2017 Volume 81 Issue 11 Pages 1580-1588
2017 Volume 81 Issue 11 Pages 1580-1588
Background: An atrial fibrillation (AF) risk score for a non-Western general population has not been established.
Methods and Results: A total of 6,898 participants (30–79 years old) initially free of AF have been prospectively followed for incident AF since 1989. AF was diagnosed when AF or atrial flutter was present on ECG at a biannual health examination; was indicated as a current illness; or was in the medical records during follow-up. Cox proportional hazard ratios were analyzed after adjusting for cardiovascular risk factors at baseline. During the 95,180 person-years of follow-up, 311 incident AF events occurred. We developed a scoring system for each risk factor as follows: 0/−5, 3/0, 7/5, and 9/9 points for men/women in their 30 s–40 s, 50 s, 60 s, and 70 s, respectively; 2 points for systolic hypertension, overweight, excessive drinking, or coronary artery disease; 1 point for current smoking; −1 point for moderate non-high-density lipoprotein-cholesterol; 4 points for arrhythmia; and 8, 6, and 2 points for subjects with cardiac murmur in their 30 s–40 s, 50 s, and 60 s, respectively (C-statistic 0.749; 95% confidence interval, 0.724−0.774). Individuals with score ≤2, 10–11, or ≥16 points had, respectively, ≤1%, 9%, and 27% observed probability of developing AF in 10 years.
Conclusions: We developed a 10-year risk score for incident AF using traditional risk factors that are easily obtained in routine outpatient clinics/health examinations without ECG.
Over the past 2 decades, the global estimated age-adjusted prevalence rates (/1,000 population) of atrial fibrillation (AF) increased from 5.7 to 6.0 in men and from 3.6 to 3.7 in women.1 AF is one of the most frequent types of arrhythmia and is a risk factor for cardiovascular disease (CVD)2 and all-cause death.3 The prevention of AF is an important first step for the extension of healthy life expectancy, but the evidence of AF risk factors is limited and almost exclusively derived from Western populations. The reported data include the AF risk score in the Framingham Heart Study (FHS),4 the Atherosclerosis Risk in Communities (ARIC) Study,5 the Women’s Health Study (WHS),6 and a pooled data analysis.7 Compared with Westerners, Asians have a high prevalence of hypertension and stroke, and a low prevalence of coronary artery disease (CAD), obesity, and AF.1,8 Therefore, an AF risk score is needed to account for these characteristics in Asians, but currently there is no AF risk score for any non-Western general population.
Editorial p 1574
For the prevention of stroke, we need not only hypertension prevention and salt reduction but also prevention of AF. AF is a high-risk factor for cardioembolism.9 Since 2008 ECGs have not been required in medical examinations in Japan, and thus there is concern about the potential for an increase in the incidence of AF. However, AF can be identified and strokes could be prevented if ECG was performed for individuals with a high AF risk score, which could be determined as part of their medical examination. We conducted the present study to develop a risk score for incident AF in an urban Japanese population, with the goal of identifying individuals who are at high risk of AF during a general health examination and in routine outpatient clinics.
The design and selection criteria of the Suita Study original cohort have already been described.10,11 As a baseline, 12,200 and 3,000 participants (age 30–79 years) were randomly selected in 1989 and 1996, respectively, from the municipality population registry of Suita City and stratified into groups by sex and age in 10-year increments. Of these, participants attending the baseline examination of the original (n=6,485) and the secondary cohort (n=1,329) were eligible for the present investigation from 1989–1996 and 1996–1998, respectively. In the present study, the baseline examination of a volunteer group (n=546, in 1992–2006) was also included. Informed consent was given by all participants.
We compared the baseline characteristics of the 3 cohort groups (original, secondary, and volunteer). Although the prevalences of hyperlipidemia and overweight were higher in the volunteer group, lifestyle habits (i.e., smoking and drinking) and the mean systolic and diastolic blood pressures (SBP and DBP) were similar among the 3 groups.11 These evaluations are referred to as the baseline examination for the present study, which was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center, Suita, Japan.
We excluded participants for the following reasons: prior or current AF or atrial flutter (n=42) or ≥80 years old (n=34) at baseline, missing covariate (n=2), and failure to complete the baseline examination (n=2) or the follow-up health surveys (n=1,382). This resulted in a final sample of 6,898 participants.
Blood Pressure and Physical MeasurementsWell-trained physicians measured each participant’s BP 3 times using a mercury column sphygmomanometer, an appropriately sized cuff, and a standard protocol.10 Before the initial BP reading was obtained, participants were seated at rest for at least 5 min. BP values were taken as the average of the 2nd and 3rd measurements, which were recorded more than 1 min apart. At the time of the baseline examination, each participant was classified into 1 of 3 SBP and DBP categories: normal (<120 mmHg and <80 mmHg), systolic/diastolic prehypertension (120‒139/80‒89 mmHg) and systolic/diastolic hypertension (≥140/90 mmHg). Categories of body mass index (BMI), calculated as weight (kg) divided by height (m) squared, were defined by the following criteria: underweight (<18.5 kg/m2), normal weight (18.5 to <25 kg/m2), and overweight (≥25 kg/m2).12 The prevalence of obesity among all the participants was <1.6%, and the obese participants were thus included in the overweight category. Cardiac murmur was defined as past/present history of any valvular heart disease (n=8) or cardiac murmur (n=159) identified if any systolic or diastolic murmur was auscultated by trained clinicians during the baseline survey. Arrhythmia was defined as current illness with any type of arrhythmia other than AF or the presence of arrhythmia during the physical examination, which included BP measurement, thoracoabdominal auscultation, and a pulse measurement by hand at baseline.
Biochemical Measurements and QuestionnaireIn the baseline examination, we performed routine blood tests that included serum total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C) and glucose levels. An individual’s non-HDL-C level was calculated by subtracting the HDL-C from the TC. Glucose categories were defined as follows: diabetes mellitus (DM: fasting plasma glucose levels [FPG] ≥126 mg/dL, non-FPG ≥200 mg/dL, or DM medication); impaired FPG (FPG=100–125 mg/dL and non-FPG=140–199 mg/dL); and normal glucose tolerance (FPG <100 mg/dL and non-FPG <140 mg/dL). The glomerular filtration rate (GFR, mL/min/1.73 m2) of each participant was calculated using the Modification of Diet in Renal Disease equation modified by the Japanese coefficient (0.881), as follows:13
GFR=0.881×186×(age)−0.203×(serum creatinine)−1.154(×0.742 for women).
Chronic kidney disease (CKD) was defined as an estimated GFR <60 mL/min/1.73 m2.
Physicians and nurses administered questionnaires covering personal habits and present illness. Smoking and drinking habits were classified as current, quit, and never smoking/drinking. The questionnaire asked the respondent about his or her past/present histories of stroke (cerebral infarction, intracerebral hemorrhage, and subarachnoid hemorrhage) and CAD (including myocardial infarction, angina pectoris, and coronary intervention).
Definition of AF and Follow-upStandard 12-lead ECGs were obtained from all participants while in the supine position. Each record was coded independently using the Minnesota Code by 2 well-trained physicians. Participants were diagnosed with AF when AF (Minnesota Codes 8-3-1 and 8-3-3) or atrial flutter (Minnesota Codes 8-3-2 and 8-3-4) was present (n=168) on an ECG from the routine Suita health check-up examination (every 2 years) or when AF was indicated as being present at the health check-up examination (n=54), on hospital medical records (n=80), and/or death records (n=9) during follow-up. The endpoint of the follow-up period for each participant was whichever of the following options occurred first: (1) the date of the first AF event, (2) the date of the last health examination and medical records, or (3) December 31, 2015 (censored).
Statistical AnalysisAnalysis of variance and chi-square tests were used to compare the mean value and frequencies according to incident AF during follow-up. We examined the associations between cardiovascular risk factors and the risk of incident AF using multivariable-adjusted Cox proportional hazard regressions after adjusting for the participants’ sex and age at baseline. There was no interaction between follow-up year and BP for the prediction of AF in the primary Cox model, suggesting that the proportional hazards assumption was appropriate. All possible interactions between the risk factors identified in our multivariate model and each of age and sex were then tested. We tested for interaction terms generated by strata of age (10-year intervals)×sex or age (10-year intervals)×cardiac murmur in the Cox model. Significant risk factors from the age- and sex-adjusted models were then pooled into 1 multivariate Cox model to calculate points associated with each level of the current risk factors and to determine the 10-year probability of incident AF by total points. We calculated a score for all participants in the present dataset by calculating a point total based on the developed risk score. We evaluated the model discrimination by determining the area under the receiver-operating characteristic curve. All analyses were performed with SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA).
The baseline characteristics of the study participants according to incident AF during the follow-up are presented in Table 1. On average, subjects with AF were older and had higher SBP and DBP, BMI, and higher prevalences of antihypertensive drug use, current smoking, excessive drinking, CAD, cardiac murmur, and arrhythmia.
| AF (−) | AF (+) | P value | |
|---|---|---|---|
| n | 6,553 | 311 | |
| Age, years | 55.4±12.7 | 62.5±9.8 | |
| Sex (male, %) | 46.1 | 65.6 | |
| SBP, mmHg | 127.0±21.6 | 136.7±22.5 | <0.001 |
| DBP, mmHg | 77.8±12.1 | 81.0±13.7 | 0.054 |
| BMI, kg/m2 | 22.6±3.1 | 23.3±3.2 | 0.003 |
| TC, mg/dL | 208.5±36.2 | 207.5±34.2 | 0.271 |
| HDL-C, mg/dL | 54.4±14.2 | 52.1±14.4 | 0.647 |
| Non-HDL-C, mg/dL | 154.0±37.1 | 155.4±35.2 | 0.394 |
| GFR, mL/min/1.73 m2 | 91.0±30.4 | 84.9±23.6 | 0.342 |
| CKD, % | 8.5 | 10.6 | 0.975 |
| Antihypertensive drug use, % | 11.7 | 22.8 | <0.001 |
| Blood glucose category | 0.001 | ||
| Diabetes mellitus, % | 4.8 | 7.4 | |
| Impaired fasting glucose, % | 26.1 | 33.4 | |
| Smoking status | <0.001 | ||
| Current smoking, % | 28.6 | 33.6 | |
| Quit smoking, % | 16.7 | 27.3 | |
| Drinking status | 0.004 | ||
| Current drinking, % | 51.4 | 59.3 | |
| Quit drinking, % | 2.6 | 3.6 | |
| Excessive drinking, % | 9.5 | 16.7 | 0.005 |
| Coronary artery disease, % | 1.8 | 5.5 | 0.001 |
| Cardiac murmur, % | 2.2 | 6.1 | <0.001 |
| Arrhythmia (other than AF), % | 3.5 | 11.6 | <0.001 |
| Chronic heart failure, % | 0.1 | 0.3 | 0.072 |
| Stroke, % | 1.6 | 1.9 | 0.285 |
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol.
During the 95,180 person-years of follow-up, 311 incident AF events occurred. Figure 1 shows the age- and sex-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for incident AF according to the traditional risk factors. Advancing age, sex, overweight, SBP, DBP, pulse pressure (PP), and antihypertensive drug use were associated with AF. Among the BP variables, after multivariate-adjustment including BP parameters, only SBP was independently associated with AF.11 Among the lipid variables, only the normal range of non-HDL-C (130–189 mg/dL) was inversely associated with AF (HR=0.74, 95% CI, 0.57–0.97), when compared with the lower range of non-HDL-C (<130 mg/dL). Current smoking, excessive drinking, CAD, cardiac murmur, and arrhythmia were associated with increased risk of incident AF.
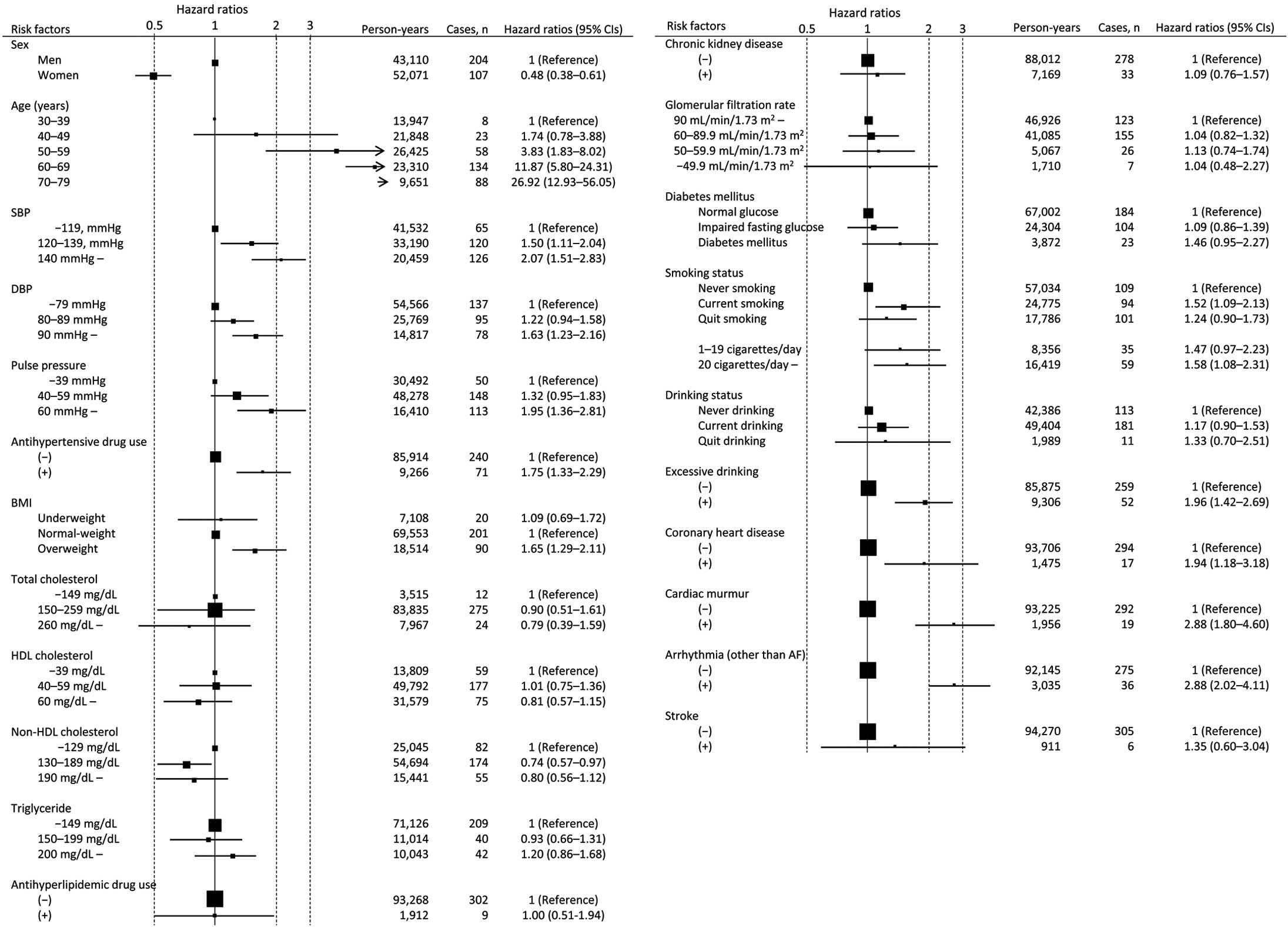
Basic risk factors and age- and sex-adjusted hazard ratios for incident atrial fibrillation. Area of each square is proportional to number of subjects. Horizontal lines indicate 95% confidence interval (CI). BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure.
The final multivariable-adjusted Cox proportional hazard regression coefficients for the risk of incident AF included age, sex, SBP and BMI categories, antihypertensive drug use, current smoking, excessive drinking, non-HDL-C, CAD, arrhythmia (other than AF), and cardiac murmur (Table 2). The interactions of sex and cardiac murmur with age were accommodated by adding interaction terms (P for interaction <0.001 and 0.002, respectively). We developed a scoring system for each risk factor (Table 3). The 10-year predicted probability of incident AF by total risk score is presented in Table 4 (C-statistic 0.749; 95% CI, 0.724–0.774). Participants with a score ≤2 points had a 10-year predicted probability of incident AF of ≤1%. Participants with a score of 12 or ≥16 points had a respective 12% and 27% predicted probability of incident AF during 10 years. Figure 2 shows the 10-year risk of incident AF based on traditional risk factors, including age categories, sex, the “Cardiovascular Risks” axis (systolic hypertension, overweight, arrhythmia other than AF, and CAD), and the “Lifestyle and Lipids” axis (current smoking, excessive drinking, and moderate non-HDL-C) according to the presence of cardiac murmur.
| β coeff. | SE | P value | |
|---|---|---|---|
| Age (years) | |||
| Men | |||
| 30–49 | Ref. | – | – |
| 50–59 | 0.871 | 0.269 | 0.003 |
| 60–69 | 1.860 | 0.251 | <0.001 |
| 70–79 | 2.428 | 0.280 | <0.001 |
| Women | |||
| 30–49 | −1.329 | 0.440 | 0.003 |
| 50–59 | −0.198 | 0.362 | 0.585 |
| 60–69 | 1.410 | 0.285 | <0.001 |
| 70–79 | 2.434 | 0.304 | <0.001 |
| SBP | |||
| <120 mmHg | Ref. | – | – |
| 120–139 mmHg | 0.297 | 0.163 | 0.069 |
| ≥140 mmHg | 0.544 | 0.178 | 0.002 |
| Antihypertensive drug use | 0.249 | 0.153 | 0.105 |
| BMI | |||
| Underweight | 0.007 | 0.247 | 0.976 |
| Normal weight | Ref. | – | – |
| Overweight | 0.427 | 0.131 | 0.001 |
| Excessive drinking | 0.409 | 0.173 | 0.018 |
| Current smoking | 0.336 | 0.141 | 0.017 |
| Non-HDL-C | −0.309 | 0.142 | 0.029 |
| Coronary artery disease | 0.606 | 0.256 | 0.018 |
| Cardiac murmur (by age interval) | |||
| 30–49 | 2.209 | 0.547 | <0.001 |
| 50–59 | 1.720 | 0.480 | <0.001 |
| 60–69 | 0.607 | 0.422 | 0.150 |
| 70–79 | Ref. | – | – |
| Arrhythmia (other than AF) | 1.112 | 0.188 | <0.001 |
SE, standard error. Other abbreviations as in Table 1.
| Factor | Score | |
|---|---|---|
| Age, years | ||
| 30–49 | 0 (men) | −5 (women) |
| 50–59 | 3 (men) | 0 (women) |
| 60–69 | 7 (men) | 5 (women) |
| 70–79 | 9 (men) | 9 (women) |
| Systolic hypertension | 2 | |
| Overweight (BMI ≥25 kg/m2) | 2 | |
| Excessive drinking | 2 | |
| Current smoking | 1 | |
| Non-HDL-C (130–189 mg/dL) | −1 | |
| Arrhythmia (other than AF) | 4 | |
| Coronary artery disease | 2 | |
| Cardiac murmur (by age interval) | ||
| 30–49 | 8 | |
| 50–59 | 6 | |
| 60–69 | 2 | |
| 70–79 | 0 | |
Abbreviations as in Table 1.
| Risk score | Predicted risk, % |
|---|---|
| <0 | <0.5 |
| 0 | 0.8 |
| 1–2 | 1 |
| 3 | 2 |
| 4 | 3 |
| 5–7 | 4 |
| 8–9 | 7 |
| 10–11 | 9 |
| 12 | 12 |
| 13 | 16 |
| 14–15 | 20 |
| ≥16 | 27 |
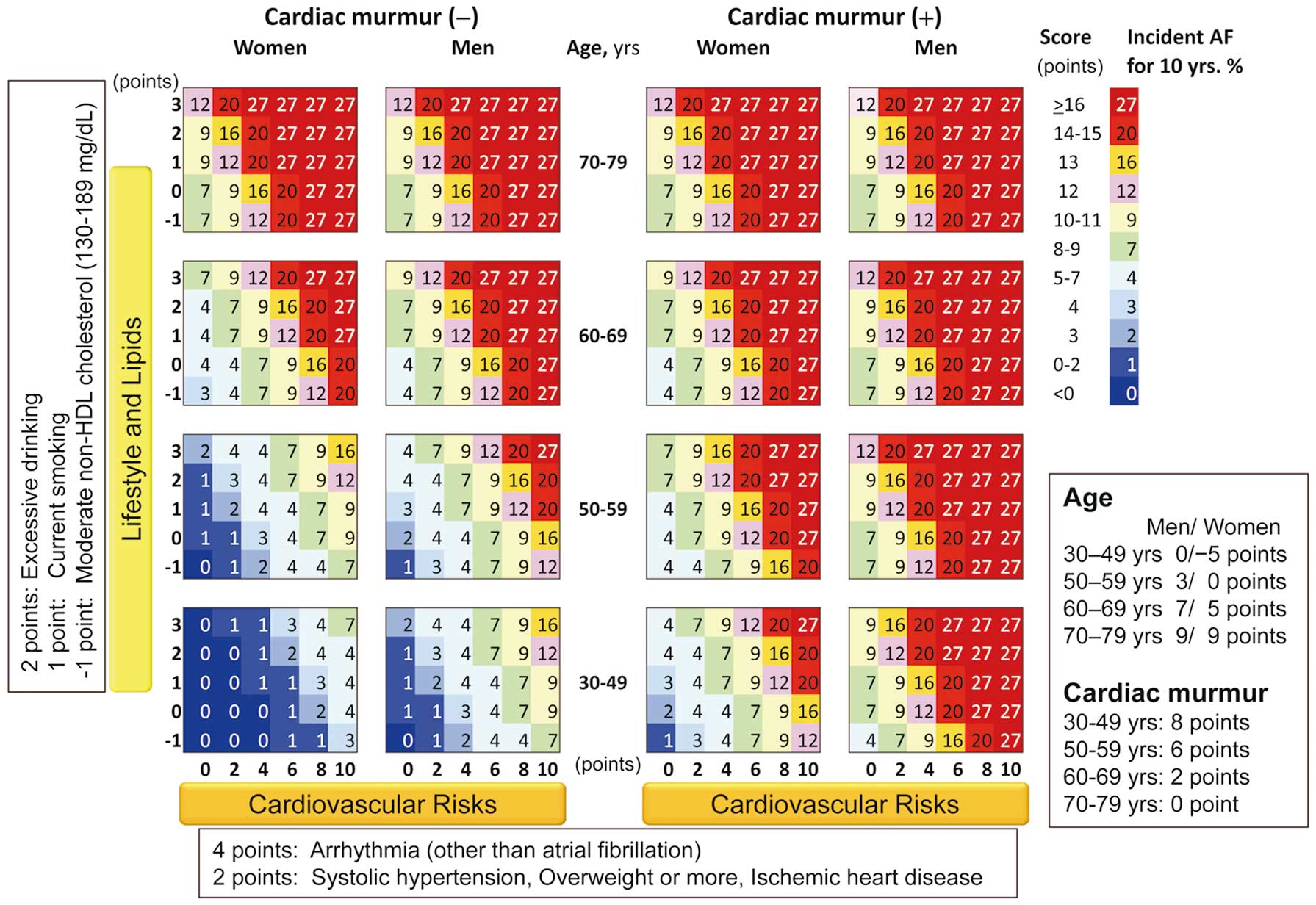
The 10-year risk of incident atrial fibrillation (AF) based on traditional risk factors, including age, sex, cardiac murmur, and the 2 axes of “Lifestyle and Lipids” and “Cardiovascular Risks”. The Lifestyle and Lipids axis consists of excessive drinking (2 points), current smoking (1 point), and moderate non-high-density lipoprotein-cholesterol (−1 point). The Cardiovascular Risks axis consists of arrhythmia other than AF (4 points) and systolic hypertension, overweight, and coronary artery disease (2 points each). The value shown in each colored square is the percentage of incident AF within 10 years.
We developed a 10-year risk score for incident AF in a general Japanese population by using traditional risk factors commonly measured in routine health examinations in Japan. This is the first risk score of incident AF in a non-Western population. This new risk score for predicting AF in the next 10 years may be useful in health examinations and routine outpatient clinical encounters in Asian populations to identify individuals at high risk of developing AF, who can then be assessed using ECG.
AF risk scores based on US populations have been reported by the FHS4 and ARIC study.5 The risk-score components common to these 2 cohorts were age, sex, BMI, SBP, and cardiac murmur, which were also found to be significant risk-score components in the present study, suggesting that these components may be common to all ethnicities. Treatment for hypertension and heart failure were also components of the risk score in the 2 Western cohorts, but in the present study they were not significantly associated with AF. A pooled data analysis from 3 large cohorts provided a simple risk model for primary care settings that adequately predicted AF in diverse populations from the USA and Europe.7 The WHS was developed as a simple model for the prediction of postoperative AF and consists of age, SBP, weight, height, alcohol use, and smoking.6 in the present model we used BMI rather than height and weight, although both height and weight were associated with increased risk of incident AF in our preliminary data (data not shown). When our present study was applied to the FHS, ARIC, WHS, and CHARGE-AF (simple model) risk scores, the C-statistics of these risk scores tended to be lower than that of our risk score (Table S1), because the prevalences of AF, obesity, and CAD in our study were lower than those in the previous studies. Stroke was not significantly associated with AF in this study and nor has it been observed as a risk in the risk score studies published so far.4–7
The prevalence of AF in women (2.1/1,000 person-years) was lower than in men (4.7/1,000 person-years) in the present study, but women showed a larger gradient of incident AF with increasing age compared with men, which was compatible with the FHS results.4 After menopause, the prevalence of hypertension and overweight, which are risk factors for AF, are known to increase steeply in women.
High-normal SBP has been reported as associated with incident AF,14,15 and after further adjustment, systolic hypertension was still associated with incident AF,14 which is compatible with the present findings.11 In another report, SBP and PP were positively associated with AF,16 but when DBP was added to the model, only PP was associated with AF. In our present investigation, after further adjustment for SBP, the association between PP and AF was attenuated. Prospective studies in Japan have shown that, among the various types of BP, SBP is the most important predictor of CVD, and PP less important, even when older people are included.17
In the present study, the association between hypertension and AF was weaker in women than in men (Tables S2,S3) because of the lower incidence of AF in women, the shorter period of hypertension exposure in women due to postmenopausal effects, and the high prevalence of overestimated BP in women because of the high prevalence of “white coat” hypertension in women.
Overweight18 and obesity18–20 were associated with incident AF in previous cohort studies, and our findings are compatible with those associations. We could not calculate the association between obesity and the risk of AF, because of the very small number of obese participants (1.6%). The pathogenesis of overweight/obesity is related to increasing BP21 and the sympathetic nervous system,22 and it involves metabolic dysregulation.23
Smoking is a modifiable risk factor for AF.24,25 A recent meta-analysis revealed that current smokers (relative risk=1.39; 95% CI, 1.11–1.75) and former smokers (1.16; 1.00–1.36) had an increased risk of AF compared with never-smokers.26 The nicotine content of cigarettes increases the heart rate, BP,27 and plasma catecholamine levels28 and acts a potent inhibitor of cardiac A-type K+ channels. This action contributes to the ability of nicotine to affect cardiac electrophysiology and induce arrhythmia,29 which chronically contributes to the development of atrial fibrosis and atrial arrhythmias.30 Our present analyses demonstrated that current smoking was a risk factor for AF. The AF risk from smoking was stronger in women than in men, and the CVD risk presented by smoking was also greater in women than in men. Here we observed that the risk of smoking in men was attenuated, partly because during the follow-up approximately half of the smokers gave up smoking (data not shown) because of a smoking cessation promotion in Japan, but we kept smoking as a component of the AF risk score because it was significant in the overall analysis and there is still a very high prevalence of smoking in Asia.
In Western populations, excessive drinking was associated with a 45–46% increased risk of AF in men, but no association between moderate drinking and the risk of AF in women.31,32 A meta-analysis showed that the pooled estimate for a 10 g ethanol/day increment for the risk of AF was 1.08 (95% CI, 1.05–1.10). Our present findings showed that excessive drinking posed an increased risk of AF, but moderate drinking and having quit drinking were not associated with incident AF. Long-term excess alcohol consumption could act as a direct cardiotoxin affecting the structure and size of the human atria, as suggested by the findings of a rat experiment,33 and could also have direct proarrhythmic effects.34 A case-crossover study indicated that alcohol consumption could cause brief or asymptomatic episodes of AF to become persistent.35 Excessive drinking has been linked to an increased risk of hypertension,36 which is a risk factor for AF.11,14,15
One research group reported that high triglycerides and low HDL-C levels were associated with an increased risk of AF.37 Other studies showed an inverse association between TC38–40 and low-density lipoprotein-cholesterol (LDL-C)39,40 levels and incident AF. A longitudinal study of the results of health examinations in Japan revealed an inverse association between TC, LDL-C, and non-HDL-C and incident AF, and a positive association between HDL-C and AF.41 In the present study, moderate non-HDL-C showed the lowest risk of incident AF. Low HDL-C may contribute to the risk of incident AF via an increase in the prevalence of CAD and heart failure as risk factors of AF.4 Mora et al conjectures that the association between LDL and AF could be related to the stabilizing effect of cholesterol on cardiomyocyte membranes.39 It is also generally accepted that there is an association between lower cholesterol and hyperthyroidism, which is a risk factor for incident AF.42 Lower cholesterol levels may thus be involved with high incident AF. Different ethnicities, lifestyles, age ranges, and study designs may partly explain the inconsistencies among the findings of the past and present studies.
In the previous prospective studies, inconsistent results were observed regarding the association between DM and AF, although a meta-analysis revealed that DM is associated with an increased risk of AF.43 DM has been reported to be associated with an increased risk of AF mediated by increasing body weight and BP in individuals with DM.44 In other studies, impaired glucose tolerance and insulin resistance were associated with left ventricular mass,45 but no significant association was observed.46 In our present investigation, there was no association between DM and AF, probably because obesity is much less prevalent in Japanese than among Westerners.
Arrhythmia other than AF, which in our cohort consisted primarily of premature beats, was a strong risk factor for AF in the present study. Most cases of arrhythmia involve frequent atrial premature complexes (APC), which is a risk factor for future AF47 as well as a surrogate marker of paroxysmal AF. A hospital-based cohort study showed that patients with >100 APC/day had higher incident AF.48 Findings of arrhythmia at a medical examination are thus extremely important for the prediction of AF.
Cardiac murmur is a component of some AF risk scores.4,5 In the present study, cardiac murmur was strongly associated with incident AF, and the association interacted with young age and was diminished with old age, which was compatible with the FHS results.4 Cardiac murmur is caused by heart valvular disease, congenital heart disease, and functional murmur. Functional murmurs are identified primarily in young individuals. In our study, there were only 5 cases of congenital heart disease at baseline, which was not associated with AF because of the small sample size. We do not know whether AF caused by a cardiac murmur interacted with the age categories, but the long-term cumulative effect of atrium load caused by a cardiac murmur (i.e., heart valve disease) may cause incident AF.
Study Strengths and LimitationsThis study has several strengths. First, it is the first study of an AF risk score in a population other than Westerners. Most of the previously reported common components of AF risk scores were examined in our analyses. Second, the same followed-up cohort members were randomly selected from the municipality population registry of Suita City and stratified into groups by sex and 10-year age increments in a premeditated manner. This cohort is representative of the Japanese urban population, which comprises two-thirds of the population of Japan. Third, we determined the incidence of AF from not only the standard ECG from every 2-year routine check-up examination and/or present illness, but also from hospital medical records and/or cause of death records during follow-up, which is an improvement over the previous cohort studies that relied only on an ECG definition. More than 50% of the present participants with CVD were admitted to hospital, and almost all of them were examined by Holter ECG during their admission. Most cases of paroxysmal or intermittent AF are diagnosed during emergency transportation or in conjunction with incident CVD.
Our study also has several limitations. First, we did not perform Holter ECG for all participants, and even if it had been performed, it may have missed participants with paroxysmal AF. We identified incident AF by the use of not only ECG in medical examinations, but also from medical records and by the cause of death (26% of the whole) in order to identify AF as precisely as possible. Second, our risk score was developed in a Japanese urban population, and thus, although this score might be useful for Asian populations, it might not be useful for other ethnicities and racial backgrounds. Nonetheless, this risk score is the first to be developed in non-Westerners. Moreover, most of the components of the AF risk score were also components of the AF risk scores for Westerners. Further studies will be needed to elucidate an AF risk score for non-Westerners. Third, we did not conduct an external validation of our risk score using other cohorts, because this is the first such risk score for AF. We are currently preparing an external validation study of other Japanese cohorts. Fourth, we could not use the grade of cardiac murmur in this study. It is better for subjects who exhibit cardiac murmur at screening to consult a doctor, because all types of cardiac murmur were found to confer high risk for incident AF in the present study. Fifth, heart failure could not be used in this risk score, partly because we only determined the present illness of heart failure among the participants, rather than using a clinical diagnosis of heart failure. Sixth, our analysis of AF subtypes is not included, because of the small sample size. A study with a larger sample size is required to validate the associations for AF subtypes. Seventh, we did not include subjects older than 80 years of age, because it is difficult to follow them and their health is complicated by various diseases and treatments. In cohort studies of general populations, the initial AF is regarded as an endpoint. Generally, approximately half of patients with AF have no symptoms, so the average age of patients in registry studies tends to be older than that of the general population.49 Finally, we did not have the participants’ information about the types of antihypertensive agents used, obstructive sleep apnea, thyroid disease, family histories of arrhythmia, sudden cardiac death, or cardiovascular disease, or any other biomarkers at baseline. Thus, our analysis used only the basic cardiovascular risks to predict a 10-year risk score for incident AF. We will consider whether further studies on the association between these risk factors and incident AF can contribute to improving the risk score.50
We developed a 10-year risk score for incident AF in Japanese using traditional risk factors that are widely available and easily obtained in routine outpatient clinic and health examinations without the need for ECG. This risk score may be useful for the identification of individuals at higher risk of AF, such as through a 10-year risk of incident AF calculator on a website or an application on a mobile phone, in order to encourage lifestyle improvements before the onset of AF.
We thank Dr. Katsuyuki Kawanishi, the President of the Suita Medical Association, for his support of the Suita Study. We are also grateful to the members of the Suita City Health Center, the Suita Medical Association, and all the researchers and staff of the Department of Preventive Cardiology for performing medical examinations and follow-up. We thank Dr. Shiro Kamakura for his valuable discussion. Finally, we thank Satsuki-Junyukai, the volunteers involved in the administration of the Suita Study.
This work was supported by the Intramural Research Fund of the National Cerebral and Cardiovascular Center (22-1-2), and by Grants-in-Aid for Scientific Research (B, Nos. 25293147 and 16H05252) and Challenging Exploratory Research (No. 16K15365) in Japan. This study is partially supported by the Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from Japan Agency for Medical Research and Development, AMED (15656344).
The authors declare no conflicts of interest.
Supplementary File 1
Table S1. AF risk score components according to study group
Table S2. Basic risk factors and age- and sex-adjusted HRs for incident AF
Table S3. Multivariable-adjusted cox proportional hazard regression coefficients for risk of incident AF
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0277