2017 Volume 81 Issue 4 Pages 558-566
2017 Volume 81 Issue 4 Pages 558-566
Background: The clinical significance of left ventricular (LV) diastolic function (DF) improvement after transcatheter aortic valve implantation (TAVI) is not fully elucidated. We sought to investigate the long-term clinical and hemodynamic effects of post-TAVI DF improvement and to identify its determinants.
Methods and Results: Clinical and serial echocardiographic data before and after TAVI were retrospectively reviewed together with measurement of mitral annular displacement (MAD) representing longitudinal myocardial function in 98 patients with severe aortic stenosis and ejection fraction >55%. DF grade was determined as 0–III according to the current guideline. Patients with ≥1-grade improvement in DF at 1 year (n=59, 60%) showed a better recovery of New York Heart Association functional class, plasma B-type natriuretic peptide level, stroke volume index, and averaged e’, and a greater decrease in systolic pulmonary artery pressure than those without DF improvement (all P<0.05). Coronary artery disease (odds ratio (OR), 0.30; 95% confidence interval (CI), 0.11–0.84; P=0.022) and averaged MAD (per 2.5 mm increase; OR, 4.11; 95% CI, 2.18–7.72; P<0.001) were predictors of post-TAVI DF improvement. An averaged MAD cutoff value of 10.7 mm discriminated cases of improvement with a sensitivity of 88% and specificity of 64%.
Conclusions: Post-TAVI DF improvement may be associated with preserved LV longitudinal function and when present may be accompanied by more favorable clinical and hemodynamic changes.
Transcatheter aortic valve implantation (TAVI) improves survival in patients with symptomatic severe aortic stenosis (AS) who are considered at high surgical risk or who have contraindications for surgery.1 In recent, large TAVI registry, however, approximately 15% of patients were rehospitalized because of heart failure during the first year despite relief of valvular obstruction.2 One of the potential factors related to heart failure following TAVI is left ventricular (LV) diastolic dysfunction. Previous studies suggested that LV diastolic dysfunction often persists postoperatively despite a reduction in LV mass (LVM) and is related to worse outcome in patients with severe AS.3 This finding suggests the hypothesis that LV diastolic function (DF) improvement positively affects prognosis in patients undergoing TAVI. However, the changes in DF following TAVI have received less attention than LV systolic function.4–6
Movement of the mitral annulus towards the LV apex, which is referred to as mitral annular displacement (MAD), has been demonstrated as a result of the contraction of longitudinally oriented fibers across the LV wall.7 Recent studies investigating the LV remodeling process induced by AS consistently indicated the importance of early histological changes in endocardial tissue such as interstitial fibrosis.8–11 MAD may reflect histological changes in endocardial tissue that can potentially depress longitudinal function. Therefore, in the present study, we sought to examine the serial changes in DF after TAVI, investigate the long-term clinical and hemodynamic effects of post-TAVI DF improvement, and identify its determinants, focusing on MAD representing longitudinal myocardial function.
We retrospectively reviewed 425 consecutive severely symptomatic AS patients who underwent TAVI with a balloon-expandable Edwards Sapien or Sapien XT transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) via a transfemoral approach between September 2011 and August 2013 at Cedars-Sinai Heart Institute. Inclusion criteria was an aortic valve area ≤0.8 cm2, and a resting or inducible peak transaortic velocity >4 m/s or a mean pressure gradient ≥40 mmHg. Exclusion criteria were any of the following: previous mitral valve replacement (n=8); bicuspid aortic valve (n=3); severe mitral stenosis or regurgitation (n=46); chronic or paroxysmal atrial fibrillation (n=129); concomitant coronary artery stenting (n=28); TAVI-related complication including new-onset conduction disturbance (n=10); lack of echocardiographic record at any follow-up (n=22); or a follow-up period <1 year (n=48). In total, 131 patients met the inclusion criteria. We also excluded 33 patients with LV ejection fraction (EF) ≤55% to simply clarify the effect of TAVI on DF.12 The remaining 98 patients eligible for analysis formed the study group.
EchocardiographyAll patients were examined using transthoracic echocardiography at baseline and at the 1-day, 1-month, and 1-year follow-up (iE33; Philips Medical Systems, Andover, MA, USA). The aortic valve area was calculated using quantitative Doppler and the continuity equation, and was indexed for body surface area. Peak transaortic velocity was determined in the apical, right parasternal or right subcostal window by continuous-wave Doppler, and the mean pressure gradient was estimated using the modified Bernoulli equation.13 At 1-year follow-up, an indexed effective orifice area (EOA) <0.65 cm2/m2 was considered as severe prosthesis-patient mismatch.14 An integrative, semiquantitative approach was used to assess the severity of aortic or mitral regurgitation.14 Severity of aortic valve calcification was classified as Grade 0–3+ from the parasternal short-axis view.15 Mitral annular calcification was also classified as Grade 0–3+ according to an established grading method, with calcification of less than one-third of the posterior annulus graded as 1+, and greater than two-thirds graded as 3+.16
LVEF and left atrial volume were measured using the biplane Simpson formula.17 LVM was calculated using 2D-derived linear dimensions according to the Cube formula. LV hypertrophy was defined as LVM index (LVMI) >115 g/m2 in men and >95 g/m2 in women. Relative wall thickness was calculated as 2×(posterior wall thickness/end-diastolic diameter) and considered abnormal when >0.42.17 The stroke volume (SV) was measured as the product of the LV outflow tract area and its velocity-time integral. Systolic pulmonary artery pressure was derived from the maximal tricuspid regurgitation (TR) jet velocity and right atrial pressure estimated from the response of the inferior vena cava to the patient sniffing. End-systolic meridional wall stress was calculated according to previous studies: end-systolic meridional wall stress=systolic blood pressure (BP)×a×1/4 h(1+h/a), where a=LV end-systolic dimension and h=posterior wall thickness at end-systole.18 Systemic arterial compliance (SAC) were assessed as the ratio of SV index (SVI) to brachial pulse pressure. As a measure of global hemodynamic load, valvuloarterial impedance (Zva) was calculated as (systolic BP+mean pressure gradient)/SVI.
DF GradeTransmitral early (E), late (A) diastolic velocities and deceleration time (DT) were measured in the apical 4-chamber view using pulsed-wave Doppler. Pulmonary venous flow velocities during systole (S) and diastole (D) were also recorded. Early diastolic (e’) velocity of the septal and lateral mitral annulus was measured and then the E/e’ ratio was calculated. By integrating these variables, DF grade was classified as follows: normal function (Grade 0) if left atrial volume index ≤34 mL/m2, preserved e’ (septal e’ ≥7 cm/s, lateral e’ ≥10 cm/s, and averaged E/e’ ≤14), and peak velocity of TR jet ≤2.8 m/s; mild diastolic dysfunction (Grade I) if E/A ratio ≤0.8 together with a peak E velocity of ≤50 cm/s; moderate diastolic dysfunction (Grade II) if E/A ratio ≤0.8 together with a peak E velocity >50 cm/s, or an E/A ratio >0.8 but <2, with additional parameters including peak velocity of TR jet >2.8 m/s, average E/e’ >14, and left atrial volume index >34 mL/m2; and severe diastolic dysfunction (Grade III, restrictive) if E/A ratio ≥2 with DT <160 ms, as recommended by the American Society of Echocardiography.19 From the echocardiographic record at each follow-up, DF improvement was determined as ≥1-grade improvement in DF grade.
Measurement of MADMAD was measured by total excursion of the septal or lateral mitral annulus from its highest position after atrial ascent to the maximal descent during systole, as measured from the apical 4-chamber view by observing the annular motion carefully frame-by-frame in B-mode image planes (Figure 1). The end of diastole was defined as the frame before mitral valve closure. %MAD was calculated as the percentage of averaged MAD to the LV length, which was obtained from the apical 4-chamber view on end-diastole frames.

Example of B-mode-based measurement of mitral annular displacement (MAD). MAD was determined by the total excursion of the septal or lateral mitral annulus from its highest position after atrial ascent (white circles) to the maximal descent during systole (yellow circles), as measured from the apical 4-chamber view by observing frame-by-frame in the B-mode image planes.
All-cause death or heart failure hospitalization was the primary outcome. Death and admission were confirmed by querying nationally available databases, inspection of the electronic health record, or verified by a family member. The duration of follow-up ranged from the initial TAVI to July 2015.
Intra- and Interobserver VariabilityTo assess the reproducibility of MAD, 20 randomly chosen studies were selected, and another independent observer and the original observer repeated the MAD measurements. Intraobserver variability was defined as the mean of the values obtained by dividing the absolute difference between 2 measurements by the mean of the 2 measurements made by a single observer for a given patient. Interobserver variability was similarly defined for the 2 independent observers.
Statistical AnalysisDifferences between patients with and without DF improvement were analyzed by Student’s t-test, Wilcoxon rank-sum test, and the χ2 test or Fisher exact test as appropriate. A 2-way ANOVA for repeated measures, followed by a Tukey posthoc test, was used to evaluate the effects of time and groups on New York Heart Association functional class (NYHA-FC), plasma B-type natriuretic peptide (BNP) level, echocardiographic variables, and global hemodynamic and vascular loads. Furthermore, we selected potential determinants of DF improvement noted at 1-year post-TAVI with a univariable P<0.05 with fixed adjustment for age and sex. Next, the selected univariable variables were entered into a multivariate logistic regression analysis. The independent determinants of post-TAVI DF improvement were sought by backward selection using the likelihood ratio criterion. Comparisons of the predictive power were performed by comparing the C-statistic derived from the area under the receiver-operating characteristic curves. Statistical analyses were conducted using SPSS ver. 21 (SPSS Inc., IBM, Armonk, NY, USA).
Of the 98 subjects, there were 53 men and 45 women with a mean age of 83±8 years. Mean value of the Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score was 9.2±2.1% (Table 1). The cardiac rhythm was normal sinus in 96% and paced in 4%. A history of prior myocardial infarction was present in 25%, previous percutaneous coronary intervention in 31%, and coronary artery bypass grafting in 17%. As a whole, coronary artery disease (CAD) was present in 36 of 98 subjects (37%). Transmitral diastolic velocities and e’ were feasible in all subjects, but pulmonary venous flow velocities were not available in 16 of 98 subjects (16%). After the assessment of baseline DF grade, 37 subjects (38%) were classified as Grade I, 40 (41%) as Grade II, and 21 (21%) as Grade III.
| Variable | All patients (n=98) |
With DF improvement (n=59) |
No DF improvement (n=39) |
P value |
|---|---|---|---|---|
| Age, years | 83±8 | 84±8 | 82±8 | 0.38 |
| Female sex | 45 (46%) | 26 (44%) | 19 (49%) | 0.65 |
| Systolic BP at baseline, mmHg | 132±17 | 130±17 | 137±17 | 0.09 |
| Systolic BP at 1 year, mmHg | 132±16 | 133±16 | 131±16 | 0.42 |
| NYHA-FC III or IV | 80 (82%) | 49 (83%) | 31 (79%) | 0.34 |
| STS-PROM score, % | 9.2±2.1 | 9.1±1.9 | 9.3±2.2 | 0.57 |
| Obesity (BMI ≥30 kg/m2) | 25 (26%) | 11 (19%) | 14 (36%) | 0.06 |
| Coronary artery disease | 36 (37%) | 17 (29%) | 19 (49%) | 0.045 |
| Pacer lead | 13 (13%) | 4 (7%) | 9 (23%) | 0.02 |
| Hypertension | 86 (88%) | 53 (90%) | 33 (85%) | 0.44 |
| Diabetes mellitus | 39 (40%) | 22 (37%) | 17 (44%) | 0.53 |
| Chronic kidney failure | 25 (26%) | 14 (24%) | 11 (28%) | 0.62 |
| Pulmonary disease | 20 (20%) | 13 (22%) | 7 (18%) | 0.62 |
| Plasma BNP, pg/mL | 266 (156–436) | 265 (124–463) | 267 (190–411) | 0.89 |
| Peak transaortic velocity, ms | 4.5±0.6 | 4.5±0.5 | 4.5±0.7 | 0.97 |
| Mean aortic valve gradient, mmHg | 50±13 | 51±10 | 53±13 | 0.63 |
| Indexed aortic valve area, cm2/m2 | 0.35±0.09 | 0.37±0.09 | 0.32±0.08 | 0.009 |
| Aortic valve calcification 2+ or 3+ | 91 (93%) | 54 (92%) | 37 (95%) | 0.42 |
| Mitral annular calcification 2+ or 3+ | 44 (45%) | 26 (44%) | 18 (46%) | 0.50 |
| End-diastolic diameter, cm | 4.3±0.6 | 4.3±0.5 | 4.3±0.7 | 0.73 |
| End-systolic diameter, cm | 2.8±0.6 | 2.8±0.5 | 2.8±0.7 | 0.99 |
| LV mass index, g/m2 | 125±38 | 126±34 | 122±43 | 0.71 |
| Relative wall thickness | 0.56±0.12 | 0.55±0.12 | 0.58±0.12 | 0.37 |
| LVEF, % | 67±10 | 66±10 | 68±11 | 0.48 |
| Stroke volume index, mL/m2 | 36±9 | 39±8 | 33±8 | 0.001 |
| End-systolic meridional wall stress, kdyne/cm2 | 40±16 | 39±13 | 41±20 | 0.62 |
| Septal MAD, mm | 10.7±2.5 | 11.6±1.8 | 9.3±2.7 | <0.001 |
| Lateral MAD, mm | 12.3±2.8 | 13.5±2.1 | 10.5±2.9 | <0.001 |
| Averaged MAD, mm | 11.5±2.5 | 12.5±1.8 | 9.9±2.7 | <0.001 |
| %MAD | 14±3 | 16±2 | 12±4 | <0.001 |
| Mitral E wave, ms | 1.0±0.3 | 1.1±0.3 | 1.0±0.3 | 0.45 |
| Deceleration time, ms | 235±115 | 239±136 | 229±75 | 0.68 |
| Mitral E/A ratio | 1.2±0.8 | 1.2±0.7 | 1.1±1.0 | 0.63 |
| Pulmonary S/D ratio | 0.8±0.6 | 0.8±0.5* | 0.7±0.6† | 0.55 |
| Averaged e’, cm/s | 6.1±1.7 | 6.2±1.8 | 6.0±1.6 | 0.56 |
| Averaged E/e’ ratio | 18±8 | 19±8 | 18±7 | 0.70 |
| Left atrial volume index, mL/m2 | 41±15 | 44±15 | 38±13 | 0.06 |
| Systolic PAP, mmHg | 40±17 | 40±18 | 41±14 | 0.81 |
| Zva, mmHg/mL/m2 | 5.3±1.6 | 4.9±1.3 | 6.0±1.8 | 0.001 |
| SAC, mL/mmHg/m2 | 0.59±0.19 | 0.64±0.20 | 0.51±0.17 | 0.001 |
| Aortic regurgitation, 0–4+ | 1.4±1.0 | 1.4±1.0 | 1.5±1.1 | 0.77 |
| Mitral regurgitation, 0–4+ | 1.9±0.9 | 2.0±0.9 | 1.8±0.9 | 0.42 |
Continuous variables are expressed as mean±SD or median (interquartile range). *50 of 59 subjects; †32 of 39 subjects. BMI, body mass index; BNP, B-type natriuretic peptide; DF, diastolic function; LVEF, left ventricular ejection fraction; MAD, mitral annular displacement; NYHA-FC, New York Heart Association functional class; PAP, pulmonary artery pressure; SAC, systemic arterial compliance; STS-PROM, the Society of Thoracic Surgeons predicted risk of mortality; TAVI, transcatheter aortic valve implantation; Zva, valvuloarterial impedance.
Prevalence of patients with ≥1-grade DF improvement at 1-day, 1-month, and 1-year follow-up was 22%, 34%, and 60%, respectively (Figure 2). Recovery of LVEF was slight immediately after TAVI (baseline, 67±10%; 1-day, 70±10%; 1-month, 70±10%; 1-year, 71±10%; P=0.08). Regression of LVMI occurred progressively both early and late after TAVI (baseline, 125±38 g/m2; 1-day, 126±40 g/m2; 1-month, 112±34 g/m2; 1-year, 100±31 g/m2; P<0.001).

Temporal changes in the grade of diastolic function (DF) before and after transcatheter aortic valve implantation. Prevalence of patients with ≥1-grade DF improvement at 1-day, 1-month, and 1-year follow-up was 22%, 34%, and 60%, respectively.
Patients with DF improvement noted at 1 year post-TAVI had a lower prevalence of CAD and pacer lead as compared with those without DF improvement (both P<0.05; Table 1). Patients with post-TAVI DF improvement had a larger indexed aortic valve area, higher SVI, and better preserved MAD (all P<0.05; Table 1). In contrast, STS-PROM score, LV size, mass, and EF, and systolic pulmonary artery pressure (sPAP) did not differ significantly between groups.
NYHA-FC and Plasma BNP LevelDespite similar baseline NYHA-FC and plasma BNP levels between groups (Table 1), patients with DF improvement showed better recovery of NYHA-FC (P<0.001 for interaction; Figure 3A) and greater decrease in plasma BNP level (P=0.017 for interaction; Figure 3B) than those without DF improvement.

Comparison of changes in NYHA-FC (A) and plasma BNP level (B) in patients with and without DF improvement at 1-year follow-up. BNP, B-type natriuretic peptide; DF, diastolic function; NYHA-FC, New York Heart Association functional class.
The change in indexed EOA was not statistically different between patients with and without DF improvement noted at 1 year post-TAVI (Figure 4A). In addition, the incidence of severe prosthesis-patient mismatch was similar between the groups (9% vs. 13%, P=0.36). Also, regression of the LVMI and recovery of LVEF did not differ significantly between the groups (Figure 4B,C). In contrast, greater recovery of the SVI (P=0.04 for interaction) and averaged e’ (P=0.002 for interaction), and a greater decrease in sPAP (P<0.001 for interaction) were observed in patients with post-TAVI DF improvement (Figure 4D–F).

Comparison of changes in indexed EOA (A), LV mass index (B), LVEF (C), stroke volume index (D), averaged e’ (E), and systolic pulmonary artery pressure (F) in patients with (green triangles) and without (blue squares) DF improvement at 1-year follow-up. Central triangles/squares and vertical lines represent mean value±SD. *P<0.05 between groups, †P<0.05 vs. baseline, ‡P<0.05 vs. 1 day, §P<0.05 vs. 1 month. EOA, effective orifice area; DF, diastolic function; LVEF, left ventricular ejection fraction; MAD, mitral annular displacement.
At baseline, a lower Zva and higher SAC were observed in the DF improvement group (Table 1); however, these changes did not differ significantly between the groups (Zva, P=0.19 for interaction; SAC, P=0.09 for interaction; Table S1). With regard to valvular regurgitation, there was no significant difference in aortic or mitral regurgitation grade at baseline (Table 1). At 1-year follow-up, aortic or mitral regurgitation grade significantly decreased in both groups (all P<0.05), but the changes did not differ between the groups (aortic regurgitation, P=0.47 for interaction; mitral regurgitation, P=0.53 for interaction; Table S1).
Determinants of Post-TAVI DF ImprovementIn the univariate analyses with fixed adjustment for age and sex, DF improvement noted at 1 year post-TAVI was associated with the following pre-TAVI parameters: CAD, pacer lead, indexed aortic valve area, SVI, averaged MAD, Zva, and SAC (all P<0.05). Multivariate logistic regression analysis revealed that CAD (odds ratio [OR] 0.30, 95% confidence interval [CI] 0.11–0.84, P=0.022) and averaged MAD (per 2.5 mm increase, OR 4.11, 95% CI 2.18–7.72, P<0.001) were independently associated with post-TAVI DF improvement (Table 2).
| Variable | Univariate* | Multivariate† | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Coronary artery disease | 0.33 (0.12–0.88) | 0.027 | 0.30 (0.11–0.84) | 0.022 |
| Pacer lead | 0.24 (0.07–0.86) | 0.028 | ||
| Indexed aortic valve area (per 0.1 cm2/m2) | 1.78 (1.12–2.81) | 0.014 | – | – |
| Stroke volume index (per 8.7 mL/m2) | 2.13 (1.31–3.46) | 0.002 | – | – |
| Averaged MAD (per 2.5 mm) | 3.81 (2.08–6.96) | <0.001 | 4.11 (2.18–7.72) | <0.001 |
| Zva (per 1.6 mmHg/mL/m2) | 0.47 (0.29–0.76) | 0.002 | – | – |
| SAC (per 0.2 mL/mmHg/m2) | 2.19 (1.32–3.62) | 0.002 | – | – |
*Trivariate logistic regression analysis with fixed adjustment for age and sex for all variables. †Using fixed adjustment for age and sex, independent determinants (coronary artery disease and averaged MAD) were sought by backward selection using the likelihood ratio criterion (Pin=0.05, Pout=0.1). CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
Figure 5 demonstrates the receiver-operating characteristic curves for septal and lateral MAD, averaged MAD, and %MAD. Despite the highest area under the curve, C-statistics for lateral MAD demonstrated nonsuperiority to averaged MAD (P=0.52). A cutoff value of averaged MAD >10.7 mm had the sensitivity (88%) and specificity (64%) for post-TAVI DF improvement.

Receiver-operating characteristic curves evaluating the predictive value of septal MAD, lateral MAD, averaged MAD, and %MAD for post-TAVI DF improvement. AUC, area under the curve; CI, confidence interval; DF, diastolic function; MAD, mitral annular displacement; TAVI, transcatheter aortic valve implantation.
During a median follow-up period of 2.6 years (interquartile range, 2.1–3.1 years), 26 patients (27%) met the primary outcome: 10 died and 16 were admitted for heart failure. Kaplan-Meier analysis showed patients with post-TAVI DF improvement had a significantly better outcome than those without it (Figure 6A). In addition, patients with averaged MAD >10.7 mm also showed better outcome than those with averaged MAD ≤10.7 mm (Figure 6B). After multivariate adjustment for STS-PROM score, baseline indexed aortic valve area, sPAP, recovery of LVEF, and regression of LVMI, post-TAVI DF improvement was associated with a decreased risk of all-cause death or heart failure hospitalization (hazard ratio 0.10, 95% CI 0.03–0.29, P<0.001).
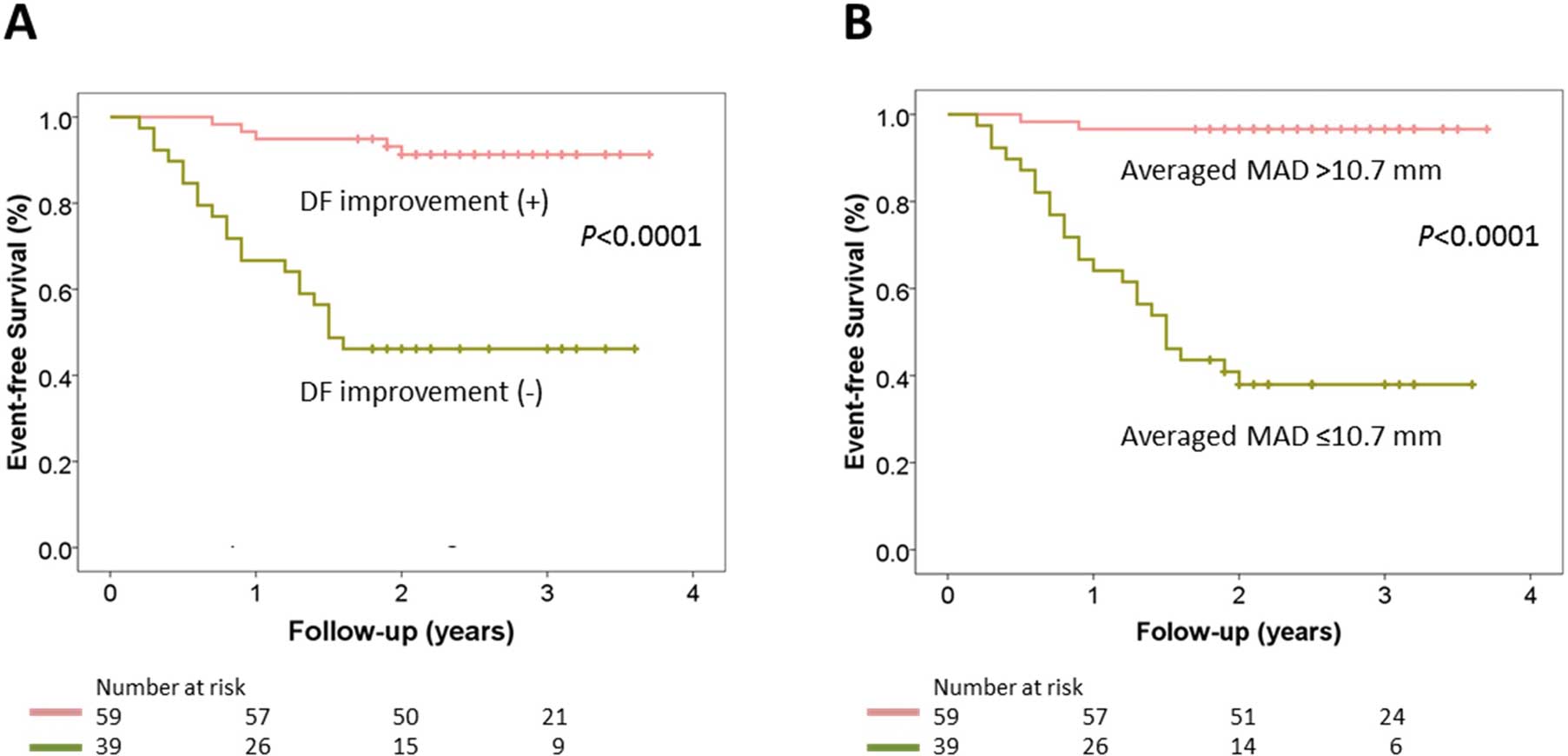
Kaplan-Meier plots for cardiac events. (A) Prognostic effect of post-TAVI DF improvement. (B) Prognostic impact of averaged MAD (cutoff 10.7 mm). Abbreviations as in Figure 5.
The intraobserver variability in the measurements was 5.1±4.6% and 4.8±4.5% for septal MAD and lateral MAD, respectively, and the corresponding interobserver variability was 8.7±7.2% and 8.5±7.6%.
The present study demonstrated (1) an improvement in DF grade in 60% of patients at 1 year post-TAVI among those with severe symptomatic AS and preserved LVEF; (2) patients with post-TAVI DF improvement had more favorable clinical, hemodynamic and prognostic outcomes; and (3) on multivariable analysis, absence of CAD and preserved MAD were independently associated with the occurrence of post-TAVI DF improvement.
Value of Post-TAVI DF ImprovementThe persistent risk of cardiac death or heart failure in patients undergoing TAVI has been recently indicated.2 Presence of significant aortic regurgitation, use of transapical approach, and baseline comorbidities such as depressed LVEF, atrial fibrillation, and pulmonary hypertension, predict death.20 However, the prognostic value of post-TAVI DF improvement has been unknown. The present study showed that among the changes in LV indices, only post-TAVI DF improvement was associated with better outcome in patients with severe AS and preserved LVEF, even after adjustment for pre-TAVI variables including STS-PROM score, indexed aortic valve area, and sPAP. In contrast, recovery of LVEF was not predictive because it appears to be relatively small in patients with preserved LVEF.
The present study showed that improvement in NYHA-FC was related to DF improvement following TAVI, which is consistent with the recent publication by Muratori and colleagues.21 Another clinical effect of post-TAVI DF improvement observed in this study was a decrease in plasma BNP. We observed favorable hemodynamic changes in patients with post-TAVI DF improvement through greater recovery of the SVI and averaged e’ (improved relaxation) and greater decline in sPAP. Thus, it is reasonable to assume that improvement in LV relaxation and compliance results in increased ventricular filling and decreased end-diastolic pressure.
Cause of Post-TAVI DF ImprovementTzikas et al5 reported an unchanged DF grade at 1 year post-TAVI despite significant regression of LVM. That study differed from ours with respect to statistical strategy; in the previous study, DF grade was considered as a continuous variable whereas we considered it to be an ordinal variable. Vizzardi et al6 demonstrated an improvement in DF grade in 72% at 6 months post-TAVI with significant regression of LVM and that report is consistent with the results of the present study. However, these investigators included patients with reduced LV systolic function and also atrial fibrillation, which may have complicated the interpretation of DF improvement.
The improvement in DF grade observed in the present study was shown in a stepwise fashion, namely 22%, 34%, and 60% at 1-day, 1-month, and 1-year follow-up, respectively. Acute DF improvement noted at 1-day post-TAVI may be related to rapid reduction in afterload. The reduction in afterload by TAVI may lead to shortening of the LV ejection time and improvement in LV relaxation with a subsequent decrease in end-diastolic pressure. In contrast, chronic DF improvement noted at 1-month and 1-year post-TAVI may be caused by normalization of LV structural remodeling. Flett et al11 suggested that regression of LV hypertrophy at 6 months after AS treatment is mainly caused by cell volume reduction while interstitial fibrosis remains unchanged. It appears, therefore, that patients with greater fibrosis may be burdened with a persistent risk of heart failure after TAVI.
Longitudinal Myocardial Function and Post-TAVI DF ImprovementAnatomical studies indicate that longitudinally oriented myocardial fibers are located in the subepicardial and subendocardial layers of the LV wall.22 MAD is a well-known but infrequently used surrogate for LV longitudinal myocardial function, which is mainly generated by myocardial fibers located in the subendocardium.7 Our current data and that from some previously published works18,23,24 indicate that longitudinal myocardial contractility in the chronic pressure-overloaded LV can be depressed in the presence of a normal LVEF. Interestingly, MAD did not correlate with end-systolic meridional wall stress (r=−0.12, P=0.25). In this respect, longitudinal shortening differs from circumferential shortening at the midwall.18 This finding would be undetectable with solely a stress-strain response of depressed longitudinal myocardial function.
Notably, recent studies have investigated inducible LV fibrosis in patients with severe AS. Herrmann et al10 estimated LV longitudinal function using MAD in 86 subjects with AS and also found that this variable inversely correlated with the degree of interstitial fibrosis in biopsy samples. Heymans and colleagues25 demonstrated in severe AS that collagen deposition was most prominent in the subendocardial area. Their results were consistent with the following theoretical considerations, as well as with those recently reported by Weidemann et al8 utilizing late-enhancement magnetic resonance imaging. Theoretically, the highest stress is on myocardial fibers with the largest radial curvature, where the LV is “least spherical”. That explains why longitudinal fibers are always exposed to disproportionally greater wall stress than circumferential fibers. Because the wall stress is highest at the subendocardium of the LV base, these investigators suggested that the longitudinal fibers of the basal subendocardium are the first to be affected by inducible fibrotic processes. Therefore, we speculate that low MAD caused by fibrosis in the subendocardium results in less DF improvement in patients with severe AS and preserved LVEF. Based on our findings, the measurement of MAD may be helpful in identifying patients who will have DF improvement and better cardiac outcomes after TAVI.
CADRisk factors for AS and CAD frequently coexist. Beach et al26 demonstrated increased deaths of patients with severe AS and CAD and its association with the effects of myocardial ischemia. The results of our study suggest that active investigation of preexisting ischemic myocardial damage in elderly patients with severe AS and risk factors for CAD would be desirable so that timely TAVI is performed before ischemic myocardial damage occurs.
Study LimitationsFirst, presence of severe mitral stenosis or regurgitation would complicate accurate evaluation of DF. Therefore, we had to exclude such patients from our analysis. Second, MAD is not the most contemporary assessment of LV performance in an era in which speckle-tracking echocardiography may be used. Most recently, the prognostic effect of basal longitudinal strain in AS patients has been reported.27 However, excellent image quality is indispensable for LV strain and strain rate analyses. In the present study, we were able to measure MAD easily in all cases even if the LV endocardial border was unclear. Third, our study is limited by the lack of tissue characterization by cardiac magnetic resonance imaging or pathological confirmation, so the interpretation of low MAD as increased endocardial fibrosis remains to be elucidated. Both fibrosis and unrecognized amyloidosis might lead to persistent diastolic dysfunction and low MAD. Fourth, this study did not include patients with paradoxical low-flow, low-gradient severe AS who have been associated with increased risk for adverse outcomes.20 Additionally, we excluded patients with reduced LVEF and atrial fibrillation to simply clarify the effect of TAVI on DF. Therefore, the fact that the study sample is highly selected should be recognized. To confirm our findings, multicenter and prospective studies are mandatory.
In summary, post-TAVI DF improvement is often observed at 1 year in patients with severe AS and preserved LVEF, and when present may be accompanied by more favorable clinical, hemodynamic, and prognostic outcomes. Preserved LV longitudinal myocardial function, as assessed by MAD, had a strong association to post-TAVI DF improvement, which is related to a decreased risk of cardiac events. Thus, a comprehensive assessment of cardiac function, including the measurement of MAD, would be useful for risk stratification following TAVI in this population.
The authors thank Miki Mizuta and Takayuki Hataoka, from Philips Electronics Japan, for their technical assistance.
H.U. holds a Banyu Fellowship Program sponsored by Banyu Life Science Foundation International. H.J. is a consultant for Edwards Lifesciences, St. Jude Medical, and Venus Medtech. R.J.S. is a consultant to Abbott and a speaker for Philips Ultrasound. R.R.M. receives research grants from Edwards, Medtronic, Abbott, Capricor, and St. Jude Medical; and is a proctor for Edwards and consultant to Medtronic. T.S. is a speaker for Philips Ultrasound.
Supplementary File 1
Table S1. Global hemodynamic and vascular load and valvular regurgitation
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-1105