) | 126 (119–132) | 138 (132–145) | 154 (141–163) | <0.01 |
| Daytime DBP (mmHg) | 69±8 | 75±9 | 80±11 | 83±12 | <0.01 |
| Night-time SBP (mmHg) | 104 (97–109) | 121 (118–124) | 134 (131–138) | 152 (147–164) | <0.01 |
| Night-time DBP (mmHg) | 63 (56–68) | 71 (66–76) | 76 (68–82) | 81 (74–88) | <0.01 |
| Night-to-day ratio of SBP | 0.91 (0.85–0.96) | 0.96 (0.92–1.00) | 0.98 (0.93–1.02) | 1.03 (0.97–1.07) | <0.01 |
| Dipper, n (%) | 44 (40) | 13 (13) | 16 (16) | 6 (6) | <0.01 |
| Non-dipper, n (%) | 52 (47) | 66 (63) | 54 (52) | 30 (29) | <0.01 |
| Extreme dipper, n (%) | 5 (5) | 0 (0) | 1 (1) | 0 (0) | 0.01 |
| Riser, n (%) | 9 (8) | 25 (24) | 32 (31) | 68 (65) | <0.01 |
| Non-dipping, n (%)a | 61 (55) | 91 (88) | 86 (84) | 98 (94) | <0.01 |
| Antihypertensive drugs, n (%) | 70 (64) | 82 (79) | 92 (89) | 98 (94) | <0.01 |
| Antihypertensive drugs only in the daytime, n (%) |
46 (42) | 41 (39) | 41 (40) | 42 (40) | 0.99 |
| Antihypertensive drugs only at night, n (%) |
5 (5) | 2 (2) | 2 (2) | 1 (1) | 0.35 |
| Antihypertensive drugs in both the daytime and at night, n (%) |
19 (17) | 39 (38) | 49 (48) | 55 (53) | <0.01 |
| RAAS inhibitors, n (%) | 60 (55) | 64 (62) | 71 (69) | 83 (80) | <0.01 |
| CCB, n (%) | 39 (35) | 68 (65) | 81 (79) | 86 (83) | <0.01 |
| α-blocker, n (%) | 2 (2) | 8 (8) | 12 (12) | 20 (19) | <0.01 |
| β-blocker, n (%) | 15 (14) | 19 (18) | 23 (22) | 18 (17) | 0.43 |
| Diuretics, n (%) | 25 (23) | 32 (31) | 37 (36) | 44 (42) | 0.02 |
Values are expressed as mean±SD, number (%) or median (interquartile range). aNon-dipping, non-dipper+riser. Abbreviations as in Table 1.
The median follow-up period was 24.3 months (full range, 1.2–87.5 months). At the end of follow-up, 150 patients had reached the composite renal endpoint (ESRD, n=130; death, n=20). With regard to dialysis, 123 patients started maintenance hemodialysis and 7 started peritoneal dialysis. The causes of death were infectious disease in 6, sudden death in 3, malignancy in 2, heart failure in 2, multiple organ failure in 2, and uremia, respiratory failure, brain hemorrhage, old age, and an unknown cause in 1 patient each.
Kaplan-Meier analysis showed significantly higher rates of renal events in both higher daytime and night-time SBP quartiles (Figure 2).
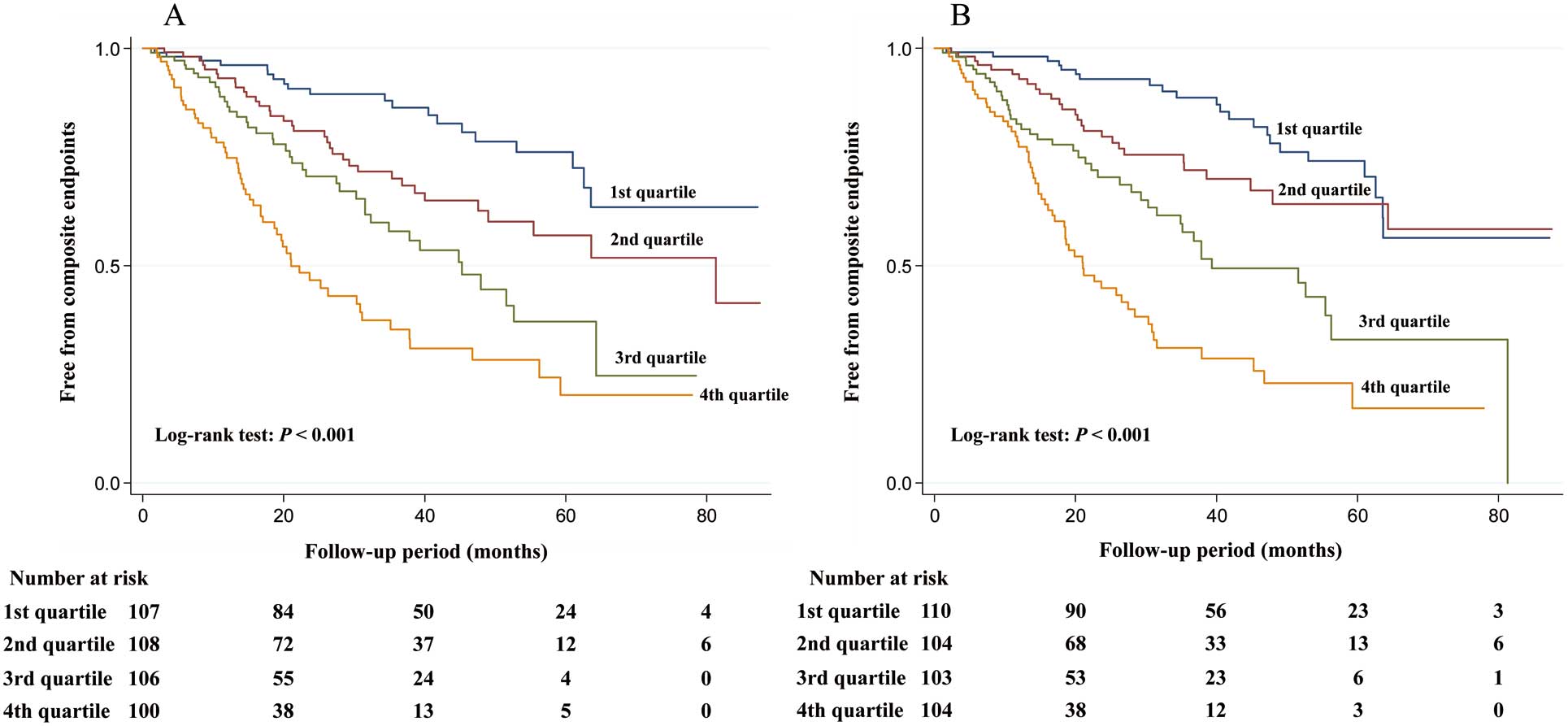
Kaplan-Meier curves for freedom from adverse composite renal outcomes in patients stratified by quartiles of daytime SBP (A) and night-time SBP (B) and compared using log-rank tests. SBP, systolic blood pressure.
Table 3 shows the HR for composite renal outcomes according to each component of ABPM. In the multivariable analysis (model 2), higher daytime and night-time SBP levels (every 10-mmHg increase) were associated with an increased risk for composite renal outcomes, whereas independent associations of higher daytime or night-time DBP levels (every 10-mmHg increase) with composite renal outcomes were not found. Patients in the 4rth night-time SBP quartile had significantly poorer renal outcomes than those in the 1st quartile; conversely, the 4th quartile of daytime SBP was not associated with adverse outcomes compared with the 1st quartile. There were no independent associations of the night-to-day ratio of SBP or non-dipping status with composite renal outcomes. Restricted multivariable cubic spline plots for composite renal outcomes are presented in Figure 3. The risk for composite renal outcomes increased in a linear fashion with an increment in daytime and night-time SBP levels.
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | P for trend | HR | 95% CI | P value | P for trend | |
| Daytime SBP (every 10-mmHg increase) | 1.37 | 1.26–1.49 | <0.01 | – | 1.13 | 1.02–1.26 | 0.02 | – |
| Daytime SBP | <0.01 | 0.16 | ||||||
| Q1 (n=107, 94–119 mmHg) | (Ref.) | (Ref.) | ||||||
| Q2 (n=108, 120–131 mmHg) | 1.78 | 1.02–3.10 | 0.04 | 1.25 | 0.70–2.25 | 0.45 | ||
| Q3 (n=106, 132–143 mmHg) | 2.81 | 1.63–4.85 | <0.01 | 1.09 | 0.61–1.94 | 0.77 | ||
| Q4 (n=100, 144–188 mmHg) | 4.95 | 2.93–8.35 | <0.01 | 1.58 | 0.88–2.85 | 0.13 | ||
| Night-time SBP (every 10-mmHg increase) | 1.28 | 1.20–1.37 | <0.01 | – | 1.15 | 1.05–1.27 | <0.01 | – |
| Night-time SBP | <0.01 | 0.03 | ||||||
| Q1 (n=110, 84–113 mmHg) | (Ref.) | (Ref.) | ||||||
| Q2 (n=104, 114–127 mmHg) | 1.46 | 0.83–2.58 | 0.19 | 1.09 | 0.61–1.96 | 0.78 | ||
| Q3 (n=103, 128–141 mmHg) | 2.75 | 1.63–4.66 | <0.01 | 1.31 | 0.76–2.28 | 0.33 | ||
| Q4 (n=104, 142–194 mmHg) | 5.19 | 3.13–8.60 | <0.01 | 1.82 | 1.00–3.30 | 0.049 | ||
| Daytime DBP (every 10-mmHg increase) | 1.19 | 1.02–1.38 | 0.03 | – | 1.18 | 0.996–1.39 | 0.06 | – |
| Daytime DBP | <0.01 | 0.08 | ||||||
| Q1 (n=118, 44–69 mmHg) | (Ref.) | (Ref.) | ||||||
| Q2 (n=95, 70–76 mmHg) | 1.07 | 0.67–1.70 | 0.78 | 1.61 | 0.98–2.65 | 0.06 | ||
| Q3 (n=113, 77–84 mmHg) | 1.18 | 0.76–1.83 | 0.46 | 1.12 | 0.69–1.81 | 0.65 | ||
| Q4 (n=95, 85–114 mmHg) | 2.08 | 1.31–3.29 | <0.01 | 1.97 | 1.13–3.45 | 0.02 | ||
| Night-time DBP (every 10-mmHg increase) | 1.25 | 1.09–1.44 | <0.01 | – | 1.20 | 1.02–1.42 | 0.03 | – |
| Night-time DBP | <0.01 | 0.04 | ||||||
| Q1 (n=111, 39–64 mmHg) | (Ref.) | (Ref.) | ||||||
| Q2 (n=103, 65–71 mmHg) | 1.54 | 0.97–2.46 | 0.07 | 1.70 | 1.03–2.80 | 0.04 | ||
| Q3 (n=103, 72–79 mmHg) | 1.98 | 1.25–3.16 | <0.01 | 1.50 | 0.90–2.51 | 0.12 | ||
| Q4 (n=104, 80–112 mmHg) | 2.12 | 1.31–3.44 | <0.01 | 1.89 | 1.10–3.26 | 0.02 | ||
| Night-to-day ratio of SBP | 0.07 | 0.21 | ||||||
| Q1 (n=107, 0.66–0.91) | (Ref.) | (Ref.) | ||||||
| Q2 (n=104, 0.91–0.96) | 1.59 | 0.98–2.58 | 0.06 | 1.48 | 0.89–2.47 | 0.13 | ||
| Q3 (n=105, 0.96–1.03) | 1.37 | 0.86–2.18 | 0.19 | 1.46 | 0.89–2.38 | 0.13 | ||
| Q4 (n=105, 1.03–1.27) | 1.62 | 1.02–2.56 | 0.04 | 1.40 | 0.85–2.31 | 0.19 | ||
| Non-dippinga (vs. Dippingb) | 1.43 | 0.93–2.18 | 0.10 | – | 1.29 | 0.83–2.02 | 0.26 | – |
Model 1: adjusted for age and sex. Model 2: model 1 plus adjusted for diabetes mellitus, smoking, dyslipidemia, ischemic heart disease, use of immunosuppressants, use of RAAS inhibitors, use of antihypertensive drugs only in the daytime, only at night or use in both the daytime and at night, body mass index, daily proteinuria, hemoglobin, eGFR, serum phosphorus, and serum albumin. aNon-dipping, non-dipper+riser; bDipping, dipper+extreme dipper. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
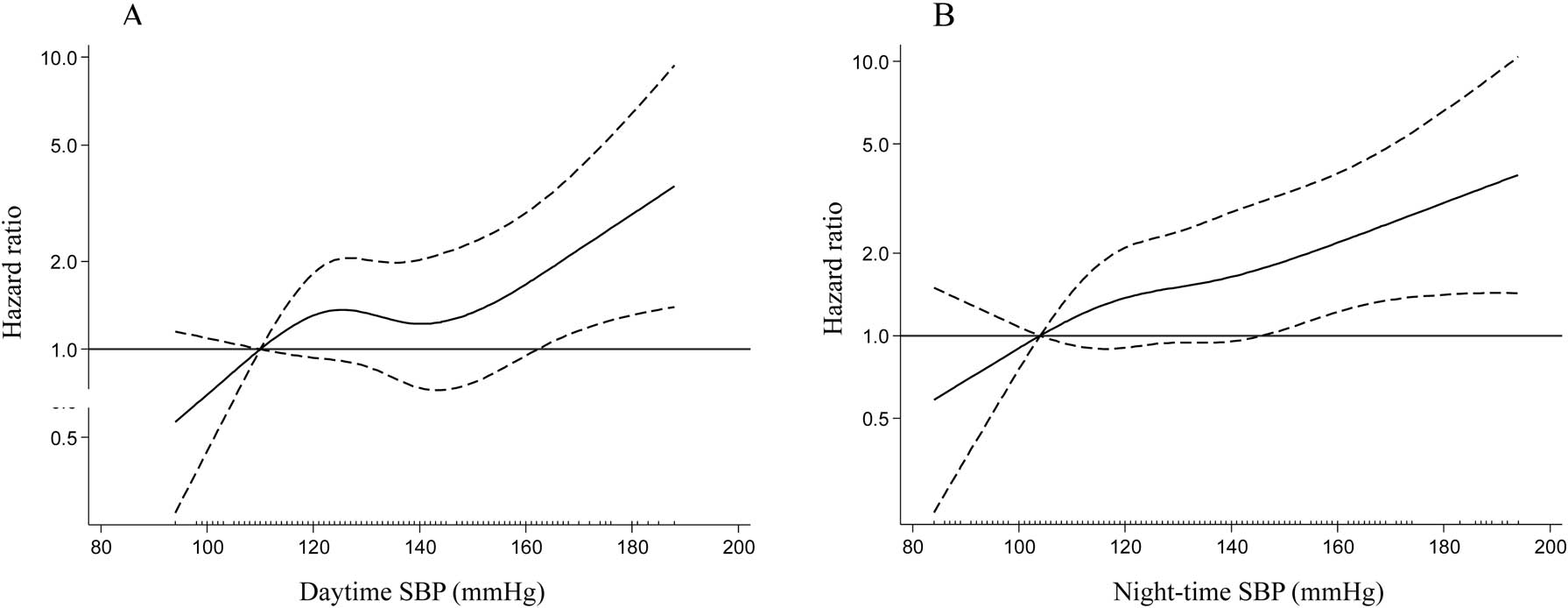
Adjusted hazard ratios for composite renal outcomes in daytime SBP (A) and night-time SBP (B). The solid line indicates the hazard ratio; dashed lines, 95% confidence interval. SBP, systolic blood pressure.
Figure 4 summarizes the adjusted HR according to subgroups stratified by demographic and clinical characteristics. With regard to daytime SBP, in older patients and in patients with diabetes mellitus, lower eGFR, lower serum albumin, higher serum phosphorus, and lower hemoglobin, higher daytime SBP levels significantly increased the risk for composite renal outcomes. However, significant associations of night-time SBP with composite renal outcomes were found in all subgroups showing significant associations between daytime SBP and composite renal outcomes. Additionally, higher night-time SBP levels increased the risk for adverse renal outcomes in male and female patients and in patients without diabetes mellitus. No significant interactions for renal outcomes were observed between daytime or night-time SBP and other baseline clinical characteristics (P for interaction, 0.10–0.94).

Adjusted hazard ratios and 95% confidence intervals for composite renal outcomes for every 10-mmHg increase in daytime SBP (A) and night-time SBP (B) in subgroups stratified according to baseline characteristics. Adjusted for age, sex, diabetes mellitus, smoking, dyslipidemia, ischemic heart disease, use of immunosuppressants, use of renin–angiotensin-aldosterone system inhibitors, use of antihypertensive drugs only in the daytime, only at night or use in both the daytime and at night, body mass index, daily proteinuria, hemoglobin, eGFR, serum phosphorus, and serum albumin. *P<0.05, **P<0.01. eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
We investigated the associations of each component of ABPM with progression to ESRD (n=130). Table S1 shows the HR for ESRD according to each component of ABPM. Compared with the 1st quartile of daytime or night-time SBP, the 4th quartile of night-time SBP, but not daytime SBP, significantly increased the risk for ESRD (daytime SBP: HR: 1.71, 95% CI: 0.89–3.30, P=0.11; night-time SBP: HR: 2.03, 95% CI: 1.05–3.93, P=0.04), similar to the relationship between composite renal outcomes, daytime or night-time SBP, as shown in Table 3.
This study investigated the independent associations of daytime and night-time SBP with composite renal outcomes, and compared the predictive values of these 2 parameters on adverse renal outcomes. By dividing night-time SBP into quartiles and comparing them with the 1st quartile (≤113 mmHg), patients in the 4th quartile (≥142 mmHg) showed a significantly increased risk for composite renal outcomes (by 82%). Conversely, patients in the 4th quartile with daytime SBP ≥144 mmHg showed an increased risk of 58%, compared with those in the 1st quartile (≤119 mmHg), but did not reach significance.
Previous studies have demonstrated that night-time BP has more predictive value for cardiovascular and renal events and death compared with daytime BP in both hypertensive subject6,8–10 and those with CKD.14,17 In the general population, night-time BP has been found to be a better predictor for the development of CKD.16 In the current study, night-time SBP was also found to have prognostic superiority for composite renal outcomes compared with daytime SBP.
There has been no definite explanation of why night-time BP has a stronger predictive value for adverse outcomes compared with daytime BP. Several hypothetical mechanisms of how night-time BP affects a poor prognosis have been proposed. In terms of physical and mental activities, BP levels are more variable during the daytime period than during the night-time period, and therefore night-time BP is a better standardization of BP measurement compared with daytime BP.5 It is also suggested that night-time BP could reflect more precisely the hemodynamic effects on target organs. During the night-time period, afferent arteriolar tone in glomerular capillaries is at its lowest, leading to a more direct transmission of high night-time systemic BP to the glomeruli. Therefore, maintaining high night-time BP causes intraglomerular hyperfiltration, resulting in kidney disease progression.19 However, higher night-time BP is also linked to changes in the sympathetic modulation of night-time BP,20 disturbed baroreflex sensitivity,21 sleep apnea syndrome,22 and disturbed circadian rhythm of natriuresis.23–25 Despite previous research, the reasons for the associations of these alterations with poor renal outcomes remain unclear and further studies are required.
Non-dipping is reported to be associated with CKD. In previous reports, the prevalence of non-dipping in CKD patients varied markedly, ranging from 47% to 80%.13–15,17,26,27 The current study showed a high prevalence (80%) of non-dipping, similar to previous studies.13,15,26 This wide range in the prevalence of non-dipping might be attributable to differences in the studied populations, sample sizes, time of setting of ABPM, proportions of patients treated with antihypertensive agents, or the degree of kidney dysfunction in each cohort.
The prognostic value of non-dipping on renal outcomes is controversial. In CKD patients, non-dipping was found to be associated with poor renal outcomes,13,14 but other studies report no relationship.15,17 Additionally, in people with preserved kidney function, one study reported a significant association of non-dipping with deterioration of kidney function,28 but another study did not.16
High nocturnal BP is, in some cases, accompanied by a non-dipping pattern, but both patterns are not always present together and there are differences in the significance between nocturnal BP and the non-dipping pattern.17,29 In the current study, 336 patients with non-dipping comprised the following quartiles of night-time SBP with a full range of 91–194 mmHg: 1st quartile, 18%; 2nd quartile, 27%; 3rd quartile, 26%; and 4th quartile, 29%. These results indicated that patients with a disturbed dipping status had a range of night-time SBP from lower to higher levels. The findings suggested that higher night-time SBP levels increase the risk for renal outcomes, but non-dipping or the night-to-day ratio of SBP does not, similar to other studies.15–17 Based on these findings, it is suggested that absolute night-time SBP itself predicts renal outcomes, but disturbed dipping status does not. However, studies using larger cohorts are needed to clarify whether night-time SBP can provide more predictive information on renal outcomes compared with disturbed dipping status.
This study also investigated associations of daytime and night-time SBP with composite renal outcomes using stratified analyses. In addition to all subgroups in which daytime SBP was associated with composite renal outcomes, night-time SBP was identified as an independent factor for adverse outcomes in both sexes and in patients without diabetes mellitus. Both daytime and night-time SBP were associated with composite renal outcomes in patients with lower eGFR, lower serum albumin, higher serum phosphorus, and lower hemoglobin, but not in those with higher eGFR, higher serum albumin, lower serum phosphorus, and higher hemoglobin. The former parameters are known risk factors for kidney disease progression. In the current study, the incidence rates of renal events in patients with the former parameters were high compared with those with the latter parameters (Figure 4). Given these findings, it is suggested that patients with a high risk for deterioration of kidney function are more susceptible to the effects of daytime or night-time SBP on adverse outcomes.
Study LimitationsFirst, all study participants were recruited at a single regional hospital, so the selection of patients was limited and the sample size was relatively small. Furthermore, there is the possibility that the findings obtained from this population could be affected by selection bias. Our study recruited consecutive patients who were admitted to hospital. As a result, patients were relatively old, and the number of male patients was approximately twice the number of female patients. Nevertheless, in the stratified analyses, higher night-time SBP levels increased the risk for renal outcomes in older patients and in both males and females, while a significant relationship between higher daytime SBP and renal outcomes was observed in older patients. Second, we did not examine home or clinic BP. Therefore, we could not evaluate whether the predictive values for each component of ABPM on kidney disease progression are stronger than those of home or clinic BP, and the effects of white-coat or masked hypertension on renal outcomes could not be investigated. Third, we assessed BP variability and dipping status using a single measurement of 24-h ABPM, which might have low reproducibility, in particular, for dipping status in CKD.27,30 Fourth, there was a lack of validity of the 2 devices used for ABPM in this study. A previous report demonstrated that the ES-H531 device offered acceptable validity for practical use,31 but in another validation study, the FM-800 device was also recommended for clinical use.32 However, in this study, we did not simultaneously perform the standard auscultation method; therefore, the validity of these 2 devices could not be examined in the current Japanese CKD patients. Furthermore, the findings in this study might be affected by the use of 2 devices for ABPM over different periods with a difference in intervals for BP monitoring at night. Fifth, the results of this cohort study might be biased by the inclusion of the 72 censored people. Because a Cox proportional hazard model was used to determine risk factors for renal outcomes, inclusion of these censored people could reduce the influence on renal outcomes. Finally, all participants were Japanese, and it has not yet been determined whether the findings of this study can be generalized to other ethnic groups.
This study examined whether daytime or night-time SBP was associated with a composite endpoint of ESRD or death and compared the predictive values of these 2 parameters on adverse outcomes in CKD patients. Compared with the 1st quartile of daytime or night-time SBP, patients in the 4th quartile of night-time SBP showed a significantly increased risk for composite renal outcomes, but patients in the 4th quartile of daytime SBP did not. Given these findings, it is suggested that night-time SBP measurement is more useful for predicting composite renal outcomes compared with daytime SBP measurement.
We express our gratitude to the 421 participants in the study.
No potential conflicts of interests relevant to this article were reported.
Supplementary File 1
Table S1. Adjusted HRs for ESRD in each component of ABPM
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0063