2018 Volume 82 Issue 4 Pages 999-1007
2018 Volume 82 Issue 4 Pages 999-1007
Background: Oral administration of tolvaptan, a vasopressin V2 receptor antagonist, significantly reduces deterioration of renal function, which has recently been highlighted as an exacerbating factor for adverse events in patients with acute heart failure. In the present study we tested the hypothesis that concomitant administration of tolvaptan with a conventional diuretic is beneficial for perioperative body fluid management in patients who have undergone cardiac surgery.
Methods and Results: In all, 280 patients who underwent cardiac surgery were prospectively randomized to concomitant treatment with tolvaptan and a conventional diuretic (tolvaptan group; 147 patients) or treatment with a conventional diuretic alone (control group; 133 patients). Groups were compared in terms of the time required to restore preoperative body weight and the incidence of worsening renal function (WRF), defined as an increase in the serum creatinine level ≥0.3 mg/dL. The time required to restore preoperative body weight was significantly shorter in the tolvaptan than control group (mean [±SD] 3.97±1.95 vs. 5.02±2.83 days, respectively; P<0.001). The incidence of WRF was significantly lower in the tolvaptan than control group (n=11 [7.5%] vs. n=25 [18.8%], respectively; P=0.011).
Conclusions: Administration of tolvaptan with conventional diuretics in the early postoperative period after cardiac surgery could be beneficial in maintaining urine output without affecting renal function and may thus help avoid WRF.
Maintaining adequate body fluid levels is an extremely essential component in the treatment of patients who have undergone cardiac surgery. Fluid accumulation in the body often occurs after cardiac surgery due to surgical stress and the use of extracorporeal circulation, and could lead to congestive heart failure. Moreover, Hobson et al reported that acute renal failure increases the mortality and morbidity of patients after cardiac surgery.1 Thus, preventing acute renal dysfunction in the postoperative period following cardiac surgery is crucial.
Loop diuretics have been widely used for the management of body fluid balance; however, several side effects have been reported, such as exacerbation of renal function or hypotension, and the excess use of diuretics often results in worsening renal function (WRF), defined as an increase in serum creatinine levels ≥0.3 mg/dL, or >50%, relative to baseline values. Tolvaptan is an aquaretic that promotes the excretion of free water only from the body by inhibiting vasopressin V2 receptors. This unique mechanism of action of tolvaptan does not affect renal function or hemodynamics.2
Recent studies have shown that WRF prolongs the hospital stay and exacerbates the long-term prognosis of patients with heart failure.3,4 Matsue et al reported that tolvaptan significantly reduces the incidence of WRF in patients with acute heart failure.5 Thus, we hypothesized that oral administration of tolvaptan in the early postoperative period of cardiac surgery may be useful for body fluid management and prevention of renal dysfunction. In addition, the efficacy and safety of tolvaptan after cardiac surgery remain unclear. Thus, the aim of the present study was to investigate the efficacy and safety of tolvaptan administration in the early postoperative period following cardiac surgery.
The present study was a prospective single-center randomized open-label study of patients who underwent cardiac surgery with cardiopulmonary bypass. Because our primary concern was postoperative renal function, patients undergoing surgery accompanied by renal artery reconstruction and the placement of a cardiac assist device were excluded from the study. Patients were randomized to concomitant treatment with tolvaptan and a conventional diuretic (tolvaptan group) or treatment with a conventional diuretic alone (control group). The present study was performed in accordance with the principles outlined in the Declaration of Helsinki, and was approved by the Ethics Committee of Tottori University Faculty of Medicine. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (ID: UMIN000025594). All enrolled patients provided written informed consent.
PatientsAdult patients who were undergoing cardiac surgery with cardiopulmonary bypass were enrolled in the present study. Specifically, 324 consecutive patients who underwent open cardiac surgery at Tottori University Hospital between July 2013 and August 2016 were enrolled. Twenty patients who underwent off-pump coronary artery bypass grafting, left ventricular assist device implantation, and thoracoabdominal aorta replacement were excluded. Preoperative and postoperative exclusion criteria were as follows: (1) under dialysis treatment preoperatively; (2) presence of renal dysfunction (serum creatinine concentration ≥3.0 mg/dL); and (3) unable to take oral medication on postoperative day (POD) 1. Another 16 patients who were undergoing dialysis preoperatively or who had renal dysfunction with a serum creatinine concentration ≥3.0 mg/dL were also excluded, as were 8 patients who were unable to take oral medication on POD1. Consequently, 280 patients were randomized to 2 groups: the tolvaptan group (n=147 patients) and the control group (n=133 patients; Figure 1A).
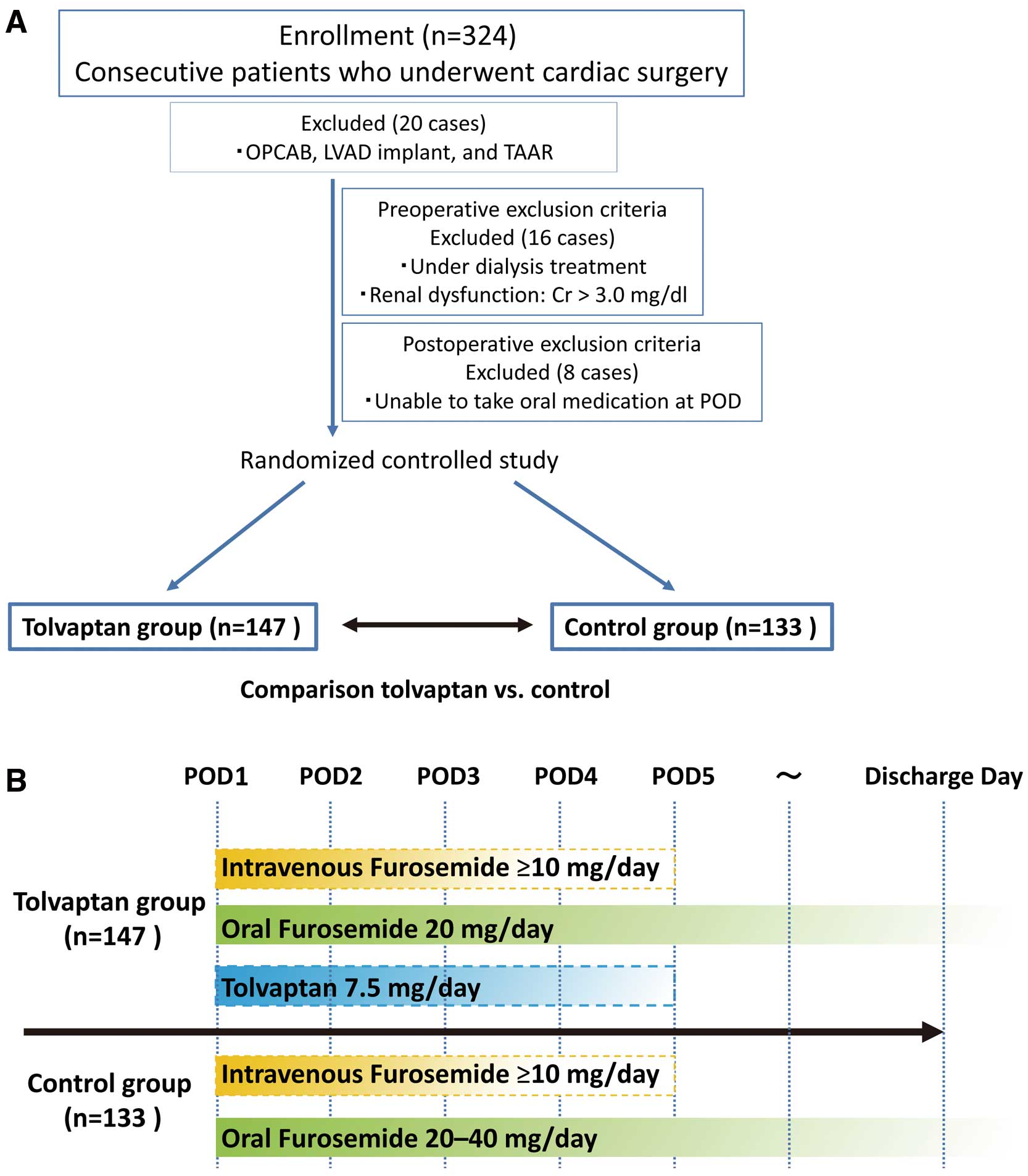
(A) Patient enrollment. In all, 324 consecutive patients who underwent open cardiac surgery with cardiopulmonary bypass between 2013 and 2016 were enrolled in the present study; 20 patients who underwent off-pump coronary artery bypass (OPCAB), left ventricular assist device (LVAD) implantation, or thoracoabdominal aorta replacement (TAAR) were excluded, as were 16 patients who were undergoing dialysis preoperatively or had renal dysfunction (serum creatinine [Cr] ≥3.0 mg/dL) and 8 patients who were unable to take oral medication. Finally, 280 patients were randomized to 2 groups: a tolvaptan group (n=147) and the control group (n=133). POD, postoperative day. (B) Study protocol. In the tolvaptan group, tolvaptan was administered orally (7.5 mg/day) from POD1 until the time when body weight returned to the preoperative level or POD5. Furosemide was administered orally (20 mg/day) concomitantly, and was administered intravenously as necessary. In the control group, furosemide was administered orally (20–40 mg/day) from POD1, and was administered intravenously as necessary. In both groups, the dose of intravenous furosemide and proper duration of oral furosemide administration were determined by individual physicians based on postoperative urine output.
In the tolvaptan group, tolvaptan was administered orally at a daily dose of 7.5 mg from POD1 until the time at which the body weight returned to the preoperative level or until POD5. To prevent hypernatremia, tolvaptan administration was discontinued when the serum sodium concentration was ≥147 mEq/L. In the tolvaptan group, furosemide (20 mg/day) was administered orally as a concomitant medication. When necessary, furosemide was administered intravenously as well until the time at which the body weight returned to the preoperative level or until POD5. Similarly, in the control group, furosemide (20–40 mg/day) was administered orally from POD1, and was administered intravenously as necessary until the time at which body weight returned to the preoperative level or until POD5. In both groups, the dose of intravenous furosemide was determined by individual physicians based on postoperative urine output. The appropriate duration of oral furosemide administration was also determined by individual physicians; when necessary, oral furosemide was administered until the day of discharge. A standard was established for starting intravenous furosemide administration or increasing the dose of furosemide, namely when urine output was <0.5 mL·kg−1·h−1). Individual physicians determined the starting dose/dosage of administration. In the present study, only furosemide was used as the conventional diuretic; no other diuretics, such as human atrial natriuretic peptide (hANP) and spironolactone, were used. The study design is shown in Figure 1B.
The primary study endpoint was the incidence of WRF, and the secondary endpoint was body fluid management in the early postoperative period. Specifically, we examined urine output, the time required to restore preoperative body weight, the total amount of furosemide administered, maximum serum creatinine concentrations, rate of increase in creatinine concentrations, changes in the serum sodium and potassium concentrations, and incidence of WRF. In addition, we examined the factors associated with WRF, defined as an increase in the serum creatinine concentration ≥0.3 mg/dL relative to the preoperative value at any given day until POD5. WRF was classified into Stage 1, 2, and 3 acute kidney injury (AKI), according to the Kidney Disease: Improving Global Outcomes clinical practice guideline for AKI.6 Stage 1 AKI was defined as an increase in serum creatinine concentrations ≥0.3 mg/dL or a ≥1.5- to 2-fold increase from the baseline value; Stage 2 AKI was defined as a >2- to 3-fold increase from baseline values; and Stage 3 AKI was defined as a >3-fold increase from baseline values or absolute serum creatinine level ≥4.0 mg/dL, with an acute increase of at least 0.5 mg/dL, or on renal replacement therapy.
Perioperative ManagementFollowing surgery, patients were admitted to the intensive care unit (ICU) where various hemodynamic functions (i.e., systemic arterial pressure, pulmonary arterial pressure, cardiac index, central venous pressure) and hourly urine volume were monitored. Subsequently, patients were transferred to a monitored intermediate care unit. The postoperative care was performed according to established standards of care for cardiac surgery patients. The ICU discharge criteria were as follows: cooperative patient, SpO2 >90% with an FiO2 <0.5, no ventricular arrhythmias, no mechanical circulatory support, urine output >0.5 mL·kg−1·h−1, and no signs of ischemia on electrocardiography, which were all to be achieved within 3 successive hours. A standard was established for the administration of intravenous potassium at a dose of 20 mEq per event, specifically when serum potassium concentrations were <3.7 mEq/L, from POD1 until POD3. In the ward, blood pressure, heart rate, and body weight were checked each day. Chest radiographs and blood samples were also obtained daily for 1 week after surgery.
Statistical AnalysisAll numerical data are shown as the mean±SD. All data were analyzed using PASW Statistics ver. 24 (IBM SPSS, Chicago, IL, USA). Unpaired t-tests were used for comparisons of continuous variables, and Fisher’s exact test was used for comparisons of frequencies between groups. The distribution of ICU stay and postoperative hospital stay was not normal; therefore, the Wilcoxon signed-rank test with Bonferroni adjustment, a non-parametric method, was used to examine these variables. Kaplan-Meier curves and the log-rank test were used to compare endpoints between groups. Two-way analysis of variance (ANOVA) for repeated measures, followed by Tukey’s post hoc test, was used to evaluate changes in serum sodium and potassium concentrations. Univariate logistic regression analysis was performed with all variables to identify potential risk factors for WRF incidence, followed by multivariate logistic regression with the univariate predictors satisfying an entry criterion of P<0.05. In all analyses, P<0.05 was considered statistically significant.
There were no significant group differences in age, sex, preoperative New York Heart Association class, the number of patients who received preoperative oral diuretic therapy, preoperative cardiac index, left ventricular ejection fraction, creatinine concentrations, estimated glomerular filtration rate, European system for cardiac operative risk evaluation (EuroSCORE) II score, the types of surgical procedures, the duration of surgery, cardiopulmonary bypass time, or bleeding volume (Table 1).
| Tolvaptan group | Control group | P value | |
|---|---|---|---|
| Age (years) | 70.8±11.4 | 69.5±12.2 | 0.330 |
| Age ≥75 years | 66 (45) | 53 (40) | 0.393 |
| No. males/females | 91/56 | 74/59 | 0.287 |
| Body height (m) | 1.60±0.09 | 1.59±0.10 | 0.474 |
| BW (kg) | 58.3±10.3 | 58.7±11.8 | 0.751 |
| BSA (m2) | 1.60±0.17 | 1.60±0.18 | 0.920 |
| Hypertension | 86 (59) | 80 (60) | 0.164 |
| Diabetes | 31 (21) | 25 (19) | 0.174 |
| Hyperlipidemia | 66 (45) | 55 (41) | 0.170 |
| COPD | 46 (31) | 39 (29) | 0.182 |
| Cerebral infarction | 25 (17) | 24 (18) | 0.178 |
| AF | 32 (22) | 27 (20) | 0.183 |
| Preoperative NYHA class | 1.96±0.56 | 1.94±0.72 | 0.794 |
| Preoperative NYHA class ≥3 | 13 (9) | 17 (13) | 0.310 |
| Diuretics | 53 (36) | 47 (35) | 0.187 |
| β-blockers | 35 (24) | 37 (28) | 0.127 |
| Preoperative renal function | |||
| Cr (mg/dL) | 0.89±0.36 | 0.88±0.52 | 0.738 |
| BUN (mg/dL) | 18.8±7.6 | 19.6±11.1 | 0.479 |
| eGFR (mL/min/1.73 m2) | 66.7±21.6 | 69.3±23.7 | 0.333 |
| Preoperative hemodynamics | |||
| Heart rate (beats/min) | 70.7±12.6 | 70.8±11.7 | 0.951 |
| SBP (mmHg) | 122.4±11.7 | 120.4±17.9 | 0.340 |
| DBP (mmHg) | 69.0±13.7 | 67.2±15.4 | 0.313 |
| PAP (mmHg) | 35.4±11.3 | 34.7±11.3 | 0.704 |
| PCWP (mmHg) | 16.8±7.3 | 16.1±6.8 | 0.532 |
| CVP (mmHg) | 9.8±4.6 | 8.7±3.8 | 0.097 |
| CI (L·min−1·m−2) | 2.9±0.6 | 2.8±0.6 | 0.207 |
| Preoperative echocardiographic data | |||
| LAD (mm) | 41.5±7.9 | 42.0±8.9 | 0.648 |
| LVDd (mm) | 48.1±7.9 | 49.1±8.9 | 0.339 |
| LVDs (mm) | 31.2±8.4 | 31.9±8.7 | 0.501 |
| EF (%) | 62.9±10.5 | 62.5±10.1 | 0.763 |
| EF <50% | 19 (13) | 15 (12) | 0.744 |
| Surgical procedure | |||
| AVR | 49 (33.3) | 48 (36.1) | 0.628 |
| MVR | 5 (3.4) | 5 (3.8) | 0.872 |
| DVR | 0 (0) | 2 (1.5) | 0.136 |
| MVP | 19 (12.9) | 26 (19.5) | 0.145 |
| CABG | 35 (23.8) | 23 (17.3) | 0.179 |
| ASD closure | 3 (2.0) | 4 (3.0) | 0.605 |
| AAR or TAR | 26 (17.7) | 19 (14.3) | 0.439 |
| Bentall or David | 6 (4.2) | 5 (3.8) | 0.890 |
| LA myxoma resection | 4 (2.7) | 1 (0.8) | 0.214 |
| Concomitant procedure | |||
| AVR | 0 (0) | 1 (0.8) | 0.475 |
| MAP | 2 (1.4) | 0 (0) | 0.499 |
| TAP | 6 (4.2) | 13 (9.8) | 0.094 |
| Maze or PVI | 17 (11.6) | 24 (18.0) | 0.132 |
| CABG | 9 (6.1) | 8 (6.0) | 1.000 |
| AAR or TAR | 3 (2.0) | 9 (6.8) | 0.074 |
| EUROSCORE II | 3.88±3.59 | 4.45±4.11 | 0.219 |
| EuroSCORE II ≥10 | 13 (8.8) | 14 (10.5) | 0.634 |
| Surgical data | |||
| Surgical time (min) | 273±83 | 283±103 | 0.354 |
| CPB time (min) | 135±41 | 141±46 | 0.206 |
| Bleeding volume (mL) | 251±189 | 228±133 | 0.262 |
Unless indicated otherwise, data are given as the mean±SD or as n (%). AAR, ascending aorta replacement; AF, atrial fibrillation; ASD, atrial septal defect; AVR, aortic valve replacement; BSA, body surface area; BUN, blood urea nitrogen; BW, body weight; CABG, coronary artery bypass grafting; CI, cardiac index; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; Cr, creatinine; CVP, central venous pressure; DBP, diastolic blood pressure; DVR, double valve replacement; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EuroSCORE, European system for cardiac operative risk evaluation; LA, left atrium; LAD, left atrium diameter; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; MAP, mitral annuloplasty; MVP, mitral valve plasty; MVR, mitral valve replacement; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVI, pulmonary vein isolation; SBP, systolic blood pressure; TAP, tricuspid annuloplasty; TAR, total arch replacement.
Operative mortality was 0% in both groups. In the tolvaptan group, the total amount of tolvaptan administered to each patient was 22.04±6.70 mg. No significant group differences were noted in comorbidities (e.g., those requiring thoracentesis, reintubation or mechanical circulatory support with intra-aortic balloon pumping), postoperative hospital stay, or the number of patients who received oral diuretics at discharge. With regard to oxygenation, FiO2 at POD3 and the time taken to stop oxygen administration were significantly lower in the tolvaptan than control group (P=0.009 and P=0.018, respectively). The ICU stay was significantly shorter and significantly fewer patients required transfer to a rehabilitation facility in the tolvaptan compared with control group (Table 2).
| Tolvaptan group | Control group | P value | |
|---|---|---|---|
| 30-day operative mortality | 0 (0) | 0 (0) | 1.000 |
| Thoracentesis | 7 (4.8) | 7 (5.3) | 0.848 |
| Reintubation | 1 (0.7) | 5 (3.8) | 0.133 |
| Postoperative MCS | 7 (4.8) | 5 (3.8) | 0.773 |
| Duration of MCS (h) | 68.6±35.1 | 67.2±20.1 | 0.934 |
| ICU stay (days) | 2.59±1.08 | 2.96±1.96 | 0.044 |
| FiO2 at POD3 | 0.29±0.10 | 0.32±0.11 | 0.009 |
| Time to stop oxygen (days) | 3.5±2.5 | 4.4±3.8 | 0.018 |
| Total amount of tolvaptan (mg) | 22.04±6.70 | – | – |
| Total amount of furosemide at POD3 | |||
| Oral furosemide (mg) | 51.2±15.6 | 70.7±21.1 | <0.001 |
| Intravenous furosemide (mg) | 63.4±60.4 | 81.5±62.3 | 0.014 |
| Intravenous potassium administration | 88 (60) | 85 (64) | 0.539 |
| Total intravenous potassium at POD3 (mEq) | 24.5±32.4 | 28.6±36.1 | 0.319 |
| Dopamine administration | 74 (50) | 81 (61) | 0.092 |
| Total amount of dopamine at POD3 (mg) | 119.6±214.8 | 188.4±336.2 | 0.045 |
| Dobutamine administration | 24 (16) | 33 (25) | 0.102 |
| Total amount of dobutamine at POD3 (mg) | 32.8±104.4 | 66.8±188.7 | 0.067 |
| Time to preoperative BW (days) | 3.97±1.95 | 5.02±2.83 | <0.001 |
| Postoperative renal function | |||
| Maximum Cr (mg/dL) | 1.05±0.55 | 1.09±0.74 | 0.582 |
| Rate of Cr increase (%) | 115.6±25.3 | 122.7±31.2 | 0.045 |
| Minimum eGFR (mL/min/1.73 m2) | 59.3±22.4 | 58.9±24.0 | 0.906 |
| AKI staging | |||
| Stage 1 | 6 (4.1) | 17 (12.8) | 0.009 |
| Stage 2 | 4 (2.7) | 7 (5.3) | 0.360 |
| Stage 3 | 1 (0.7) | 1 (0.8) | 1.000 |
| Diuretics at discharge | 50 (34) | 52 (39) | 0.377 |
| Postoperative hospital stay (days) | 20.8±7.8 | 22.4 ±11.5 | 0.166 |
| Transfer to rehabilitation facility | 12 (8.2) | 22 (16.5) | 0.032 |
Data are given as the mean±SD or as n (%). AKI, acute kidney injury; FiO2, fraction of inspired oxygen; ICU, intensive care unit; MCS, mechanical circulatory support; POD, postoperative day. Other abbreviations as in Table 1.
The mean cumulative urine output from POD0 to POD3 was 2,496±547 and 2,243±549 mL in the tolvaptan and control groups, respectively (P<0.001). Urine output tended to be higher in the tolvaptan than control group on POD1 (although the difference was not significant), but was significantly higher in the tolvaptan than control group on POD2 and POD3 (Figure 2A). The time required to restore preoperative body weight in the tolvaptan and control groups was 3.97±1.95 and 5.02±2.83 days, respectively (P<0.001; Table 2). The log-rank test using the Kaplan-Meier method also showed a significant difference between the 2 groups with regard to the number of days to return to preoperative body weight (Figure 2B).

(A) Total urine output. On postoperative day (POD) 1 urine output on tended to be higher in the tolvaptan (TV) than control (C) group, although the difference was not statistically significant. However, on POD2 and POD3, the difference in urine output between the 2 groups was significant (P=0.003 and P=0.034, respectively). The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. (B) Results of a log-rank test using the Kaplan-Meier method also showing a significant difference between the TV and C groups with regard to the number of days to return to preoperative body weight.
Figure 3A shows the distribution of the duration of tolvaptan administration. The mean duration of tolvaptan administration was 2.96±0.86 days, and no patients were receiving tolvaptan after POD5. At POD3, the total amount of oral and intravenous furosemide administered was significantly lower in the tolvaptan than control group (P<0.001 and P=0.014, respectively; Table 2; Figure 3B). Furthermore, at POD3, the total amount of postoperative dopamine was significantly lower in the tolvaptan than control group (P=0.045; Table 2).

(A) Duration of tolvaptan administration. The mean (±SD) duration of tolvaptan administration was 2.96±0.86 days. (B) Total amount of furosemide administered over 3 days postoperatively in the tolvaptan (TV) and control (C) groups. On postoperative day 3, the total amount of furosemide administered was significantly lower in the TV than C group (P<0.001). The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range.
Serum sodium concentrations from POD2 to POD5 were significantly lower in the control than tolvaptan group (Figure 4A). However, there were no significant group differences in serum potassium concentrations (Figure 4B). After tolvaptan administration, the serum sodium concentration in 5 patients increased to ≥147 mEq/L; thus, tolvaptan administration was discontinued. The following day, sodium concentrations were ≤147 mEq/L in all 5 patients. Hence, no patient in the tolvaptan group showed clinically significant hypernatremia (i.e., sodium >149 mEq/L).

(A) Serial changes in the serum sodium levels in the tolvaptan (TV) and control (C) groups. Serum sodium concentrations from postoperative day (POD) 2 to POD5 were significantly lower in the C than TV group. (B) Serial changes in the serum potassium concentrations. There were no significant differences in serum potassium levels between the TV and C groups. Data are the mean±SD. *P<0.05, †P<0.001 compared with the C group.
The maximum creatinine level in the tolvaptan group was lower than in the control group (although the difference was not significant). The rate of increase in the creatinine concentration was significantly lower in the tolvaptan than control group (P=0.045; Table 2). In addition, the incidence of WRF was significantly lower in the tolvaptan than control group (10 patients [7.5%] vs. 25 patients [18.8%]; P=0.011; Figure 5). The occurrence of Stage 1 AKI was significantly lower in the tolvaptan than control group (P=0.009), but there was no significant difference in the occurrence of Stage 2 and 3 AKI between the 2 groups (P=0.360 and P=1.000, respectively; Table 2).

Incidence of worsening renal function (WRF) in the tolvaptan (TV) and control (C) groups. The incidence of WRF was significantly lower in TV than C group (7.5% vs. 18.8%, respectively; P=0.011). *P<0.05.
The results of univariate and multivariate analyses of factors associated with the incidence of WRF are given in Table 3. The absence of tolvaptan treatment, NYHA class ≥3, preoperative atrial fibrillation, chronic obstructive pulmonary disease, and preoperative creatinine concentration were significantly associated with an increased incidence of WRF.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Age >75 (years) | 0.008 | 2.74 (1.3–5.8) | 0.245 | 2.22 (0.6–8.5) |
| BSA (m2) | 0.704 | 1.49 (0.2–11.4) | – | – |
| Male | 0.173 | 0.59 (0.3–1.3) | – | – |
| COPD | 0.008 | 2.63 (1.3–5.4) | 0.017 | 4.87 (1.3–17.8) |
| Diabetes | 0.460 | 1.36 (0.6–3.1) | – | – |
| Preoperative AF | <0.001 | 4.30 (2.1–9.0) | 0.010 | 6.14 (1.6–24.3) |
| NYHA class >3 | <0.001 | 7.33 (3.0–18.1) | 0.045 | 3.96 (1.0–15.2) |
| Preoperative EF <50% | 0.356 | 1.57 (0.6–4.1) | – | – |
| Preoperative Cr (mg/dL) | <0.001 | 32.05 (9.9–103.5) | <0.001 | 39.3 (7.7–199.9) |
| EuroSCORE II >10 | <0.001 | 9.29 (3.9–22.1) | 0.676 | 1.40 (0.3–6.8) |
| CPB time | 0.014 | 1.01 (1.0–1.02) | 0.779 | 1.00 (0.99–1.02) |
| No tolvaptan treatment | 0.006 | 2.86 (1.3–6.1) | 0.047 | 4.00 (1.2–15.6) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio. Other abbreviations as in Table 1.
In the present study, tolvaptan was found to effectively reduce the amount of furosemide administered while maintaining urine output and renal function and was able to prevent WRF. These results suggest that tolvaptan is especially beneficial for postoperative fluid management in patients who have undergone cardiac surgery with cardiopulmonary bypass.
As reported previously, WRF is one of the most significant adverse effects of diuretics for body fluid management in patients with acute heart failure because it prolongs the hospital stay and exacerbates the long-term prognosis.3,4,7 Furthermore, renal dysfunction is considered to be associated with a poor prognosis in patients who have undergone cardiac surgery.1 Lassnigg et al reported that serum creatinine changes predict the prognosis of patients after cardiothoracic surgery,8 and Dini et al observed that a high dose of loop diuretics worsened the survival of ambulatory patients with chronic heart failure.9 Moreover, several studies have shown that tolvaptan improves the mid-term prognosis of patients with acute congestive heart failure, prevents acute renal insufficiency, and reduces the amount of furosemide administered.10–12 In the present study, tolvaptan reduced the amount of furosemide administered while maintaining sufficient urine output and reducing the incidence of WRF. Thus, tolvaptan administration during the early phase after cardiac surgery may improve the mid- to long-term prognosis of patients. Further studies are needed to confirm the long-term outcomes in patients receiving tolvaptan treatment.
The rate of postoperative creatinine level increase was significantly lower in the tolvaptan than control group in the present study. This result is consistent with previous studies showing that tolvaptan, compared with conventional therapy, prevents WRF in patients with acute heart failure,5,13 which could be attributed to tolvaptan acting as a diuretic, while maintaining renal blood flow without activating the renin-angiotensin-aldosterone system. In addition, the use of tolvaptan can reduce the amount of furosemide administered, which is also a kidney-protecting effect of tolvaptan. Therefore, tolvaptan has the potential to preserve renal function. Furthermore, Yamada et al reported that tolvaptan could be safely used in patients with chronic kidney disease undergoing open heart surgery, providing adequate urine volume without leading to a deterioration of renal function.14 In addition, Yamada et al14 reported that the renal protective effect appeared to become stronger as the stage of chronic kidney disease increased. Matsue et al reported that adding tolvaptan to a conventional treatment achieves greater diuresis and better relieves dyspnea symptoms in patients with acute heart failure and renal dysfunction;15 however, they also reported that the rate of WRF was similar between the tolvaptan and conventional treatment groups. The results of the present multivariate analysis indicated that the preoperative creatinine concentration was significantly associated with WRF. Thus, further studies are needed to fully demonstrate the efficacy of tolvaptan in patients with chronic renal dysfunction. We are currently conducting a prospective study on patients with preoperative renal dysfunction to clarify the efficacy of tolvaptan according to each stage of chronic kidney disease.
Hypernatremia is a well-known adverse effect of tolvaptan.16 In the present study, no clinically significant hypernatremia was induced after tolvaptan administration, which could be due to the low starting dose. Furthermore, all patients in the tolvaptan group received a concomitant loop diuretic, which often causes hyponatremia, and this may have contributed to the prevention of clinically significant hypernatremia. Consistent with hyponatremia being a side effect of loop diuretics, in the control group the sodium concentration decreased at POD4. Hyponatremia during the perioperative period renders appropriate body fluid management difficult.
Several studies have assessed the efficacy of tolvaptan after cardiac surgery.14,17–19 Nishi et al reported that tolvaptan was effective in treating fluid retention, without inducing renal failure or abnormal electrolyte levels, during the postoperative stage following cardiac surgery.17 Furthermore, Kato et al reported that tolvaptan administration in patients undergoing cardiac surgery improves renal perfusion.18 The results of the present study are consistent with these previous findings. However, few studies have clarified the efficacy of tolvaptan in patients who have undergone open heart surgery using a prospective randomized design.19 To the best of our knowledge, the present prospective randomized study on the efficacy of tolvaptan in patients undergoing cardiac surgery uses the largest patient population to date. Thus, we believe that the present study provides important evidence, further clarifying the efficacy of tolvaptan with regard to its effectiveness and safety for the management of body fluid levels in patients during the early postoperative period of cardiac surgery.
Slight et al reported that patient body weight approximated its preoperative value at POD5 following cardiac surgery.20 Therefore, in the present study tolvaptan was administered for only 5 days postoperatively to avoid the development of extreme dehydration, hypernatremia, and renal dysfunction.
It is obvious that patients with higher volume overload tend to retain greater fluid levels. Therefore, urine output is dependent on the extent of fluid retention before and during surgery. Excessive fluid retention can lead to a deterioration in respiratory status, prolong the duration of oxygenation, and oppose early ambulation. Tolvaptan administration is known to induce the instantaneous osmotic movement of extravascular fluid into intravascular compartments.21 In the present study, tolvaptan administration shortened the time required to restore preoperative body weight, as well as the time taken to stop oxygenation, compared with conventional treatment. Early fluid removal due to tolvaptan administration may contribute to improve pulmonary congestion during early the postoperative period of cardiac surgery, which may be the reason for the shortened ICU stay of patients administered tolvaptan in the present study. In addition, earlier body weight restoration to preoperative levels and improvement in respiratory function could contribute to early ambulation. Thus, tolvaptan administration may contribute to a shorter hospital stay. However, the use of tolvaptan did not lead to a significantly shorter hospital stay in the present study. This may be due to other factors, such as the surgical procedures, postoperative recovery, and conditions of patient care by family members, which were not assessed in the present study. However, early ambulation following tolvaptan administration may have reduced the number of patients who were transferred to a rehabilitation facility.
Endogenous arginine vasopressin levels have been reported to increase markedly (by a factor of 5–10) during and after cardiac surgery, but decrease gradually within a few days.22,23 Thus, we expected that the effect of tolvaptan would be minimal at POD1. Although no significant difference in urine output was noted at POD1, tolvaptan administration at POD1 was beneficial in terms of protection of renal function after cardiac surgery, as evidenced by the adequate average urine output in the tolvaptan group compared with that in the control group, and the reduced doses of furosemide on POD1.
Study LimitationsThe present study has some limitations. First, the study was a prospective randomized study with a small patient population from a single institution, which may have resulted in selection bias. Second, the furosemide dose was determined by individual physicians who were not blinded to the treatment arm; therefore, a bias in fluid removal therapy after the operation is possible. In addition, some factors reported to predict responses to tolvaptan, such as osmolality and urinary aquaporin 2 levels, were not investigated.24 Furthermore, there may be some patients who would have benefited from taking tolvaptan for more than 5 days. Finally, the long-term efficacy of tolvaptan treatment has not been examined. Further studies are needed to confirm the long-term outcomes in patients receiving tolvaptan treatment, especially after cardiac surgery.
Tolvaptan treatment in patients during the early phase after cardiac surgery maintained sufficient urine output without affecting renal function and serum sodium concentrations, and reduced the incidence of WRF. Furthermore, tolvaptan treatment shortened the time required to restore preoperative body weight. Thus, tolvaptan is effective and safe for the management of body fluid levels in patients during the early postoperative period of cardiac surgery.
The authors have no conflicts of interest to disclose.