Abstract
Background:
This study evaluated the safety and efficacy of venous figure-of-eight (FoE) suture to achieve femoral venous hemostasis after radiofrequency (RF) catheter ablation (CA) for atrial fibrillation (AF).
Methods and Results:
We retrospectively examined 517 consecutive patients undergoing RFCA for AF. The control group (n=247) underwent manual compression for femoral venous hemostasis after sheath removal with 6 h of bed rest. The FoE group (n=270) underwent FoE suture technique with 4 h of bed rest. All patients achieved successful hemostasis within 24 h after CA. Although the incidence of hematoma was similar between the groups, the incidence of rebleeding was lower in the FoE group than in the control group (FoE vs. control, 3.7% vs. 18.6%, P<0.001). The post-procedural use of analgesic and/or anti-emetic agents was less frequent in the FoE group (19.3% vs. 32.0%, P<0.001). On multiple logistic regression analysis after adjustment for age and sex, the use of a vitamin K antagonist (OR, 2.42; 95% CI: 1.18–4.99, P=0.02) and the FoE suture technique (OR, 0.17; 95% CI: 0.08–0.35, P<0.001) were independent predictors of rebleeding after CA.
Conclusions:
FoE suture technique effectively achieved femoral venous hemostasis after RFCA for AF. It reduced the risk of rebleeding, shortened bed rest duration, and relieved patient discomfort.
Catheter ablation (CA) is being increasingly used for the treatment of atrial fibrillation (AF). Vascular access site complications are the most common complications of AF ablation, with an incidence of up to 13%, and have been a cause of substantial morbidity.1–5
Procedures that involve the use of large-caliber sheaths, multiple vascular sites of entry, and full-dose periprocedural anticoagulation may increase the risk of vascular access site complications.
Currently, manual compression is the standard procedure for hemostasis of the femoral vein and is followed by prolonged compression using gauze-balls, extended periods of bed rest, and immobilization in the supine position. Although immobilization is considered essential to reduce the risk of developing access site complications, it causes patient discomfort by inducing back pain.6
Furthermore, immobilization is a well-known risk factor for deep vein thrombosis and embolism.7
The figure-of-eight (FoE) suture is a surgical technique for closing a skin wound. The time to immediate hemostasis was reported to be shorter when using FoE suture than that using conventional manual compression in patients undergoing cryoballoon-based AF ablation8,9
or radiofrequency CA (RFCA).10
Previous studies have reported the efficacy of the FoE suture to achieve hemostasis without increasing puncture site complications and to possibly shorten the duration of bed rest. Therefore, we evaluated hemostasis following RFCA using a simple protocol: hemostasis of 3 femoral sheaths with 2 FoE sutures followed by a short duration of bed rest (4 h) in patients undergoing RFCA for AF. The aim of this study was to verify the efficiency of this method by comparing rebleeding rate, complication rate, and frequency of use of analgesic and/or anti-emetic agents during bed rest in patients achieving femoral venous hemostasis via the FoE suture technique vs. manual compression.
Methods
Study Design and Subjects
This single-center, 12-month observational study examined the incidence of vascular access site hemostatic failure before and after the introduction of the FoE suture technique for hemostasis. We performed RFCA for AF in 533 patients between December 2015 and November 2016 at Sakurabashi-Watanabe Hospital in Osaka, Japan. All patients had symptomatic or asymptomatic AF documented on electrocardiogram (ECG). We excluded 16 patients with suspected coronary artery disease (CAD) who underwent diagnostic coronary angiography with a 5-Fr sheath for femoral artery access, which was not used for other patients. The remaining 517 patients who underwent RFCA for AF using the same vascular sheath for femoral access were enrolled in this study. We performed the FoE procedure before sheath removal in the femoral puncture site in the middle of May 2016. Thus, the first 247 consecutive patients were treated with the conventional manual compression approach for femoral venous hemostasis (control group), whereas the subsequent 270 consecutive patients were treated with the FoE suture technique for femoral venous hemostasis (FoE group). The same operators performed both ablation procedures, and the vascular access technique and ablation strategy were similar in both groups. Data analysis was retrospective based on medical records, and the study was approved by the institute ethics committee. All patients provided written informed consent for the ablation procedure and the use of their clinical data in a retrospective study.
Anticoagulation Strategy
All patients were treated with effective anticoagulation therapy (vitamin K antagonist [VKA] or direct oral anticoagulants [DOAC]) for at least 1 month before the procedure and underwent transesophageal echocardiogram before the procedure to exclude thrombi in the left atrium and the left atrial appendage (LAA). The target prothrombin international normalized ratio (PT-INR) was 1.6–2.5 for patients given VKA, such as warfarin. VKA was interrupted 1 day before the procedure. Patients on DOAC such as dabigatran, rivaroxaban, apixaban, and edoxaban were instructed to omit taking them only on the morning of the procedure. Concurrent antiplatelet agents were continued without interruption throughout the pre- and post-procedural periods.
During the procedure, an i.v. bolus of unfractionated heparin (UFH, 5,000 IU) was given immediately after achieving vascular access. An additional bolus of UFH (100–120 IU/kg) was given immediately after the trans-septal puncture. During the procedure, activated clotting time (ACT) was measured every 30 min, and additional heparin was used when required to maintain ACT >300 s. At the end of the procedure, the effects of heparin were empirically reversed in all patients using 40 mg protamine sulfate.
After the procedure, oral anticoagulants were resumed in the evening of the day of the procedure without continuous UFH. Patients on once-daily DOAC such as rivaroxaban and edoxaban were instructed to take once-daily DOAC in the evening following the procedure. Oral anticoagulation was continued for at least 3 months after the procedure.
Vascular Access and Ablation
All procedures were performed under minimal sedation with a combination of bolus thiamylal sodium and continuous dexmedetomidine hydrochloride. Invasive arterial blood pressure, oxygen saturation, and ECG were continuously monitored throughout the entire procedure. A 19-G long elastic catheter (EVTL-MR 19G 150 mm RB; Hakko, Tokyo, Japan) was inserted into the right femoral artery to continuously monitor blood pressure and extract blood samples for measuring ACT.
One right brachial or internal jugular (7 Fr) and 3 right femoral venous accesses (8.5 Fr or 8 Fr) were obtained using the Seldinger technique. In principle, the distance between each femoral venous puncture site was 8–10 mm, and the needle was directed along the course of the vein at a 45° angle to the skin. All operators were educated and encouraged to pay sufficient attention to avoid inadvertent punctures causing arteriovenous fistula and retroperitoneal hemorrhage. Detailed catheter settings and ablation methods were followed as previously described.11
Post-procedural Hemostasis
Control Group
After completing ablation and treatment with protamine sulfate, we removed all 3 sheaths from the femoral vein and the 19-G long elastic catheter from the femoral artery. Immediate constant manual compression was applied to the site for approximately 10 min in the control group. The compression was then decreased, and hemostasis was checked. If bleeding continued, firm compression was reapplied. The groin puncture site was reassessed for hemostasis in a similar manner every 1 min until complete hemostasis was achieved. After immediate hemostasis was achieved in the catheter laboratory, we then applied a pressure bandage using a gauze-ball followed by 6 h of bed rest. If patients complained of discomfort or pain due to the ablation procedure or bed rest, analgesic and/or anti-emetic agents such as pentazocine and metoclopramide were given as per the attending physician’s judgment.
FoE Group
The FoE suture technique is shown in
Figures 1,S1. Using a large-curved needle, a 0-silk suture was introduced 5–10 mm caudal to the 8.5-Fr sheath insertion site and advanced through the subcutaneous tissue without going deep enough to ligate the femoral vein. The needle and suture were crossed over the sheath. A second pass of the needle was performed 5 mm cranial to the insertion site of the 8 Fr SL0 sheath, which was the middle of the 3 sheaths, and advanced above the 2 sheaths and through the subcutaneous tissue. At completion, the first FoE stitch was placed across the 2 SL0 sheaths at the same time. After the initial knot, a second knot was placed over the previous knot across the most cranial SL0 sheath. Next, the sheaths were gently pulled out while applying tension to the silk thread, and the suture was tightened such that the subcutaneous tissue was folded to assist in hemostasis. Finally, we removed the 19-G long elastic catheter from the femoral artery, and manual compression was applied to arterial hemostasis for approximately 5 min. The following processes were the same as those of the control group, except the duration of bed rest was 4 h. Compression bandages were used in both groups. Sutures were removed on the following morning.

All patients were hospitalized for at least 72 h and followed up after CA to check for complications and the recurrence of AF. Hemostasis at the puncture site, groin hematoma, lower extremities, and peripheral pulses were evaluated and examined for access site complications after the prespecified duration of bed rest. After the pressure bandage was removed and the groin puncture site was reassessed for hemostasis, ambulation was permitted following an additional 1 h of bed rest. For patients with rebleeding, additional manual compression was applied until bleeding stopped. The pressure bandage was applied, and the duration of bed rest was extended for another 1 or 2 h. Similarly, for patients with large hematoma, we applied pressure bandages and extended the duration of bed rest.
Endpoints and Definitions of Complications
The aim of this study was to evaluate the safety and efficacy of the venous FoE suture technique to achieve hemostasis of the femoral vein during a 4-h bed rest protocol. Thus, the primary endpoints of the study were (1) rebleeding at the groin requiring additional manual and/or bandage compression after leaving the electrophysiology laboratory; and (2) groin hematoma requiring additional manual and/or bandage compression. These endpoints were classified as minor bleeding based on the International Society on Thrombosis and Haemostasis.12
Because of low frequency, we did not set the endpoint as major vascular access complications, which was defined as arteriovenous fistula or pseudoaneurysm formation, retroperitoneal bleeding, hematomas causing a decrease in hemoglobin ≥2.0 g/dL or requiring blood transfusion, or access site problems requiring surgical or radiological intervention or rehospitalization.12
The secondary endpoint was the post-procedural use of analgesic and/or anti-emetic agents, such as pentazocine and metoclopramide. A shorter duration of bed rest was estimated to relieve patient discomfort and reduce the use of these agents during the course.
Statistical Analysis
Normally distributed continuous variables are presented as mean±SD, and those with a non-normal distribution are presented as median (IQR). Categorical variables are presented as frequency and percentage. Parametric data were compared using Student’s t-test or paired Student’s t-test, and non-parametric data were compared using the Mann-Whitney U-test, Wilcoxon signed rank test, chi-squared test, or Fisher’s exact test, as appropriate. Multivariable logistic regression analysis was performed to determine risk factors for rebleeding using the following variables: age, sex, concurrent use of antiplatelets, use of VKA as an oral anticoagulant, and use of the FoE suture technique. Results are reported as OR with 95% CI. Subgroup analysis stratified by age, sex, body mass index, CHADS2
score, session number, use of VKA, and antiplatelet use was performed to examine whether there was a consistent efficacy of the FoE suture technique. Analyses were performed using Medcalc (version 17.2). P<0.05 was considered statistically significant.
Results
Subjects
Patient characteristics are listed in
Table 1. There were no significant differences in baseline characteristics between the first 247 patients (control group) and the subsequent 270 patients (FoE group). Congestive heart failure was defined as left ventricular ejection fraction (LVEF) ≤50% and New York Heart Association class II, III, or IV. Approximately 10% of patients in each group (8.5% in the control group and 9.4% in the FoE group) received concomitant antiplatelet therapy, mostly for the management of CAD. Aspirin was the most commonly used antiplatelet agent. Clopidogrel, prasugrel, and cilostazol were used only in 6 patients in the control group and in 9 patients in the FoE group. Two patients received dual antiplatelet therapy.
Table 1.
Subject Characteristics
| |
Control group
(n=247) |
FoE suture group
(n=270) |
P-value |
| Age (years) |
63±11 |
63±11 |
0.98 |
| Male |
189 (77) |
218 (81) |
0.24 |
| BMI (kg/m2) |
24±4 |
24±4 |
0.18 |
| Baseline diseases |
| CHF |
37 (15) |
51 (19) |
0.24 |
| Coronary artery disease |
21 (9) |
25 (9) |
0.76 |
| Hypertension |
127 (51) |
143 (53) |
0.73 |
| Diabetes mellitus |
41 (17) |
46 (17) |
0.89 |
| History of stroke or TIA |
18 (7.3) |
23 (8.5) |
0.61 |
| Hemodialysis |
5 (2.0) |
1 (0.4) |
0.08 |
| CHADS2 score |
| 0 |
77 (31) |
77 (29) |
0.38 |
| 1 |
99 (40) |
100 (37) |
| ≥2 |
71 (29) |
93 (34) |
| Type of AF |
| Paroxysmal |
125 (51) |
126 (47) |
0.49 |
| Persistent |
80 (32) |
101 (37) |
| Long-standing persistent |
42 (17) |
43 (16) |
| No. procedures |
| First session |
174 (70) |
181 (67) |
0.40 |
| Repeat session |
73 (30) |
89 (33) |
| Laboratory data |
| Serum albumin (g/dL) |
4.2±0.3 |
4.1±0.2 |
0.09 |
| Serum hemoglobin (g/dL) |
14.0±1.4 |
14.0±1.4 |
0.81 |
| Serum platelet count (10,000/μL) |
20.7±13.5 |
20.1±4.6 |
0.54 |
| Serum creatinine (mg/dL) |
0.96±0.68 |
0.90±0.33 |
0.24 |
| Serum BNP (pg/mL) |
136±154 |
115±132 |
0.10 |
| Echocardiographic parameters |
| LA diameter (mm) |
39.3±5.5 |
39.1±6.2 |
0.69 |
| Diastolic LV diameter (mm) |
47.2±5.1 |
47.6±5.7 |
0.42 |
| LVEF (%) |
64.1±9.9 |
64.5±11.2 |
0.70 |
| CT evaluation |
| Maximum LA volume (mL) |
110±32 |
116±39 |
0.13 |
| Anticoagulants |
247 (100) |
270 (100) |
|
| VKA |
41 (17) |
27 (10) |
0.08 |
| Factor IIa inhibitor |
27 (11) |
30 (11) |
| Factor Xa inhibitors |
179 (73) |
213 (79) |
| Antiplatelet agent |
19 (7.7) |
22 (8.1) |
0.85 |
| Aspirin and clopidogrel |
3 (1.2) |
2 (0.7) |
0.79 |
| Aspirin |
15 (6.1) |
14 (5.2) |
0.66 |
| Clopidogrel |
5 (2.0) |
9 (3.3) |
0.36 |
| Periprocedural anticoagulation |
| Preprocedural PT-INR† |
1.80±0.51 |
1.87±0.74 |
0.67 |
| Last ACT before reversal of heparin (s) |
327±33 |
325±34 |
0.51 |
Data given as n (%) or mean±SD. †Reported only for patients on warfarin, not for those on direct oral anticoagulants. ACT, activated clotting time; AF, atrial fibrillation; BMI, body mass index; BNP, brain natriuretic peptide; CHF, congestive heart failure; CT, computed tomography; FoE, figure-of-eight; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; PT-INR, prothrombin time-international normalized ratio; TIA, transient ischemic attack; VKA, vitamin K antagonist.
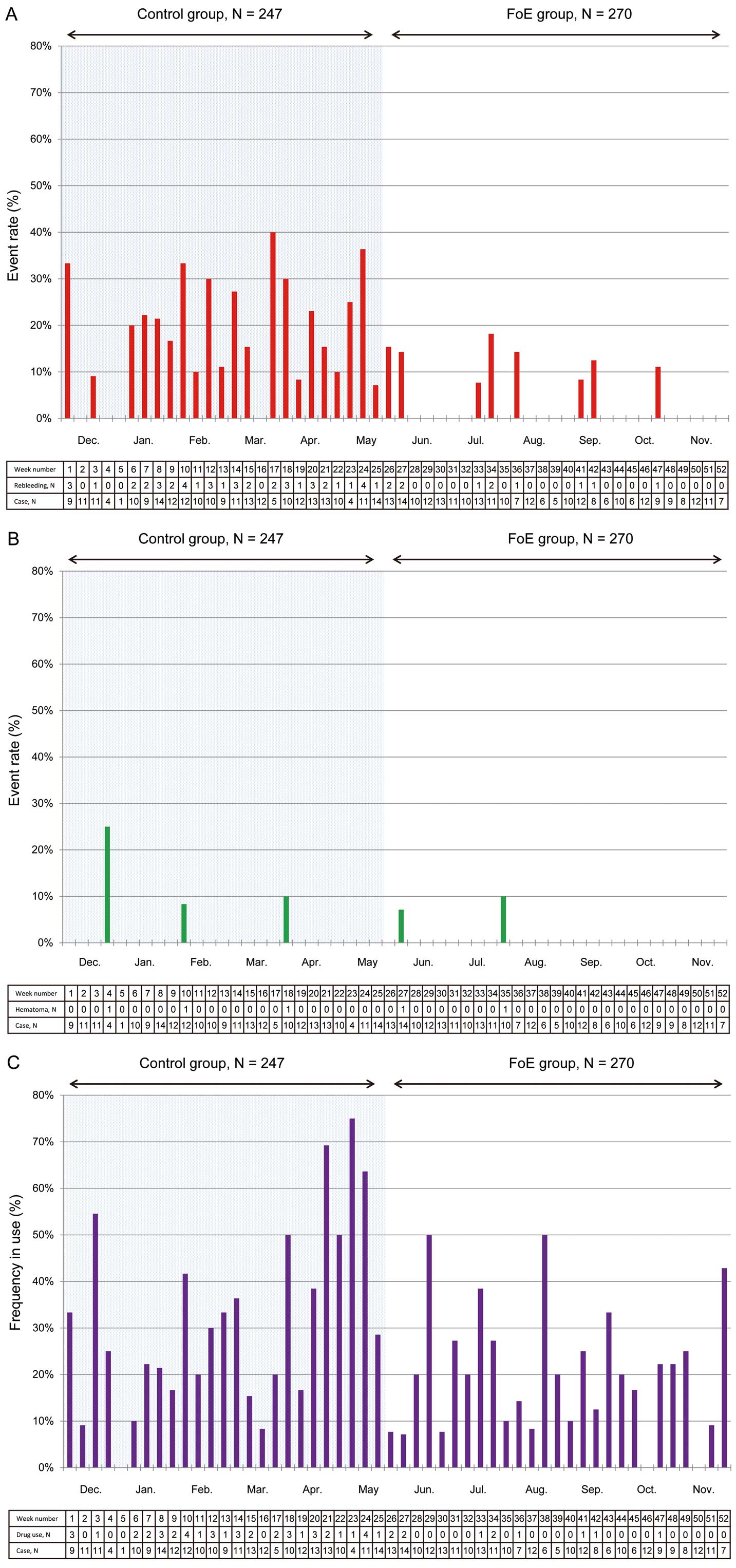
Successful PVI was achieved in all patients. Hemostasis was successfully achieved in all patients within 24 h after CA. In the FoE group, right femoral venous hemostasis was achieved immediately after tying the knot or within 5 min of arterial hemostasis.
Rebleeding requiring additional compression was diagnosed in 56 patients (10.8%). The rate of rebleeding requiring additional manual compression during the course was lower in the FoE group than in the control group (3.7% vs. 18.6%, P<0.001;
Figure 2A). Hematoma requiring additional compression, however, was diagnosed in 5 patients (1.0%): 2 hematomas (0.7%) were observed in the FoE group, and 3 (1.2%) were observed in the control group (P=0.67;
Figure 2B). Analgesic and/or anti-emetic agents were used in 114 patients (26.2%) after the procedure. The use of these agents was less frequent in the FoE group (19.3% vs. 32.0%, P<0.001;
Figure 2C). The time trends in the incidence of each outcome are presented in
Figure 3.
Other Complications Related to Anticoagulation
Although 1 patient (0.4%) in the control group developed pseudoaneurysm in the femoral artery puncture site that closed additional manual and bandage compression, there was no evidence of other vascular access site complications. Thus, major vascular access complications were observed in 2 patients (2 hematomas) in the FoE group and in 4 patients (3 hematomas and 1 pseudoaneurysm) in the control group (P=0.43).
One patient (0.2%) developed pleural effusion requiring drainage. One patient developed anemia of unknown origin requiring blood transfusion. No thrombotic events such as stroke, systemic embolism, transient ischemic attack, pulmonary embolism, or venous thrombosis were observed. After hospital discharge, 1 patient had suture abscess due to residual thread but was cured by its removal. No other unexpected adverse events related to FoE suture were observed.
Potential Risk Factors for Rebleeding
Univariate and multivariate logistic regression analyses were performed to determine risk factors for rebleeding (Table 2). Based on univariate analysis, age (OR, 1.04; 95% CI: 1.01–1.06, P=0.01), CHADS2
score (OR, 1.54; 95% CI: 1.22–1.95, P<0.001), concomitant use of antiplatelet agents (OR, 2.57; 95% CI: 1.16–5.71, P=0.02), use of VKA (OR, 3.50; 95% CI: 1.85–6.64, P<0.001), and use of the FoE suture technique (OR, 0.17; 95% CI: 0.08–0.34, P<0.001) were associated with an increased incidence of rebleeding in this study protocol. Despite adjustment for age, sex and the other covariates that were significant on univariate analysis, the use of FoE suture technique (OR, 0.17; 95% CI: 0.08–0.35, P<0.001) was significantly associated with an increased incidence of rebleeding in the multivariate model. Analysis of an alternative model adjusted for CHADS2
score indicated consistent efficacy of this technique (OR, 0.16; 95% CI: 0.08–0.33, P<0.001).
Table 2.
Risk Factors for Rebleeding
| |
Univariate |
Multivariate† |
| Model 1 |
Model 2 |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Age |
1.04
(1.01–1.06) |
0.01 |
1.03
(1.00–1.07) |
0.04 |
– |
| Female |
0.89
(0.45–1.79) |
0.75 |
0.69
(0.33–1.44) |
0.32 |
0.77
(0.37–1.61) |
0.49 |
| BMI |
1.00
(0.93–1.07) |
0.96 |
NA |
NA |
| CHF |
1.74
(0.91–3.35) |
0.10 |
NA |
– |
| CHADS2 score |
1.54
(1.22–1.95) |
<0.001 |
– |
1.52
(1.16–1.98) |
0.002 |
| Concomitant use of antiplatelet |
2.57
(1.16–5.71) |
0.02 |
1.57
(0.61–4.05) |
0.35 |
1.28
(0.49–3.37) |
0.62 |
| Use of VKA as anticoagulants |
3.50
(1.85–6.64) |
<0.001 |
2.42
(1.18–4.99) |
0.02 |
2.21
(1.06–4.59) |
0.03 |
| Repeat procedure |
1.61
(0.92–2.85) |
0.10 |
NA |
NA |
| Use of FoE technique |
0.17
(0.08–0.34) |
<0.001 |
0.17
(0.08–0.35) |
<0.001 |
0.16
(0.08–0.33) |
<0.001 |
†Multivariate logistic regression analysis was performed to assess the risk factors of rebleeding including age, sex and the other covariates significant on univariate analysis (P<0.05). In model 1, we included age and excluded CHADS2
score to avoid duplication of the variables. In model 2, we included CHADS2
score and excluded age and CHF. NA, not applicable. Other abbreviations as in Table 1.
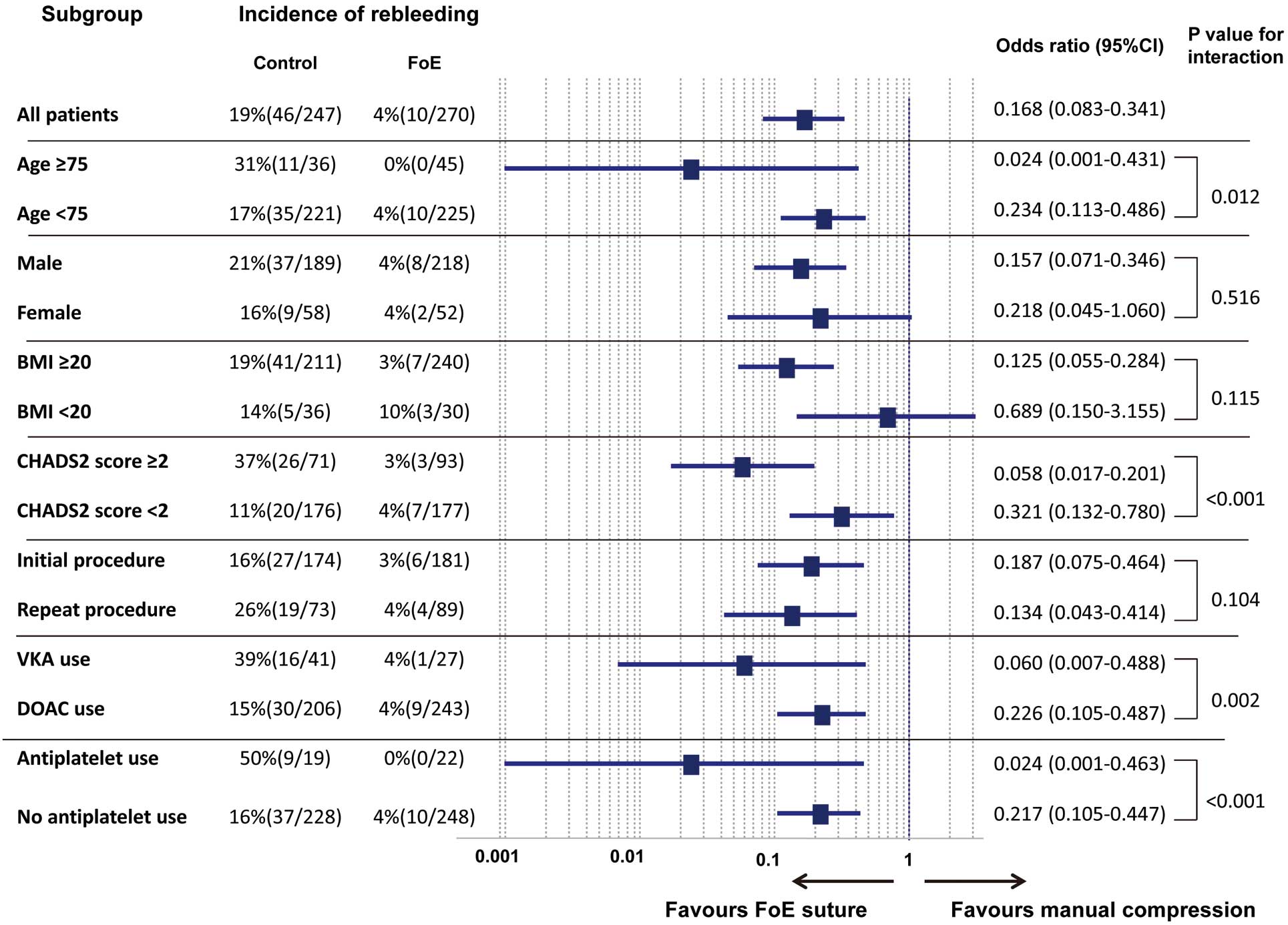
Figure 4
shows the results of the subgroup analysis. Significant interactions were found for subgroup analysis stratified by age ≥75 years, CHADS2
≥2, use of VKA, and concomitant antiplatelet use. The risk of rebleeding associated with FoE suture technique was lower (particularly in patients aged ≥75 years and those with CHADS2
score ≥2) than that associated with conventional manual compression (P for interaction=0.018 and P<0.001, respectively). The FoE suture technique resulted in a lower risk of rebleeding in patients treated with VKA as an anticoagulant (P for interaction=0.002) and concomitant antiplatelet agents (P for interaction<0.001).
Discussion
Main Findings
We demonstrated the efficacy and safety of using 2 subcutaneous FoE sutures to achieve hemostasis after the removal of 3 femoral venous sheaths in patients undergoing RFCA for AF in real-world clinical practice. This procedure successfully decreased the rate of rebleeding, even after decreasing the duration of bed rest from 6 to 4 h. Less frequent use of analgesic and/or anti-emetic agents in the FoE group suggested that this technique also decreased patient discomfort. On multiple logistic regression analysis, use of FoE suture technique was an independent negative predictor of rebleeding after CA even after adjustment for the other covariates.
Incidence of Vascular Access Site Complications and Rebleeding
In this study, major vascular access site complications occurred in 2 (0.4%) and in 4 patients (0.8%) in the FoE and control groups, respectively. The present relatively low incidence of vascular complications was consistent with that of a nationwide survey in Japan.5
Because transient rebleeding was not considered a major vascular complication, the incidence of rebleeding was not well described in previous reports. A clinical study on the efficacy of a hemostatic pad reported that the incidence of rebleeding was 46% in the conventional manual compression group after 4 h of bed rest.13
In the present study, 6 h of bed rest were used in the control group, but 18.6% of the control group experienced rebleeding and recompression at puncture sites.
Efficacy and Safety of the FoE Suture Technique
Previous studies evaluating the FoE suture technique did not prove its superiority in the incidence of major or minor vascular complications.8–10
Although the time to immediate hemostasis was shorter and the proportion of patients requiring protamine was lower in the FoE suture groups, the incidence of vascular complications was similar between the groups owing to the low frequency of complications.8–10
Thus, we set rebleeding after leaving the electrophysiology laboratory as one of the primary endpoints to evaluate the efficacy. No patient had FoE procedure-related major complications, which provided evidence to support the safety of the technique.
The previous reports indicated the efficacy of the FoE of each sheath separately,8–10
and one of the new findings in the present study was that FoE of 2 sheaths together was also effective and safe. The suture technique was easily performed by all the staff within a few minutes without a learning curve. After adopting the FoE suture technique, the frequency of rebleeding dramatically decreased (Figure 3). This technique appeared to be less time-consuming for physicians and more comfortable for patients owing to the immediate hemostasis and short bed rest time compared with conventional manual compression.
Clinical Perspectives and Remaining Problems
Although this study included only patients undergoing AF ablation, the technique could be used for all cardiac catheterization procedures using large-diameter venous sheaths, particularly for techniques without high-intensity anticoagulation such as LAA closure, atrial septal defect closure, and leadless pacemaker. FoE suture technique is considered feasible, effective, and expected to be increasingly widespread henceforth. However, there remains room for discussion in some points.
First, it remains unknown how many sutures should be used. The suture involving 3 sheaths was the next challenge that we faced. But, because there was an approximately 2-cm distance between the 3 sheaths, it was difficult to perform suturing at once. Even in cases when the suture was possible immediately, immediate hemostasis was often difficult to achieve.
Second, it is still unknown whether reversal of heparin with protamine should be performed even in the case of FoE suture. At present, the use of protamine after AF ablation is a guideline-recommended method,14
and it would reduce the risk of bleeding complications by counteracting aggressive anticoagulation with heparin. Moreover, in the present patients, no periprocedural thrombotic events were observed with the use of protamine. Therefore, the use of protamine might be reasonable.
Furthermore, the appropriate duration of bed rest has not been defined. Currently, the duration of bed rest differs according to sheath size and local policy or individual experience.6
Although both minor and major vascular complications should be prevented, prolonged bed rest is a difficult component of post-cardiac catheterization care. In this study, we set the duration of bed rest at 4 h because the ipsilateral 19-G long elastic catheter inserted into the femoral artery was removed at the same time, and we performed manual compression at the artery puncture site. It remains unknown, however, whether 4-h bed rest can be shortened any further.
Study Limitations
The main limitations of this study are associated with the retrospective non-randomized observational method. We believe, however, that the evidence obtained in our clinical practice compares favorably with a well-designed prospective study because we collected sequential data on AF ablation during a relatively short period (12 months), and the sample size was large (n=517). Second, this study was a single-center experience, and reproducibility in a multicenter setting remains to be evaluated. Third, the use of additional compression against oozing hemorrhage and/or moderate hematoma and the use of analgesic and/or anti-emetic agents for back pain and/or nausea depend on each physician’s judgment. Finally, as described here, the incidence of major bleeding at the puncture site was low, making it difficult to evaluate whether this method decreases major bleeding complications.
Conclusions
FoE suture technique performed in patients undergoing RFCA for AF reduced the incidence of rebleeding, whereas the rate of hematoma was unchanged, even after decreasing the duration of bed rest from 6 to 4 h. FoE was a simple, inexpensive, and relatively safe technique that may decrease patient discomfort by achieving immediate hemostasis.
Conflict of Interest
K. Inoue has received honoraria from Johnson and Johnson. The other authors declare no conflict of interest.
Supplementary Files
Supplementary File 1
Figure S1.
Schematization of the figure-of-eight (FoE) suture technique.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-1213
References
- 1.
Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2012; 59: 143–149.
- 2.
Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93 801 procedures. Circulation 2013; 128: 2104–2112.
- 3.
Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai K, et al. Current status of catheter ablation for atrial fibrillation: Updated summary of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF). Circ J 2014; 78: 1112–1120.
- 4.
Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai K, et al. Clinical and procedural predictors of early complications of ablation for atrial fibrillation: Analysis of the national registry data. Heart Rhythm 2014; 11: 2247–2253.
- 5.
Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai K, et al. Current status of catheter ablation of atrial fibrillation in Japan: Summary of the 4th survey of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF). J Cardiol 2016; 68: 83–88.
- 6.
Chair SY, Thompson DR, Li SK. The effect of ambulation after cardiac catheterization on patient outcomes. J Clin Nurs 2007; 16: 212–214.
- 7.
Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107: I9–I16.
- 8.
Traullé S, Kubala M, Doucy A, Quenum S, Hermida JS. Feasibility and safety of temporary subcutaneous venous figure-of-eight suture to achieve haemostasis after ablation of atrial fibrillation. Europace 2016; 18: 815–819.
- 9.
Aytemir K, Canpolat U, Yorgun H, Evranos B, Kaya EB, Şahiner ML, et al. Usefulness of ‘figure-of-eight’ suture to achieve haemostasis after removal of 15-French calibre femoral venous sheath in patients undergoing cryoablation. Europace 2016; 18: 1545–1550.
- 10.
Issa ZF, Amr BS. Venous hemostasis postcatheter ablation of atrial fibrillation while under therapeutic levels of oral and intravenous anticoagulation. J Interv Card Electrophysiol 2015; 44: 97–104.
- 11.
Tanaka N, Inoue K, Tanaka K, Toyoshima Y, Oka T, Okada M, et al. Automated ablation annotation algorithm reduces re-conduction of isolated pulmonary vein and improves outcome after catheter ablation for atrial fibrillation. Circ J 2017; 81: 1596–1601.
- 12.
Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694.
- 13.
Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, et al. Rapid hemostasis at the femoral venous access site using a novel hemostatic pad containing kaolin after atrial fibrillation ablation. J Interv Card Electrophysiol 2011; 31: 157–164.
- 14.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017; 14: e275–e444.