2017 Volume 81 Issue 10 Pages 1379-1385
2017 Volume 81 Issue 10 Pages 1379-1385
In parallel with the increase in the number of elderly people worldwide, the number of patients with heart disease is also rapidly increasing. Of the heart diseases, cardiovascular disease (CVD) and heart failure (HF) are strongly associated with adverse health outcomes that decrease productivity in later years. Recently, ANGPTL2, a secreted glycoprotein and member of the angiopoietin-like protein family, has received attention as a causal player in the development of CVD and HF. Prolonged ANGPTL2 autocrine/paracrine signaling in vascular tissue leads to chronic inflammation and pathologic tissue remodeling, accelerating CVD development. Excess ANGPTL2 autocrine/paracrine signaling induced in the pathologically stressed heart accelerates cardiac dysfunction by decreasing myocardial energy metabolism. Conversely, ANGPTL2 inactivation in vascular tissue and the heart delays development or progression of CVD and HF, respectively. Moreover, there is increased evidence for an association between elevated circulating ANGPTL2 levels and CVD and HF. Interestingly, ANGPTL2 expression is also associated with cellular senescence, which may promote premature aging and development of aging-associated diseases, including CVD and HF. Overall, ANGPTL2 autocrine/paracrine signaling is a new factor in accelerating heart disease development in the aging. Here, we focus on current topics relevant to ANGPTL2 function in heart disease.
Worldwide, the number of patients with heart disease is increasing as populations of elderly people expand. Of the heart diseases, cardiovascular disease (CVD) and heart failure (HF) are associated with adverse health outcomes that decrease a patient’s well-being and productivity.1,2 Prevention of these conditions is desirable to promote healthy aging and improve patients’ lifestyle.
The pathologic basis of CVD is atherosclerosis caused by ectopic accumulation of cholesterol in vessel walls;3 thus advent of therapies aimed at reducing low-density lipoprotein (LDL)-cholesterol levels has succeeded in decreasing the number of CVD events.4,5 However, these events continue to occur, even in patients whose LDL-cholesterol levels have been lowered by antidyslipidemia treatment,1,2,4,5 indicating that the pathologies underlying CVD are highly complex. In addition to dyslipidemia, other factors such as smoking, hypertension, diabetes, ventral obesity, male sex, aging, genetic factors, and family history of CVD are well-established risk factors,6–8 and the number of patients with dyslipidemia, diabetes, and hypertension also increases with age.9
Recently, it was shown that chronic vascular inflammation accelerates atherosclerosis development.10 Interestingly, aging, smoking, diabetes, and dyslipidemia all induce chronic inflammation,11,12 and emerging evidence reveals that senescent cells secrete factors that cause chronic local tissue inflammation associated with age-associated diseases.13–15 This newly identified cellular senescence condition, termed the senescence-associated secretory phenotype (SASP), is now recognized as creating a common pathological environment that encourages development of age-associated diseases, including CVD.13,15,16
Many of our previous studies have revealed that the expression and secretion of angiopoietin-like 2 (ANGPTL2) significantly increase in cells stressed by pathophysiologic stimuli such as hypoxia, reactive oxidative species, and pressure overload.17–21 ANGPTL2 expression also increases in cells undergoing senescence,22 suggesting that ANGPTL2 is a SASP factor. Moreover, excess ANGPTL2 signaling is pro-inflammatory in pathologic states and contributes to the development of aging-associated diseases such as CVD.23–25 Thus, to understand the mechanisms underlying these conditions, we focus our discussion here on the role of ANGPTL2 in CVD.
Around 2000, several groups independently reported secreted glycoproteins that exhibit an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain similar to angiopoietins (ANGPTs).26 A nomenclature committee designated these factors “angiopoietin-like proteins (ANGPTLs)”. The ANGPTL family now consists of 8 members (ANGPTLs 1–8),26,27 although ANGPTL8 lacks the C-terminal fibrinogen-like domain.27
ANGPTL2 is a 64-kDa glycosylated protein28 that is broadly, but not ubiquitously, expressed in adipose tissue, heart, skeletal muscle, kidney, and some hematopoietic cells.17,25,28 ANGPTL2 is secreted; the average ANGPTL2 concentration in circulation in the Japanese general population is 2.9 ng/mL with no difference between males and females.29 Circulating ANGPTL2 levels increase with age in Japanese populations25 and are high in patients with chronic, age-associated diseases, such as heart disease, kidney disease, diabetes, and cancer.21,29–35
Previous studies have shown that ANGPTL2 potently promotes pro-angiogenic and anti-apoptotic activities, as do the ANGPTs.28,36,37 Moreover, we reported that ANGPTL2 signaling through integrin α5β1 enhances cell motility by activating the Rho family GTPase Rac1 and by stimulating remodeling of the extracellular matrix (ECM) through activation of p38 mitogen-activated protein kinase (MAPK)-dependent matrix metalloproteinases (MMPs) (Figure 1).19,20,24 ANGPTL2 signaling also induces expression of inflammation-related NFκB target genes by increasing degradation of IκB, a factor that inhibits NFκB nuclear localization.17,24 More recently, we reported that ANGPTL2 promotes intestinal epithelial regeneration (Figure 1).38 Others report that ANGPTL2 signaling through leukocyte immunoglobulin-like receptor B2 (LILRB2) or paired immunoglobulin-like receptor B (PirB) plays an important role in maintaining hematopoietic stem cell stemness and in the development of leukemia (Figure 2).39,40 Moreover, ANGPTL2 signaling through PirB reportedly stimulates antithrombotic activity (Figure 2).41

Integrin α5β1-mediated ANGPTL2 signaling. In integrin α5β1-expressing cells, such as endothelial cells, macrophages, or tumor cells, ANGPTL2 promotes cell motility by activating Rac. ANGPTL2 also induces the expression and activity of matrix metalloproteinases (MMPs) via the p38 mitogen-activated protein kinase (MAPK) pathway, promoting extracellular matrix (ECM) remodeling and tumor cell invasion. ANGPTL2 also causes inflammation by activating the nuclear factor κ-B (NFκB) pathway. Moreover, in intestinal subepithelial myofibroblasts, ANGPTL2 regulates intestinal epithelial regeneration by inhibiting bone morphogenetic protein (BMP) signaling.
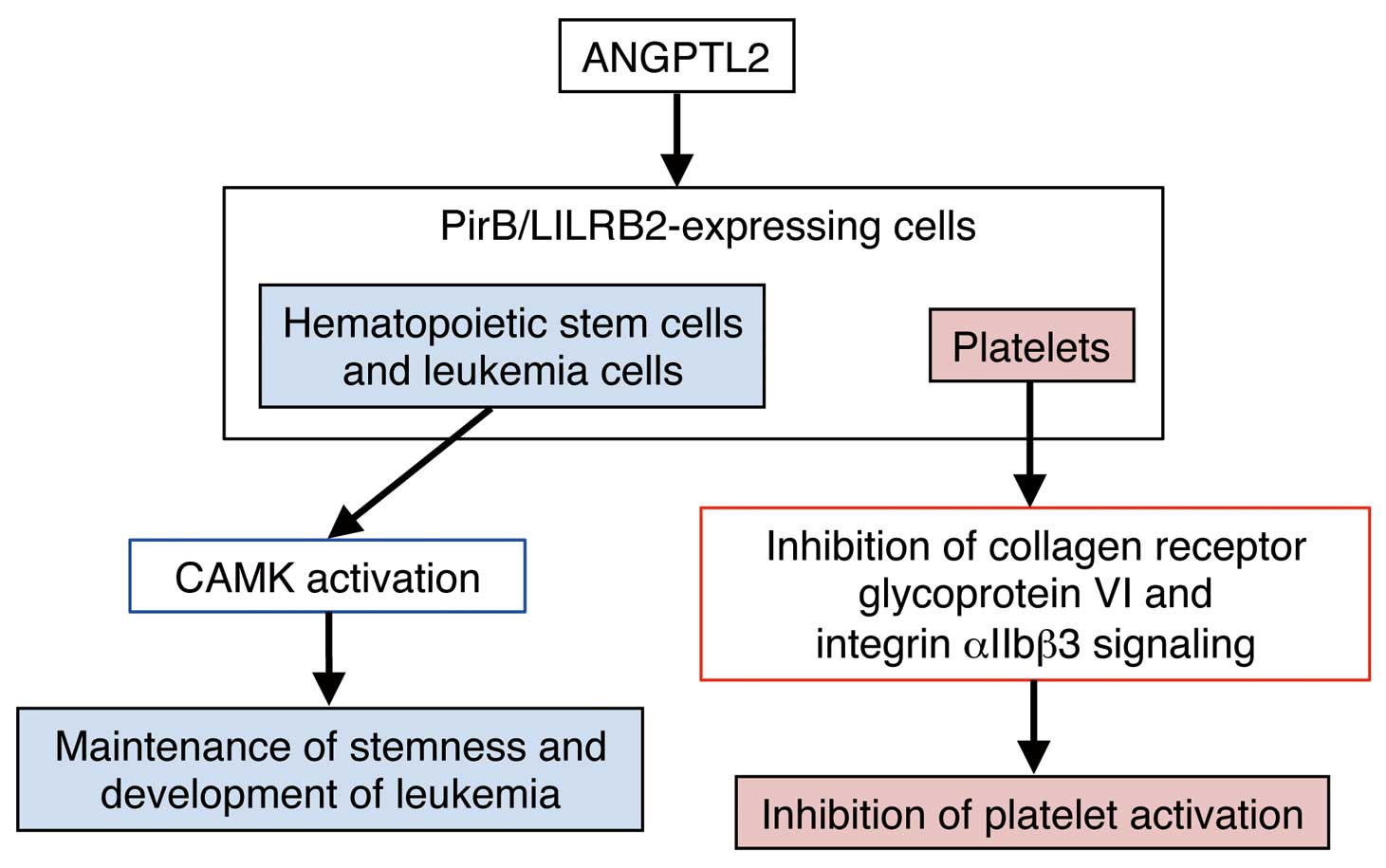
PirB/LILRB2-mediated ANGPTL2 signaling. In hematopoietic stem or leukemia cells, ANGPTL2 contributes to maintenance of respective stemness or leukemia development via the PirB/LILRB2-calmodulin-dependent protein kinase (CAMK) pathway. ANGPTL2 also inhibits platelet activation by suppressing collagen receptor glycoprotein VI and integrin αIIbβ3 signaling.
In obese mice, either hypoxia in visceral adipose tissues or activated endoplasmic reticulum stress in adipocytes increases ANGPTL2 expression and secretion by those tissues.17 Secreted ANGPTL2 then enhances adipose tissue remodeling by promoting angiogenesis, macrophage recruitment, and ECM remodeling as a way of storing excess lipids into adipocytes in response to weight gain (Figure 3).17,24 However, in very severe cases of obesity, more abundant secretion of ANGPTL2 and associated signaling causes chronic adipose tissue inflammation and irreversible tissue remodeling, leading to conditions correlated with CVD risk, such as obesity-associated metabolic disease and accompanying insulin resistant type 2 diabetes and dyslipidemia (Figure 3).17,24,25
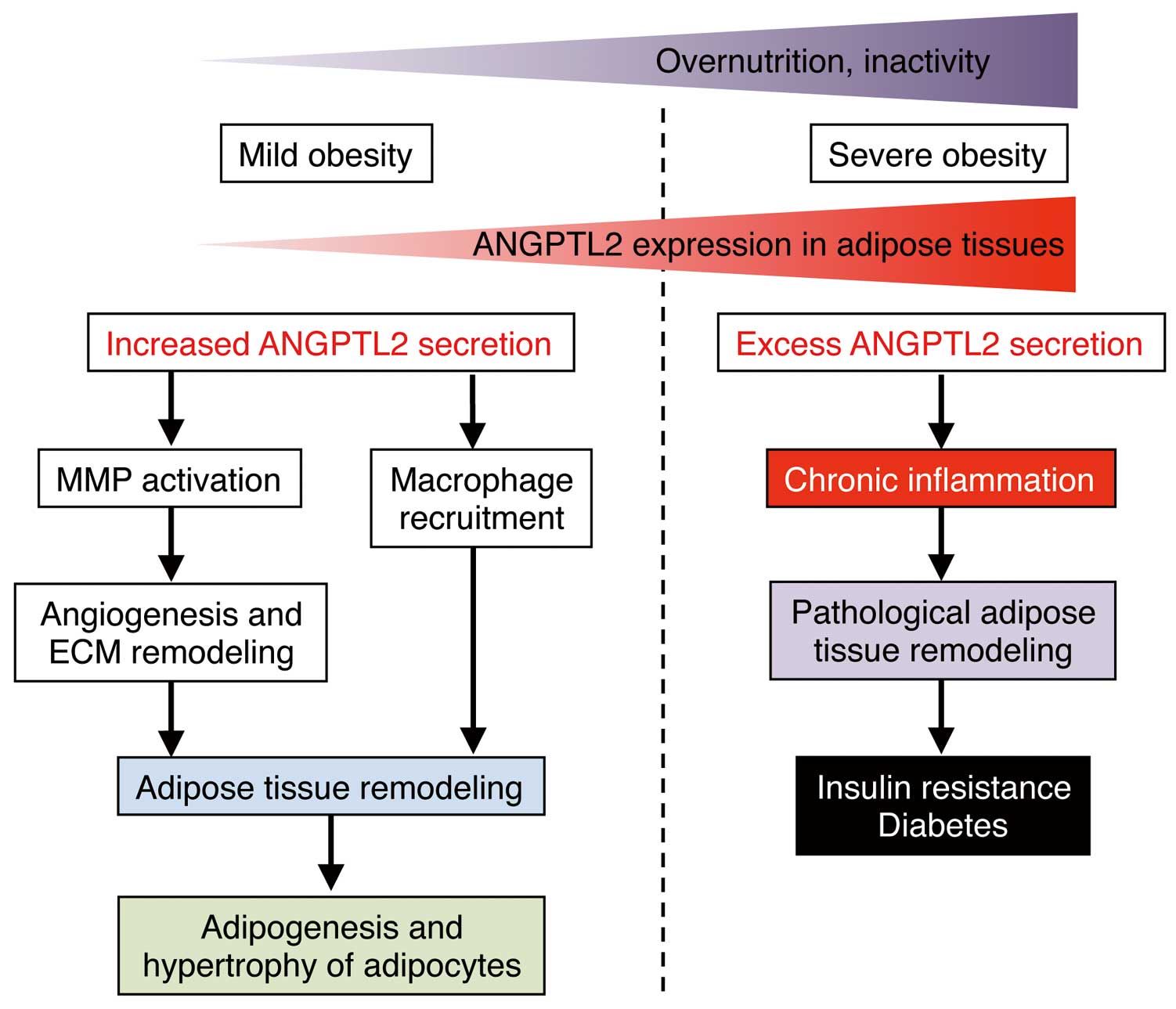
ANGPTL2 activity in mild and severe obesity. ANGPTL2 expression in adipose tissue increases under obese conditions. In the early phase (mild obesity), increased ANGPTL2 secretion from adipose tissue promotes macrophage recruitment into adipose tissue and activation of matrix metalloproteinases (MMPs) and subsequent angiogenesis and extracellular matrix (ECM) remodeling, leading to adipogenesis and adipocyte hypertrophy. Lifestyle changes, such as overnutrition or inactivity, induce higher adiposity (severe obesity) and excess ANGPTL2 secretion, leading to chronic inflammation and pathologic adipose tissue remodeling and subsequent obesity-related insulin resistance and type 2 diabetes.
Obesity and associated metabolic diseases predispose individuals to coronary artery disease (CAD),42 the major common form of CVD. In terms of the mechanisms linking these conditions, accumulating evidence suggests that inflammatory changes in perivascular fat, which is distributed ubiquitously around arteries throughout the body, may have a direct role in promoting the pathogenesis of vascular diseases accelerated by obesity.43–45 Interestingly, in obesity, chronic inflammation occurs in both visceral and perivascular adipose tissues.44,45 In obese mice, the expression of ANGPTL2 is increased in perivascular adipose tissues surrounding the femoral artery at levels equivalent to those seen in visceral adipose tissues.46 In mice, we have undertaken adipose tissue transplantation experiments that show that adipose tissue-secreted ANGPTL2 accelerates vascular inflammation, pathologic vascular tissue remodeling and subsequent CVD development.46
Moreover, atherosclerosis progression, including plaque instability, is associated with chronic vessel wall inflammation and is a risk factor for major CAD events.10,47,48 Therefore, therapies designed to inhibit chronic inflammation in vessel walls should slow atherosclerosis progression. Relevant to this, ANGPTL2 is abundantly expressed in vascular endothelial cells of CAD patients.25 ANGPTL2 expression in endothelial cells also significantly increases in subjects predisposed to atherosclerotic disease brought on by obesity or metabolic disturbances (Figure 4).25 Smoking and associated oxidative stress also predispose individuals to atherosclerotic disease.49 Interestingly, others report that current smokers show significantly higher ANGPTL2 levels in their endothelial cells than do non- or ex-smokers.50 As expected, increases in endothelial cell-derived ANGPTL2 expression in mice promote vascular inflammation and subsequent endothelial cell dysfunction and atherosclerosis development (Figure 4).25 Vascular inflammation, which underlies atherosclerotic disease, emerges from the interplay of different cell types, including endothelial cells, smooth muscle cells, and perivascular adipocytes as resident cells, and macrophages as infiltrating cells.51–54 Macrophage-secreted ANGPTL2 also accelerates atherosclerotic disease in mice (Figure 4).55 Thus, ANGPTL2-induced chronic inflammation predisposes individuals to atherosclerotic disease and to CAD development. Furthermore, inflammation also underlies plaque destabilization. Thus, the ANGPTL2-dependent chronic inflammation axis represents a potential target to prevent or treat CAD.
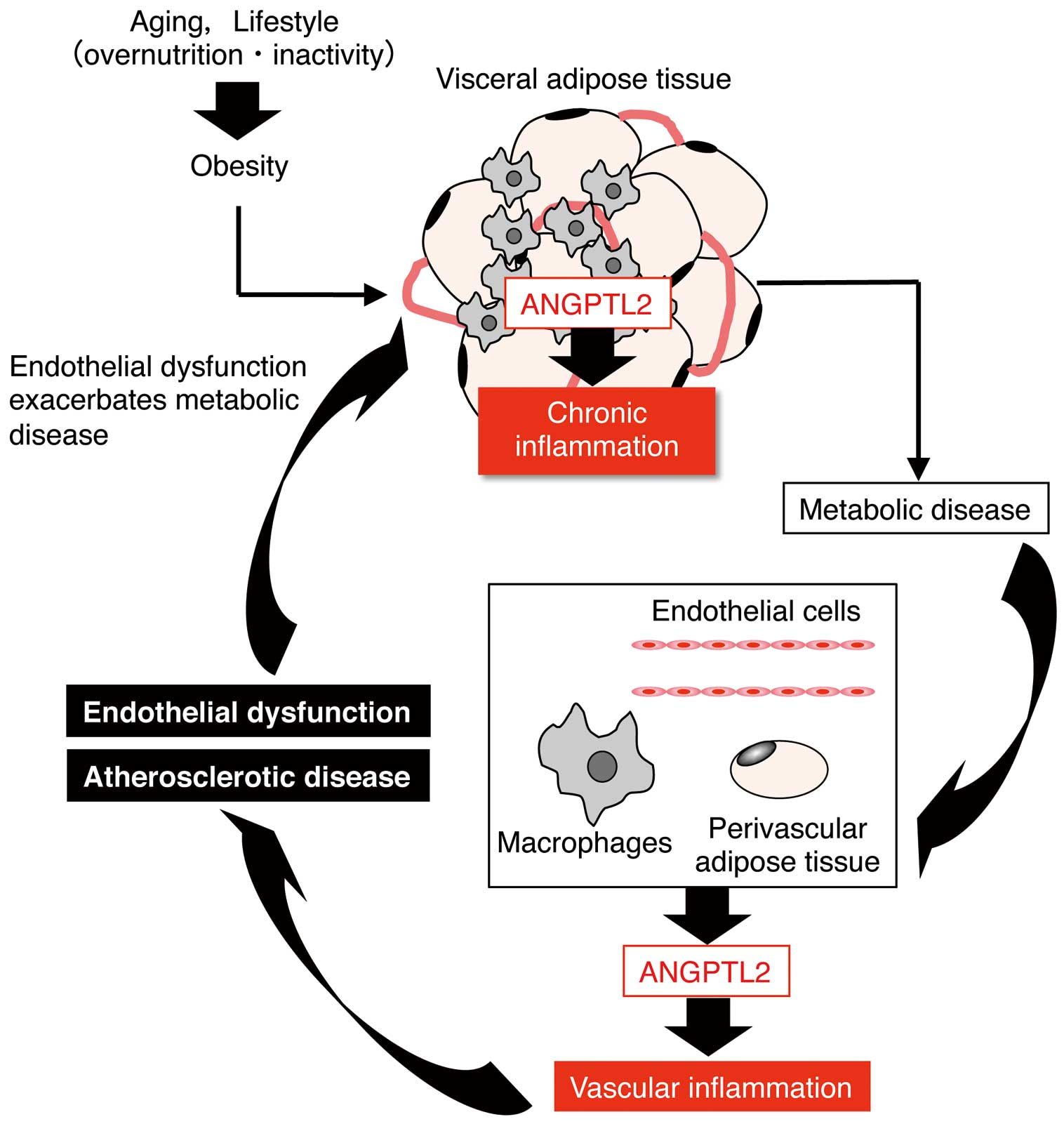
ANGPTL2 activity in cardiovascular disease (CVD). ANGPTL2 links obesity-related metabolic disorders to CVD. Perivascular adipose tissue-secreted ANGPTL2 accelerates vascular inflammation, pathologic vascular tissue remodeling and CVD development. High ANGPTL2 expression in endothelial cells and macrophages is induced by obesity and associated metabolic dysregulation, and predisposes individuals to atherosclerotic disease. Increased endothelial cell and macrophage-derived ANGPTL2 secretion induced by obesity and/or metabolic disturbance promotes vascular inflammation, leading to endothelial dysfunction and atherosclerosis.
In 1999, Dr. Koh’s group cloned the cDNA encoding ANGPTL2 protein from human and mouse adult heart tissues.28 Since then, several studies have confirmed that ANGPTL2 is expressed in the heart, but its cardiac function has not been clarified.17,28 More recently, we unexpectedly found that increased ANGPTL2 activity induced in the pathologically stressed heart accelerated cardiac dysfunction by inactivating both AKT and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a signaling and by decreasing myocardial energy metabolism in an autocrine/paracrine manner (Figure 5).21 Conversely, ANGPTL2 suppression restored cardiac function and myocardial energy metabolism, blocking pathologic remodeling.21 It is noteworthy that this suppressive activity of ANGPTL2 on cardiac function is not attributable to its pro-inflammatory effect. ANGPTL2 expression increases in pathologically remodeled hearts of mice and humans, but in mice, decreased cardiac ANGPTL2 expression occurs in physiologic cardiac remodeling induced by endurance training,21 suggesting that respective activation or inactivation of heart-derived autocrine/paracrine ANGPTL2 signaling determines whether an individual will be predisposed to or protected from HF development.
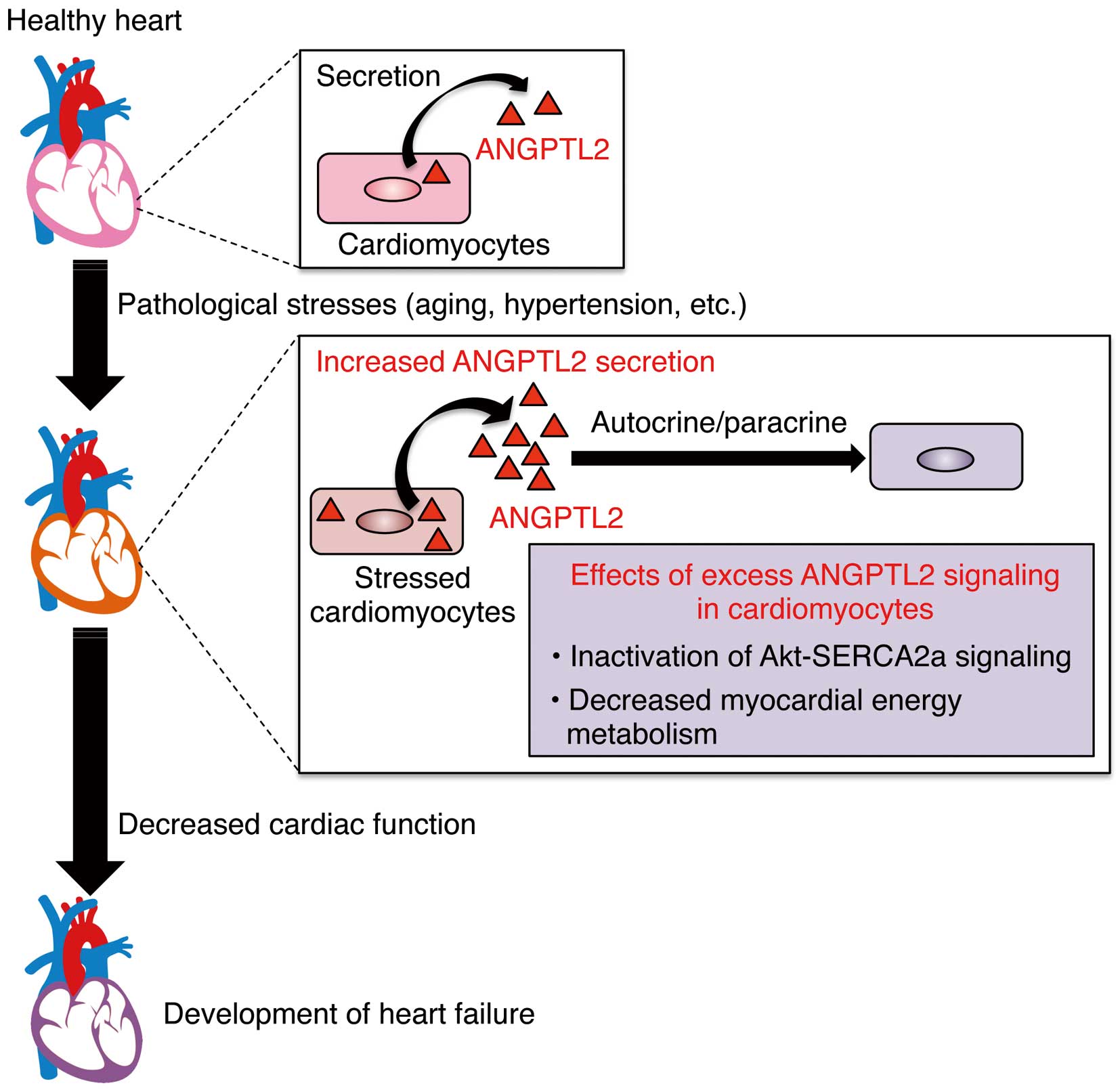
Increased ANGPTL2 activity in pathologically stressed heart accelerates cardiac dysfunction. Pathological stress, such as aging and hypertension, underlie heart failure, in part by increasing ANGPTL2 expression and secretion in cardiomyocytes. Excess ANGPTL2 causes cardiac dysfunction by inactivating Akt-sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a signaling and impairing energy metabolism in cardiomyocytes, promoting heart failure.
Many studies report a positive correlation between circulating ANGPTL2 concentrations and disease.21,29–35,56 We found that serum ANGPTL2 levels are associated with obesity, as estimated by body mass index and abdominal circumference, in the general Japanese population.17 Moreover, in overweight Japanese subjects, decreased serum ANGPTL2 levels reflect positive effects of lifestyle intervention in terms of weight loss and improved metabolic parameters, such as triglyceride (TG) and/or insulin activity and the homeostasis model assessment-insulin resistance (HOMA-IR) index.57 Others report that circulating ANGPTL2 levels decrease after bariatric surgery in severely obese patients.58 These findings strongly suggest that adipose tissue is a major source of ANGPTL2 secretion.
In Japanese patients with type 2 diabetes, circulating ANGPTL2 concentrations positively correlate with insulin resistance, as estimated by the HOMA-IR index, visceral fat volume, as assessed by magnetic resonance imaging, and systemic inflammatory status, as estimated by high-sensitivity C-reactive protein (hs-CRP) levels, and inversely correlate with insulin sensitivity, as estimated by the glucose clamp test.17 Moreover, a 7-year follow-up in an epidemiological study of individuals in the general Japanese population with no history of diabetes showed a positive association between elevated serum ANGPTL2 levels and future de novo development of type 2 diabetes, independent of other risk factors, including hs-CRP levels.59
A 10-year follow-up in an epidemiological study of the general Japanese population with no history of CVD revealed that elevated serum ANGPTL2 levels correlated with future de novo development of CVD, independent of risk factors such as high hs-CRP levels.56 Moreover, in a prospective, monocentric cohort of European patients with type 2 diabetes but without end-stage renal disease (followed for a median of 6.0 years with death as a primary endpoint and major adverse CV events (known as MACE) such as death, myocardial infarction or stroke as a secondary endpoints), serum ANGPTL2 concentrations were independently associated with death and MACE.60 Taken together with observations suggesting that ANGPTL2 promotes CVD development and progression in mice,25 circulating ANGPTL2 levels could be viewed as a candidate biomarker for risk stratification relevant to CVD development and progression in both type 2 diabetic patients and the general population. As noted, some CAD events persist even in patients whose LDL-cholesterol levels have been lowered by antidyslipidemia treatment.1,2,4,5 We propose that an ANGPTL2-dependent chronic inflammation axis may underlie such CAD events. Therefore, future studies should ask whether evaluation of circulating ANGPTL2 levels either prior to or following administration of LDL-cholesterol-lowering drugs could predict outcomes in these subjects.
Recently, a group in Taiwan reported that circulating ANGPTL2 levels in patients with HF were higher than in controls, and that cardiac function negatively correlated with those levels.30 We also observed a positive correlation of circulating ANGPTL2 levels with left atrial diameter and pulmonary capillary wedge pressure and inverse correlation with ejection fraction in patients with dilated cardiomyopathy (DCM).61 Thus, circulating ANGPTL2 levels increase in HF patients without CAD. Furthermore, in mice, circulating ANGPTL2 concentrations increase as HF induced by transverse aorta constriction develops, related primarily to increases in heart-secreted ANGPTL2.21 Circulating ANGPTL2 concentrations also inversely correlate with the percent of fractional shortening in mice. In mouse models of HF, inhibition of ANGPTL2 secretion by injection of AAV6-shANGPTL2 antagonizes cardiac dysfunction,21 suggesting that cardiomyocytes are a major source of secreted ANGPTL2 in HF conditions.
ANGPTL2 Expression Is Associated With Cellular SenescenceCellular senescence is defined as cell cycle arrest as a means to counteract DNA damage induced by aging and various stressors.14,15,62 Accumulation of senescent cells in various tissues accelerates aging and disease development. A recent report using a transgenic approach in mice demonstrated that clearance of senescent cells delays several age-associated disorders,63 suggesting that senescent cells promote these conditions. Interestingly, it has been reported that endothelial cells derived from smokers and exhibiting oxidative stress-induced premature senescence show significantly increased ANGPTL2 expression.50 Moreover, senescent fibroblasts from patients with Werner syndrome (adult progeria) show abundant ANGPTL2 expression and increased expression of other SASP factors.22 We have also reported that ANGPTL2 expression significantly increases in the hearts of aged compared with young mice.21 In a chemically induced skin carcinogenesis mouse model, dimethylbenzanthracene (DMBA) and phorbol 12-myristate 13-acetate (PMA) treatment reportedly induced DNA damage and cell senescence in the skin and subsequent development of cutaneous squamous cell carcinoma.18,64 We previously reported that ANGPTL2 expression markedly increases in the skin of DMBA/PMA-treated compared with control mice,18 indicating that ANGPTL2 expression increases in senescent cells in parallel with DNA damage. As noted earlier, circulating ANGPTL2 levels increase with age in Japanese populations25 and are high in patients with chronic, age-associated diseases such as heart disease, kidney disease, diabetes, and cancer.21,29–35 Thus, ANGPTL2 may function as a SASP in several senescent cell types. If so, markedly increased circulating ANGPTL2 levels seen in patients with chronic aging-related diseases may reflect the accumulation of senescent cells in their organs. Further investigation is required to determine whether increased circulating ANGPTL2 concentrations could serve as a biomarker for adverse health outcomes.
To date, much evidence has emerged to define the physiologic and pathologic functions of ANGPTL2. Relevant to cardiac disease, excess and prolonged ANGPTL2 autocrine/paracrine signaling in vascular tissue leads to chronic inflammation and pathologic tissue remodeling, ending in CVD development. Moreover, increased ANGPTL2 activity induced in the pathologically stressed heart alters myocardial energy metabolism, impairing cardiac function. Conversely, ANGPTL2 inactivation delays age-associated heart disease by improving tissue homeostasis and energy metabolism. Thus, although ample evidence suggests that ANGPTL2 promotes heart disease onset, several questions remain. The first is whether ANGPTL2 inactivation could serve as pharmacologic therapy against CVD and/or HF. To develop therapies targeting ANGPTL2 signaling requires defining the receptor(s) critical for ANGPTL2 function in the cardiovascular system, and among the candidates are integrins and PirB/LILRB2. Moreover, it is necessary to determine the endocrine as well as paracrine effects of circulating ANGPTL2. Because ANGPTL proteins tend to aggregate and become insoluble, preparation of high concentrations of recombinant ANGPTL2 protein required for in vivo analysis has been difficult, although studies are ongoing to resolve these issues.
Interestingly, elevated circulating ANGPTL2 concentrations are not a biomarker of a single disease but rather reflect broader systemic activity that promotes inflammation or premature aging, both of which predispose individuals to chronic disease. Thus, high levels of circulating ANGPTL2 may predict these disorders.
In conclusion, ANGPTL2 represents a new factor that accelerates heart disease development with aging. Suppression of ANGPTL2 signaling might represent a novel therapeutic strategy against heart disease and predisposing pathologic conditions. Additional preclinical studies are now required prior to clinical application.
This work was supported by the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST grant 13417915), and by the CREST program of the Japan Agency for Medical Research and Development (AMED grant 17 gm0610007 h0005) and by JSPS KAKENHI grants (nos. 17H05652 and 16K15445).
There are no conflicts of interest to be disclosed.