2017 Volume 81 Issue 8 Pages 1174-1182
2017 Volume 81 Issue 8 Pages 1174-1182
Background: Several studies have reported that colchicine attenuated the infarct size and inflammation in acute myocardial infarction (MI). However, the sustained benefit of colchicine administration on survival and cardiac function after MI is unknown. It was hypothesized that the short-term treatment with colchicine could improve survival and cardiac function during the recovery phase of MI.
Methods and Results: MI was induced in mice by permanent ligation of the left anterior descending coronary artery. Mice were then orally administered colchicine 0.1 mg/kg/day or vehicle from 1 h to day 7 after MI. Colchicine significantly improved survival rate (colchicine, n=48: 89.6% vs. vehicle, n=51: 70.6%, P<0.01), left ventricular end-diastolic diameter (5.0±0.2 vs. 5.6±0.2 mm, P<0.05) and ejection fraction (41.5±2.1 vs. 23.8±3.1%, P<0.001), as assessed by echocardiogram compared with vehicle at 4 weeks after MI. Heart failure development as pulmonary edema assessed by wet/dry lung weight ratio (5.0±0.1 vs. 5.5±0.2, P<0.01) and B-type natriuretic peptide expression in the heart was attenuated in the colchicine group at 4 weeks after MI. Histological and gene expression analysis revealed colchicine significantly inhibited the infiltration of neutrophils and macrophages, and attenuated the mRNA expression of pro-inflammatory cytokines and NLRP3 inflammasome components in the infarcted myocardium at 24 h after MI.
Conclusions: Short-term treatment with colchicine successfully attenuated pro-inflammatory cytokines and NLRP3 inflammasome, and improved cardiac function, heart failure, and survival after MI.
In acute myocardial infarction (AMI), acute ischemia causes myocardial necrosis with subsequent endogenous inflammation, leading to myocardial damage, ventricular dilation, and dysfunction.1 Sustained enhancement of inflammatory mediators induces myocardial apoptosis, a further loss of cardiomyocytes, and cardiac dysfunction.1 Although anti-inflammatory strategies after the onset of myocardial infarction (MI) could be effective at improving cardiac dysfunction, heart failure, and survival, this has not been established. Several studies using glucocorticosteroids, non-steroidal anti-inflammatory drugs, or cyclosporine have failed to show benefit in MI.2–4
Colchicine has potent anti-inflammatory properties and has been traditionally used to treat acute gout and familial Mediterranean fever.5 Colchicine is also used as a treatment for pericarditis.5,6 Several studies reported that colchicine users had a lower prevalence of MI in patients with gout.7,8
Recent studies have demonstrated that nucleotide-binding oligomerization domain-like receptors, pyrin domain-containing 3 (NLRP3) inflammasome, is associated with endogenous sterile inflammation after MI.9,10 NLRP3 is a cytosolic multiprotein complex activated by tissue danger signals and associated with the production of activated interleukin (IL)-1β and IL-18.11 Colchicine was reported to reduce inflammatory cytokines (including IL-1β and IL-18) and infarction size in MI.12,13 However, the sustained benefit of short-term colchicine treatment on survival, cardiac function, and ventricular remodeling after MI is unknown.
We hypothesized that short-term treatment with colchicine could improve adverse cardiac remodeling, dysfunction, heart failure development and survival during the recovery phase of MI by the inhibition of acute inflammation and NLRP3 inflammasome activation.
Male C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan) and used for experiments when they were 14–15 weeks of age. Mice were anesthetized with 2% isoflurane. MI was induced by permanent occlusion of the left anterior descending coronary artery, as described previously.14 Briefly, a polyethylene tube was cannulated into the trachea, and connected to a ventilator. After a left thoracotomy between the fourth and fifth ribs, the pericardial tissue was removed. Then, the left anterior descending coronary artery was identified under a microscope and permanently ligated with 8-0 silk sutures at the 1- to 2-mm inferior edge of the left atrium. Sham-operated mice underwent surgery, but the left anterior descending coronary artery was not ligated. Colchicine 0.1 mg/kg (Sigma Aldrich, St. Louis, MO, USA) was then administrated orally at 1 h after the surgery and once daily from day 2 to 7 after surgery. Survival rate was assessed until 4 weeks after surgery. At days 1, 3, 7, and 28 after surgery, mice were euthanized with pentobarbital (200 mg/kg intraperitoneally) for subsequent analyses after MI. All procedures of this study were performed in accordance with the Kumamoto University animal care guidelines (approval reference no. E25-210 and A27-022), which conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication No. 85-23, revised 1996).
Echocardiography and Hemodynamic AnalysesEchocardiography and hemodynamic measurements were performed, as described previously.14 At baseline, and on days 1, 7, 14, 21, and 28 after surgery, transthoracic echocardiography (TTE) was performed using a Xario system (Toshiba, Tokyo, Japan) with a 12-MHz linear array transducer under anesthesia with 1.5% isoflurane whilst maintaining spontaneous respiration. On day 28 after surgery, a 1.4 French micromanometer-tipped catheter (Millar Instruments, Houston, TX, USA) was inserted into the left ventricle via the right carotid artery under the same anesthesia conditions. Left ventricular (LV) pressure waves were recorded and analyzed using LabChart software V7 (AD Instruments, Dunedin, New Zealand). All echocardiographic and hemodynamic analyses were performed by investigators who were blinded to the treatment groups.
Assessment of Pulmonary EdemaPulmonary edema was evaluated as the wet/dry bilateral lung weight ratios, as described previously.15 In brief, to determine the wet lung weight, bilateral lungs were weighed soon after separate extraction. Then, each lung was dried for 72 h at 70℃, and dry lung weights were measured.
Histology and ImmunohistochemistryExtracted hearts were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. For scar size determination, each sample at 7 days’ post-infarction was divided into 6 transverse sections (5 μm) from the apex to the base of the LV with an interval of 600 μm between each section. Scar areas of each sample were measured as the collagen deposition, highlighted blue area in Masson’s trichrome stained sections. The ratio of total scar area to the total LV wall among each section was calculated.16 For immunohistochemistry, neutrophils and macrophages in the infarcted area were identified by anti-Gr-1 (Southern Biotechnology, Birmingham, AL, USA) and anti-Iba1 (Wako Pure Chemical Industries Ltd., Osaka, Japan) staining, respectively. Each section was stained with hematoxylin to visualize the nuclei. Counting the number of positive cells was performed in five different fields. All measurements were performed with Image J software (National Institute of Health, Bethesda, MD, USA).
Quantification of mRNA LevelsTotal RNA was extracted from hearts using the RNA Easy Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized using Quantitect Reverse Transcription Kits (Qiagen). Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using a TaqMan Universal Master Mix kit with a CFX384 Real-Time System (Bio-Rad, Hercules, CA, USA). Primers and probes for B-type natriuretic peptide (BNP: Mm01255770_g1), tumor necrosis factor-α (TNF-α: Mm00443258_m1), IL-1β (Mm01336189_m1), IL-18 (Mm00434225_m1), IL-10 (Mm01288386_m1), IL-4 (Mm00445259_m1), IL-6 (Mm00446190_m1), C-X-C motif ligand 2 (CXCL2: Mm00436450_m1), monocyte chemoattractant protein-1 (MCP-1: Mm00441243_g1), matrix metallopeptidase (MMP) 2 (Mm00439498_m1), MMP9 (Mm00442991_m1), transforming growth factor-β (TGF-β: Mm01178820_m1), NLRP3 (Mm00840904_m1), apoptosis speck-like protein containing caspase recruitment domain (ASC: Mm00445747_g1), caspase-1 (Mm00438023_m1), and 18S (Mm03928990_g1) were synthesized by Assays-on-Demand Gene Expression Products (Applied Biosystems, Foster City, CA, USA). The expression of each mRNA was normalized relative to the level of 18S ribosomal mRNA.
Assessment of Caspase-1 ActivityCaspase-1 activity in infarcted hearts was assessed by fluorometric substrate YVAD-AFC in infarcted hearts, as previously described.17 In brief, proteins were extracted from the LV using lysis buffer (Abcam, Cambridge, UK), and activity was measured by fluorometric substrate YVAD-AFC using a caspase-1 assay kit (Abcam). Fluorescence was measured using a Filer MAX F5 (Molecular Devices, Tokyo, Japan) at emission/excitation wavelengths of 405/500 nm.
Western Blot Analysis for Polymerized Tubulin in Infarcted HeartsPolymerized tubulin was extracted from LV, as previously described.18 In brief, 100 mg LV sample was homogenized with 4 mL of microtubule stabilizing buffer (50% glycerol, 5% dimethyl sulfoxide, 10 mmol/L sodium phosphate, 0.5 mmol/L MgSO4, and 0.5 mmol/L EGTA) and centrifuged at 100,000 g at 25℃ for 10 min. The supernatants were removed, and the pellets were resuspended and incubated at 0℃ for 1 h in 3.2 mL of microtubule depolymerizing buffer (0.25 M sucrose, 10 mmol/L sodium phosphate, and 0.5 mmol/L MgSO4). They were centrifuged at 100,000 g at 4℃ for 15 min, and the polymerized tubulin fractions were obtained from the supernatants.
Equal amounts of protein samples were separated by SDS-PAGE, and western blotting was performed to determine the protein level of polymerized tubulin. A mouse monoclonal antibody for β-tubulin (Cell Signaling Technology, Beverly, MA, USA) was used as a primary antibody in combination with a peroxidase-conjugated secondary antibody. The bands of protein were detected with chemiluminescence. An ImageQuant LAS 4000 mini-biomolecular imager (Fujifilm, Tokyo, Japan) was used for the quantification of band density.
Serum Cardiac Troponin I MeasurementBlood was collected from the right atrium into a serum separator tube (Terumo, Tokyo, Japan) and centrifuged (1,200 g for 10 min) to obtain serum, which was stored at −80℃ until analysis. Cardiac injury was assessed by measuring the serum levels of high sensitively mouse cardiac troponin-I using an enzyme-linked immunosorbent assay kit (Life Diagnostics, Inc., West Chester, PA, USA).
Myeloperoxidase Activity AssessmentAnesthetized mice were transcardially perfused with 20 mL phosphate buffered saline to clear the intravascular compartment of blood cells. For the analysis of myeloperoxidase activity in LVs, LVs were harvested and homogenized using a mechanical homogenizer (Qiagen) in 500 µL CTAB buffer (50 mmol/L cetyltrimethylammonium bromide in 50 mmol/L potassium phosphate buffer at pH=6.0), sonicated, and centrifuged at 15,000 g for 20 min. The supernatant was used for protein analysis with a BCA protein assay kit (Thermo Scientific) and for MPO activity assays. MPO chlorination activity was assessed with a Myeloperoxidase Chlorination Fluorometric Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) based on a fluorescence method. Non-fluorescent 2-[6-(4-aminophenoxy)-3-oxo-3H-xanthen-9-yl]-benzoic acid (APF) is selectively cleaved by hypochlorite (-OCl) to yield the highly fluorescent compound, fluorescein.19 Fluorescein fluorescence was measured with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
White Blood Cell and Plasma IL-1β AssessmentBlood samples were obtained by right atrial puncture and collected in EDTA-2Na tubes. White blood cells and neutrophils were automatically counted using an ADVIA®2120i hematology system (Siemens Healthcare, Germany). After centrifugation at 1,200 g for 10 min, 100 µL of plasma was obtained and used for plasma IL-1β measurement using an ELISA kit (R&D Systems, MN, USA) according to the manufacturer’s instruction.
Statistical AnalysisAll data are presented as individual samples and as mean values or the mean±SEM. The Student’s t-test was used to compare 2 groups. Differences among multiple groups were evaluated by ANOVA followed by the Bonferroni post-hoc test for equal sample sizes or the Tukey-Kramer test for unequal sample sizes. Survival rate was evaluated by the Kaplan-Meier method, and the log-rank test was used to compare survival curves of MI-operated colchicine-treated and vehicle-treated mice. P values less than 0.05 were considered statistically significant.
Colchicine significantly improved the survival rate compared with vehicle in the acute phase of MI (within 7 days). In each case, the cause of death was cardiac rupture. This efficacy on survival continued during 4 weeks after MI (colchicine, n=48: 89.6% vs. vehicle, n=51: 70.6%, P<0.01, Figure 1). Body weight, heart rate, and systolic blood pressure were not significantly different between the MI-vehicle group and the MI-colchicine group up to 4 weeks after MI (Table). Colchicine significantly inhibited the increase of heart weight at 4 weeks after MI (Figure 2A). The wet/dry lung weight ratio increased in mice with MI compared with sham operation mice, indicating the development of heart failure as pulmonary edema. Colchicine improved the increase of the wet/dry lung weight ratio compared with the vehicle control group (5.0±0.1 vs. 5.5±0.2, P<0.01, Figure 2B). The mRNA expression of B-type natriuretic peptide in the non-infarcted area was also attenuated in the colchicine group at 4 weeks after MI (Figure 2C).
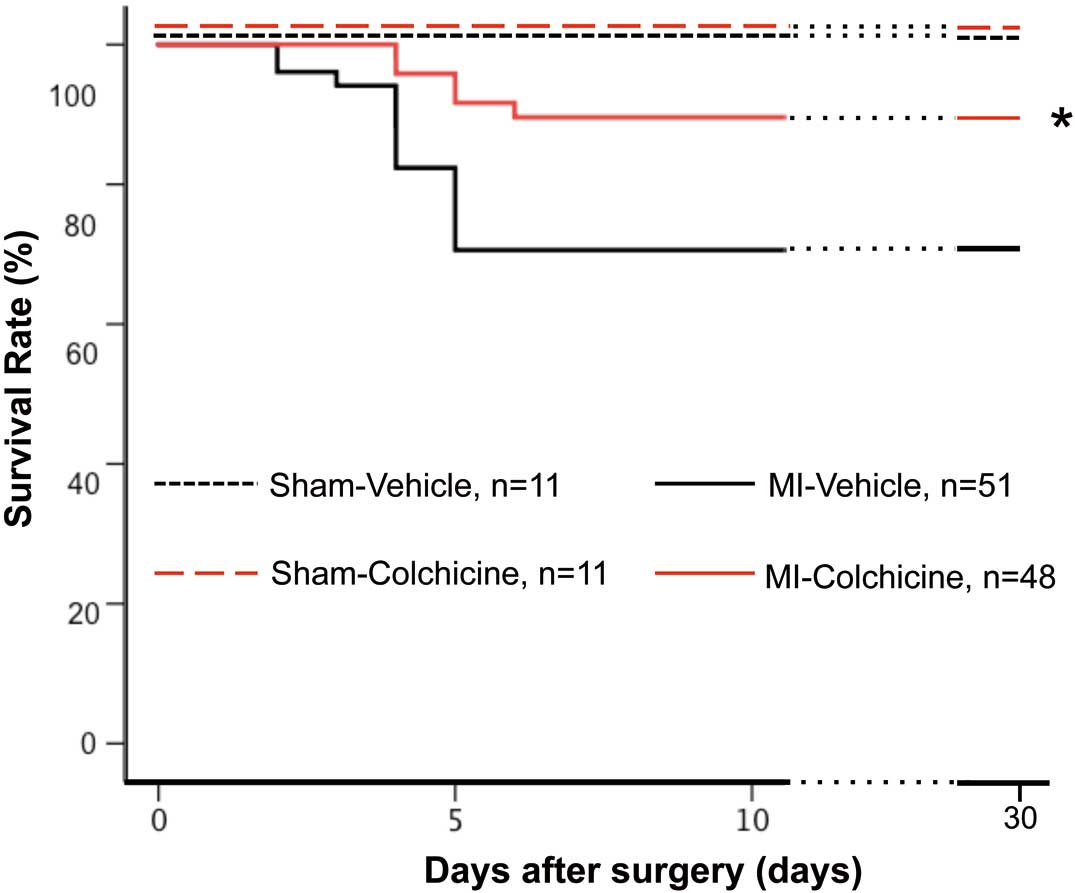
Colchicine improves survival rate in the acute phase of myocardial infarction. Survival curves of vehicle or colchicine-treated mice after surgery. Sham-vehicle, n=11; sham-colchicine, n=11; myocardial infarction (MI)-vehicle, n=51; and MI-colchicine, n=48. *P<0.01 vs. MI-vehicle mice.
| Sham vehicle | Sham colchicine | MI vehicle | MI colchicine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 7 | Day 28 | Baseline | Day 7 | Day 28 | Baseline | Day 7 | Day 28 | Baseline | Day 7 | Day 28 | |
| Body weight, g |
27.5±0.5 | 27.5±0.6 | 27.6±03 | 27.1±0.4 | 26.9±0.5 | 27.8±0.4 | 26.9±0.4 | 25.8±0.3 | 27.5±0.4 | 27.6±0.4 | 26.9±0.5 | 27.9±0.4 |
| HR, beats/min |
703±9 | 696±6 | 707±10 | 694±12 | 699±8 | 711±8 | 691±8 | 674±14 | 704±7 | 691±10 | 679±12 | 700±9 |
| SBP, mmHg |
109.7±1.3 | 107.3±1.7 | 114.8±2.0 | 106.8±1.8 | 108.5±1.8 | 115.0±1.0 | 104.4±1.5 | 105.1±1.1 | 109.6±2.2 | 105.6±3.2 | 106.3±1.6 | 110.3±1.3 |
HR, heart rate; MI, myocardial infarction; SBP, systolic blood pressure.

Colchicine inhibits heart failure development after myocardial infarction. (A) Heart weight/tibial-length ratio; and (B) wet/dry lung weight ratio in vehicle and colchicine-treated mice at 4 weeks after surgery. (A–C) Sham-vehicle and sham-colchicine, n=6; MI-vehicle and MI-colchicine, n=15. (C) The mRNA expression of myocardium for B-type natriuretic peptide in the sham operation group, and the MI group (non-infarcted area) of vehicle and colchicine-treated mice at 4 weeks after surgery (n=6 per group). Bars represent mean±SEM. §P<0.05 vs. sham-vehicle. *P<0.05 vs. MI-vehicle mice. **P<0.01 vs. MI-vehicle mice.
Serum hs-cTnI levels at 24 h after surgery, which indicate the size of cardiac injury, were increased 35–40 fold in MI mice compared with sham operation mice. The size of cardiac injury assessed by serum levels of hs-cTnI was not significantly different between the MI-vehicle group and MI-colchicine group (vehicle: 75.6 [44.7–113.4], Colchicine: 63.7 [50.8–102.2] ng/mL, P=0.11). However, colchicine inhibited the expansion of the LV scar size as assessed by Masson’s-Trichrome staining compared with vehicle control at 1 week after MI (Figure 3A).

Colchicine attenuates left ventricular remodeling and reserves contractile function after myocardial infarction. (A) Upper: representative images of Masson’s-Trichrome-stained heart sections in vehicle and colchicine-treated mice at 1 week after myocardial infarction (MI). Lower: quantitative analysis of the scar/left ventricular area percentage in vehicle and colchicine-treated mice at 1 week after MI. Scale bar represents 1,000 µm. (B) Left ventricular ejection fraction changes assessed by echocardiogram in the vehicle and colchicine groups up to 4 weeks after surgery. (C) Left ventricular end-diastolic diameter (LVDd) changes and (D) left ventricular end-systolic diameter (LVDs) changes assessed by echocardiogram in the vehicle and colchicine groups up to 4 weeks after surgery. (E) Left ventricular end-diastolic pressure and (F) max dp/dt measured by Millar catheter in vehicle and colchicine-treated mice at 4 weeks after surgery. (A–D) Sham-vehicle and sham-colchicine, n=6; MI-vehicle and MI-colchicine, n=15. Error bars are SEM. (E,F) Sham-vehicle and sham-colchicine, n=5; MI-vehicle and MI-colchicine, n=9. Bars represent mean±SEM. §P<0.05 vs. sham-vehicle, *P<0.05 vs. MI-vehicle mice. **P<0.01 vs. MI-vehicle mice.
The LV ejection fraction (LVEF), LV end diastolic diameter (LVDd), and LV end systolic diameter (LVDs) as assessed by TTE at 24 h after MI were not significantly different between the colchicine group and the vehicle control group (Figure 3B–D). However, colchicine improved LVEF (49.8±2.9 vs. 38.6±2.4%, P<0.01), LVDd (4.2±0.2 vs. 4.7±0.2 mm, P=0.06), and LVDs (3.3±0.2 vs. 3.9±0.2 mm, P<0.05) at 7 days after MI. These benefits of colchicine continued up to at least 4 weeks after MI (Figure 3B–D). We also measured LV pressure at 4 weeks after MI and observed a higher LV end diastolic pressure (LVEDP) and lower dp/dt. Furthermore, colchicine significantly improved LVEDP and dp/dt (Figure 3E,F).
Colchicine Attenuates Acute Inflammation in the Infarcted AreaThe benefit of colchicine for cardiac function and remodeling after MI was observed from 1 week after MI. Therefore, we assessed inflammatory responses in infarcted areas in the acute phase of MI to investigate the changes in mouse heart until 7 days after MI. At 3 days after MI, increased LV tubulin polymerization was observed. Colchicine significantly inhibited LV tubulin polymerization (Figure 4A). Immunohistochemistry experiments revealed that colchicine inhibited the accumulation of Gr-1-positive granulocytes into the infarcted area at 24 h and 3 days after MI (Figure 4B,C). The MPO assay also demonstrated that colchicine inhibited MPO activity in the infarcted area at 24 h after MI (Figure 4D). The number of neutrophil in the blood was not different between the vehicle control group and the colchicine group at 24 h after MI (Figure S1). Colchicine also inhibited the accumulation of Iba1-positive macrophages into the infarcted area at 3 days and 7 days after MI (Figure 4B,C). The M2 macrophage has beneficial effects on tissue repair, suppression of inflammation and activation of fibroblast.20 We assessed the phenotype of infiltrated macrophages in the infarcted area at 7 days after MI by mRNA expression of M1/M2 cytokines in the infarcted tissue (Figure S2). There was not statistically different but colchicine tended to attenuate M1 cytokines (TNF-α, IL-1β and IL-6) and tended to increase M2 cytokines (IL-10 and TGF-β, Figure S3). Both IL-4 and M2 cytokine were not detected. Because the number of differences in M2 cytokines were few, we could not confirm the phenotype of infiltrated macrophages in the infarcted area. However, colchicine significantly attenuated the ratio of TNF-α/IL-10 mRNA expression in the infarcted area at 7 days after MI (Figure S4). These results suggest that colchicine could affect the M1/M2 balance.

Colchicine attenuates acute inflammation in the infarcted area. (A) Upper: representative images of western blot analysis for left ventricular (LV) polymerized tubulin at day 3 after myocardial infarction (MI). Lower: quantitative analysis of western blot for LV polymerized tubulin at day 3 after MI (n=4 per group). (B) Representative images of immunohistochemical staining with Gr-1 and Iba1 of LV cross-sections in vehicle and colchicine-treated mice at days 1, 3, and 7 after MI. Scale bars represent 10 µm (n=6 per group). (C) The number of Gr-1-positive granulocytes (Left) and Iba1-positive macrophages (Right) in the LV infarcted area in vehicle and colchicine-treated mice at days 1, 3, and 7 after MI. White bars represent the vehicle control group, and gray bars represent the colchicine group (n=6 per group). (D) MPO activity in the infarcted area at 24 h after surgery (sham, n=6 per group; MI, n=8 per group). The mRNA expression of myocardium for tumor necrosis factor (TNF)-α (E), interleukin (IL)-1β (F), IL-18 (G), monocyte chemoattractant protein (MCP)-1 (H), C-X-C motif ligand 2 (CXCL2) (I), and matrix metalloproteinases (MMP)-9 (J) in vehicle and colchicine-treated mice at 24 h after surgery (sham, n=6 per group; MI, n=8 per group). Bars represent mean±SEM. (K) The plasma levels of IL-1β at 24 h after surgery (sham, n=9 per group; MI, n=11 per group). §P<0.05 vs. sham-vehicle, *P<0.05 vs. MI-vehicle mice. **P<0.01 vs. MI-vehicle mice.
We also assessed mRNA expression of several cytokines in the infarcted heart at 24 h after MI. Colchicine inhibited the increased mRNA expression of TNF-α, IL-1β, IL-18, MCP-1, and CXCL2 in the infarcted area (Figure 4E–I). Both MMP2 and MMP9 also play important roles in cardiac rupture and remodeling after MI.21–24 We assessed the mRNA expression of MMP2 and MMP9 in infarcted hearts after MI. It was reported that MMP2 and MMP9 show different time-courses for their expression.24 As the previous study reported, the mRNA expression of MMP9 was significantly increased in the infarcted area at 24 h after MI, and colchicine significantly attenuated MMP9 expression in the present study (Figure 4J). The mRNA expression of MMP2 was not increased in the infarcted area at 24 h after MI, which is in concordance with a previously reported study, and the effect of colchicine was not observed at this point (data not shown). However, the mRNA expression of MMP2 was increased in the infarcted area at 7 days after MI; this was not significant, but colchicine tended to attenuate the increased MMP2 expression in the infarcted area at 7 days after MI (Figure S5).
Colchicine Attenuates the Increase of NLRP3 Inflammasome After MIAt 24 h after MI, RT-PCR revealed that the mRNA expressions of NLRP3 inflammasome components (NLRP3, ASC, caspase-1) were increased in the infarcted area, and that these increased expressions were inhibited by colchicine (Figures 4F,G and 5A–C). Colchicine also inhibited increased caspase-1 activity at 24 h after MI (Figure 5D).

Colchicine attenuated the increase of the NLRP3 inflammasome after myocardial infarction. The mRNA expression of the myocardium for nucleotide-binding oligomerization domain-like receptors, pyrin domain-containing 3 (NLRP3) (A), apoptosis speck-like protein containing caspase recruitment domain (ASC) (B), and caspase-1 (C) in vehicle and colchicine-treated mice at 24 h after surgery (sham, n=6 per group; MI, n=8 per group). (D) Caspase-1 activity of the myocardium in the infarcted area in vehicle and colchicine-treated mice at 24 h after surgery (n=8 per group). Bars represent mean±SEM. §P<0.05 vs. sham-vehicle. *P<0.05 vs. MI-vehicle mice. **P<0.01 vs. MI-vehicle mice.
Although the benefit of colchicine in MI has been suggested, the pathophysiological phenomenon following treatment with colchicine has not been directly observed in the infarcted myocardium. This is the first study to demonstrate that short-term colchicine administration in the acute phase of MI improved survival, cardiac function, and LV remodeling during the recovery phase of MI. All cardiac deaths were attributed to cardiac rupture in this study, likewise previous reports from our institute.14,25 The excess acute inflammatory responses and the expression of MMP2 and MMP9 could cause the cardiac rupture.22–24,26 Previous studies have reported that MMP2 and MMP9 show different time-courses for mRNA expression.22–24 Whereas the mRNA expression of MMP2 was not significantly increased at 24 h after MI compared with sham operation mice as previously reported,24 the mRNA expression of MMP9 was significantly increased in the infarcted area at 24 h after MI. Colchicine significantly attenuated the increased mRNA expression of MMP9 in infarcted hearts at 24 h after MI. At 7 days after MI, the mRNA expression of MMP2 was also increased in the infarcted area, as the previous study reported. Colchicine tended to attenuate the increased mRNA expression of MMP2 in the infarcted area at 7 days after MI (Figure S5). We considered that colchicine inhibited the excess early inflammatory response and the expression of MMP2 and MMP9, leading to prevention of the cardiac rupture in the early phase of MI. Colchicine prevented the cardiac dysfunction within 1 week after MI, as the result of the inhibition of excess acute inflammatory response, which could be associated with the inhibition of heart failure development during 4 weeks after MI.
The accumulation of neutrophils, monocytes, and macrophages to the infarcted area expands the infarct size and the LV dimensions.27 Inflammatory cytokines including TNF-α and IL-1β directly or indirectly induce further LV dysfunction and LV expansion.27,28 Both MMP2 and MMP9 are also associated with LV remodeling, expansion and dysfunction, leading to a reduction of survival.21 The inhibition of inflammatory reactions has been proposed as a therapeutic target in AMI. However, several studies using glucocorticosteroids, non-steroidal anti-inflammatory drugs, or cyclosporine have failed to treat patients with AMI.2–4 Previous experimental studies have reported that neutrophil depletion or anti-IL-1 therapy improved LV dysfunction and remodeling after MI.29 However, to date, these therapeutic strategies have not been used as clinical therapies for AMI. Colchicine irreversibly inhibits the polymerization of microtubules by binding to unpolymerized tubulin to prevent mitotic spindle formation.5 Colchicine inhibits chemotaxis, adhesiveness, amoeboid motility, mobilization, and the degranulation of lysosomes of neutrophils, monocytes, and macropahges.5 This study demonstrated that colchicine attenuated the accumulation of neutrophils and macrophages, as well as and pro-inflammatory cytokines and chemokines in the infarcted area. This study is not enough to assess the systemic effects of colchicine (e.g., bone marrows, spleen or circulating blood cells), and the mechanism of the reduced number of Gr-1 positive cells and macrophages. A previous study reported that colchicine attenuated the number of neutrophils in the blood on the dog MI model.30 However, the number of neutrophils in the blood was not different between the vehicle control group and the colchicine group at 24 h after MI in this study (Figure S1). It has been demonstrated that IL-1β enhanced hematopoietic stem cell proliferation in bone marrows by both direct actions on hematopoietic cells and through modulating the hematopoietic micro-environment in bone marrows, and that anti-IL-1β treatment decreases leukocyte numbers in the blood and the infarcted tissue.29 In this study, plasma IL-1β levels assessed by ELISA were not significantly different between the vehicle and colchicine group (Figure 4K), although colchicine significantly decreased mRNA levels of IL-1β in the infarct area at 24 h after MI. These results suggest colchicine would affect more locally. Furthermore, colchicine inhibited the increase of MMP2 and MMP9 in the early phase after MI and reduced scar size 1 week after MI. These effects of colchicine might improve survival and heart failure by preventing adverse cardiac remodeling and LV dysfunction in the chronic phase of MI.
Recent studies reported the short-term benefit of colchicine after AMI by reducing inflammatory cytokines and infarction size, as detected by biomarkers and magnetic resonance imaging.12,13 In this study, colchicine tended to reduce the serum level of hs-cTnI at 24 h after MI, but this was not significantly different compared with the vehicle group. A previous study using a canine ischemia and reperfusion injury model concluded no myocardial protective effect of colchicine was observed, despite a reduction in neutrophil reactive oxygen species and accumulation in the myocardium.30 Although the animal species used and/or the differential induction of MI experimental models by permanent ligation or ischemia/reperfusion might cause slight different results, colchicine administration in the acute phase of MI could be expected to be a cardiac protective strategy in the chronic phase of MI.
This study demonstrated that colchicine inhibited excessive tubulin polymerization in the infarcted area. Colchicine not only inhibited the accumulation of inflammatory cells, and the increase of pro-inflammatory cytokines and chemokines, but it also attenuated the increased NLRP3 inflammasome components (NLRP3, ASC, caspase-1) after MI. Recent studies demonstrated that tubulin polymerization plays a crucial role in inflammasome activation,31 and that the activation of NLRP3 inflammasome is associated with ischemia/reperfusion injury and cardiac remodeling after MI.9,10 These studies support our results. Inhibiting activation of the NLRP3 inflammasome is expected to be a therapeutic target of MI. Several compounds that inhibit the NLRP3 inflammasome have been described; however, these have not been used for clinical applications to date.32 Colchicine has been used for more than 2,000 years, and long-term colchicine administration is a well-tolerated therapy for Familial Mediterranean Fever. Therefore, colchicine might also be beneficial for the treatment of MI.
The LoDoCo trial reported that long-term colchicine administration prevented cardiovascular events, mainly acute coronary and nonstent-related events in patients with stable coronary disease33 The chronic effects of colchicine are unclear in patients with AMI. If short-term colchicine administration in patients with AMI, even after onset, inhibits acute inflammatory responses and NLRP3 inflammasome activation, leading to improved survival, heart failure, and cardiac dysfunction in the chronic phase, this would be a significant strategy for AMI. We need to investigate the acute and chronic effects of colchicine in patients with MI in a large clinical trial.
Study LimitationsFirst, this study could not determine the additional effects of drugs such as β-blockers, angiotensin-converting enzyme inhibitors, or angiotensin-II receptor blockers, which are standard medications after MI. Second, the permanent coronary ligation mouse model we used in this study is different from the ischemia/reperfusion model. Therefore, further investigation to confirm the benefit of colchicine in the setting of ischemia/reperfusion myocardial injury is required. Third, although evaluating the effect of colchicine was only conducted in a short-term treatment study (7 days), evaluating the effect of colchicine in long-term treatment or only in late phase treatment would be important to clarify the effective targets of colchicine. Fourth, Ly6C high monocyte has been reported to cause adverse cardiac healing after MI.34 However, we could not assess the relation between Ly6C monocytes and cardiac remodeling after MI in the present study. Fifth, we could not confirm the phenotype of infiltrated macrophages in the infarcted area, which relate to cardiac remodeling. Further experiments are required. Sixth, we showed colchicine inhibited IL-1β and NLRP3 inflammasome in the infarcted heart in the early phase of MI, and that resulted in reduced cardiac remodeling. However, it still remains unclear whether decreasing the IL-1β level and NLRP3 inflammasome after colchicine treatment directly inhibited the adverse cardiac remodeling. Additional experiments using NLRP3 knock out mice or IL-1β knock out mice are required.
Short-term treatment with colchicine after the onset of MI successfully attenuated the infiltration of inflammatory cells, the increasing level of cytokines, and NLRP3 inflammasome in the infarcted myocardium, and improved adverse cardiac remodeling, cardiac function, heart failure development, and survival during the recovery phase of MI.
We thank Ms. Megumi Nagahiro, Ms. Saeko Tokunaga, and Ms. Ayuko Tateishi in our department, and Mr. Takenobu Nakagawa at the Department of Cell Pathology, Kumamoto University, for their excellent technical assistance during the study.
All authors declare that they have no conflicts of interest.
This study was supported, in part, by a Grant-in-Aid for Scientific Research (No. C25461086 for S.S.) from the Ministry of Education, Culture, Sports, Science, and Technology in Japan.
Supplementary File 1
Figure S1. Number of neutrophils in the blood at 24 h after surgery.
Figure S2. Results of M1 macrophage-derived cytokines in the infarct area at 7 days after myocardial infarction.
Figure S3. Results of M2 macrophage-derived cytokines in the infarct area at 7 days after myocardial infarction.
Figure S4. Ratio of tumor necrosis factor (TNF)-α/interleukin (IL)-10 mRNA expression in the infarcted area at 7 days after myocardial infarction.
Figure S5. mRNA expression of the myocardium for matrix metalloproteinase (MMP) 2 at 7 days after surgery.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0949