2019 Volume 83 Issue 6 Pages 1197-1199
2019 Volume 83 Issue 6 Pages 1197-1199
In the early days of percutaneous coronary intervention, interventional cardiologists intended to obtain luminal gain in the stenotic region guided only by coronary angiogram. Thereafter, introduction of intravascular ultrasound (IVUS) facilitated not only a successful procedure, but also provided information on the vessel wall. Disruption of vulnerable plaque causes ST-elevation myocardial infarction (STEMI). Given that the pathological features of plaque vulnerability consist of large lipid pool, thin fibrous cap and infiltration of inflammatory cells, coronary imaging became more important to detect vulnerable plaque and to treat it before rupture. Now, optical coherence tomography (OCT), which has outstanding spatial resolution, has enabled detection of thin-cap fibroatheroma (TCFA),1 calcified nodules and thrombi. In addition, using coronary angioscopy, we can directly observe red or white thrombus formation or yellow lipidic plaque.2
Article p 1214
Bryniarski et al observed TCFA of the non-culprit lesion in the culprit vessel in detail, using OCT, and compared this between patients with STEMI and those with stable angina (SA) pectoris. In this issue of the Journal, they showed that the lipid burden was greater and fibrous cap was thinner in the STEMI group than in the SA group.3 This may be appropriate, as demonstrated by the many prior clinical studies showing similar results.4,5 Interestingly, in the Bryniarski et al study, the baseline statin usage was significantly higher in the SA group, compared with the STEMI group, which may have affected the results.3 This bias, however, may not be an important issue. Although a thinning of fibrous cap is deeply associated with plaque rupture, OCT-based TCFA is not always a predictor of this. In most of the studies on the vulnerability of the non-culprit plaque in acute coronary syndrome (ACS) patients, TCFA was measured at an arbitrary point determined by qualitative visual assessment of 2-D cross-sectional images.4,6 As Bryniarski et al described, even though 2 lesions have TCFA with the same fibrous cap thickness, the distribution and total sum area of thin fibrous cap may be different.3 Moreover, this type of measurement is subject to greater intra- and inter-observer variability. Therefore, they furthermore assessed the 3-D structure of the TCFA, using computer-aided volumetric analysis.3 The fibrous cap thickness was mapped with respect to each distribution and the TCFA area was calculated. As a result, the largest single TCFA area and the total sum of TCFA area were significantly greater in the STEMI group than in the SA group. In addition, the proximal part of the lesion had the largest mean TCFA area.3 They interpreted these results with relation to a previous study by their group.7 Low endothelial sheer stress (ESS) is found frequently in the proximal and distal part of the lesion, whereas it is rarely seen in the MLA site. In the previous study, they applied unique methods and found a relationship between low EES and coronary atherosclerosis.8 They performed a 3-D coronary artery reconstruction using OCT and coronary angiograms, simulated the detailed characteristics of intravascular blood flow and assessed local ESS distribution using computational fluid dynamics for a non-culprit lesion in patients with ACS. The assessment was performed at the 2 points of baseline in the acute phase of ACS, and at 6-month follow-up. They found that TCFA was distributed more abundantly in the low-EES sites than in the high-EES sites. In addition, the fibrous cap was significantly thinner in the low-EES sites than in the high-EES sites. Compared with baseline, at follow-up the prevalence of TCFA was higher and the fibrous cap was thinner in the segments of persistently low EES than in other segments.
Vascular endothelial cells (EC) are constantly exposed to mechanical stress, namely, ESS due to blood flow, and stretching tension depending on blood pressure. Once the EC recognizes ESS, it is transmitted into the EC and inhibits endothelial apoptosis.9 Endothelial proliferation and/or death increase permeability, enabling the migration of lipid and inflammatory cells into the plaque. In the segment without stenosis or branching, the EC are exposed to stationary sheer stress and a microenvironment is generated in which the development of atherosclerosis is prevented.10 Meanwhile, stenosis and/or branching generate a turbulent flow and decrease the ESS, leading to hyperpermeability. Therefore, low ESS contributes to atherosclerosis.
ESS is also influenced by the structural features of the aortic wall. Although the descending thoracic aorta above the celiac trunk generally exhibits a mild degree of atherosclerosis, the infrarenal part of the abdominal aorta is a frequent site of clinically significant atherosclerosis such as true aneurysm of the aorta and Leriche syndrome. There are large differences in flow pattern, velocity distribution and wall shear stress between the suprarenal and infrarenal abdominal aorta. Pedersen et al measured the intimal thickness of postmortem aorta, obtained from young healthy accident victims without cardiovascular disease.11 They simulated wall sheer stress, using a glass model of the abdominal aorta including its major branches, blown from a cast obtained during autopsy. They found that wall sheer stress was significantly correlated with intimal thickness.11 In addition, the wall sheer stress was lower, and intima thickness was greater in the infrarenal aorta than in the suprarenal aorta.11 Recently, Komatsu et al observed the aortic wall on angioscopy in 324 patients with suspected coronary artery disease (CAD).12 They found spontaneous ruptured aortic plaque in 262 patients (80.9%), of whom 120 patients had plaque rupture below the diaphragm portion of the aorta (Figure 1).12 Figure 2 shows representative angioscopy of the descending aorta of our case. Ruptured plaque, subintimal bleeding and cholesterol crystal were observed in the infrarenal aorta but the vulnerability was lower in the suprarenal aorta. Although atherosclerosis is a multifactorial phenomenon, low-wall sheer stress may be one of the important factors involved in the silent impairment of EC. Thus, observation of the low-ESS region of the aorta, as well as of the coronary artery on imaging would be promising not only to predict but also to prevent vascular events.
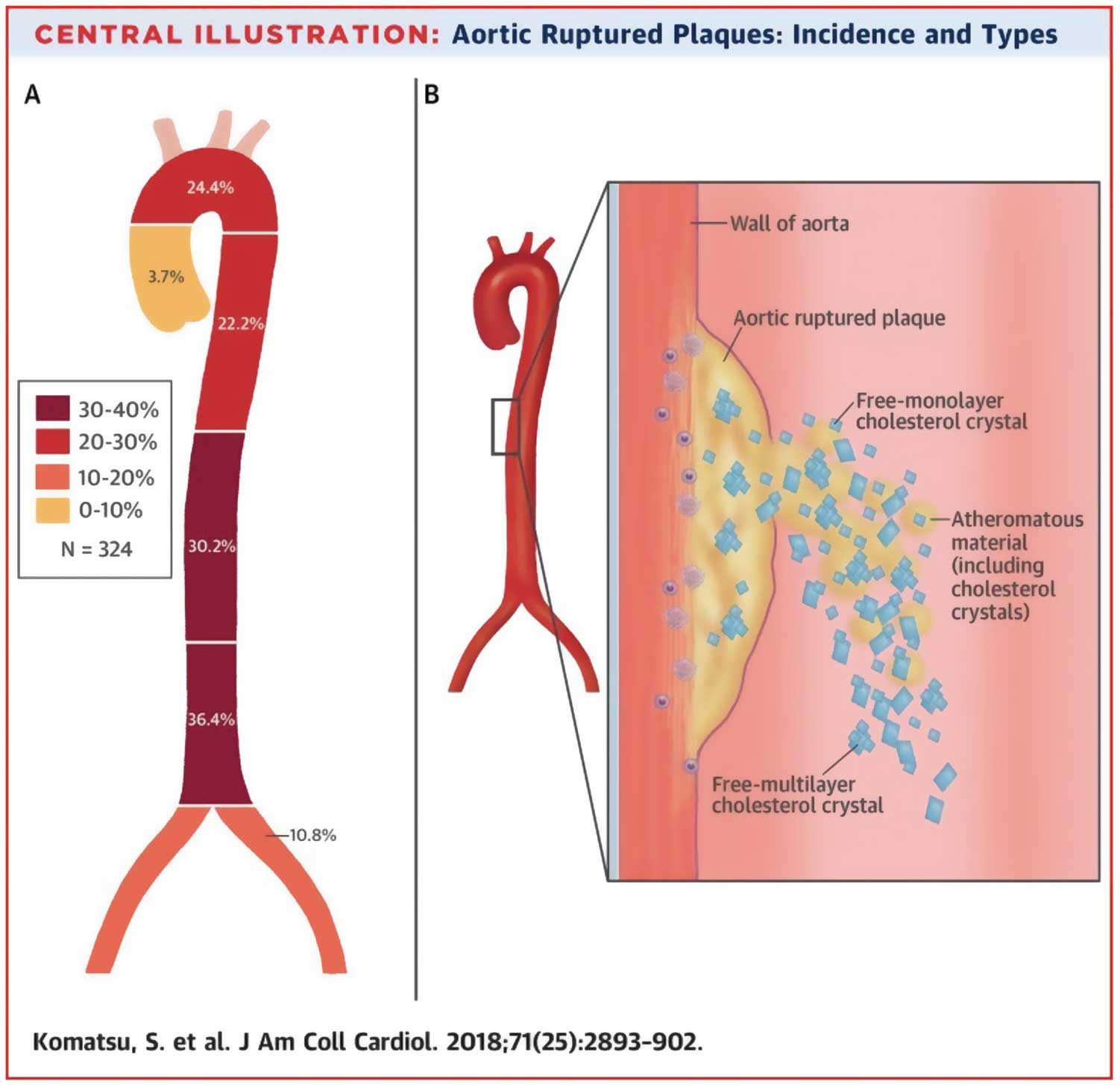
(A) Incidence of spontaneous ruptured plaques in the aorta. (B) Schematic diagram of the various types of cholesterol crystals from spontaneous ruptured plaque. Atheromatous content consists of cholesterol crystals (median length, 650 µm; median width, 313 µm), fibrin, macrophage, calcification, a mixture of which may be blown out, along with free-form monolayer (median length, 40 µm; median width, 30 µm) and multilayer cholesterol crystals. (Reproduced with permission from Komatsu S, et al.12)
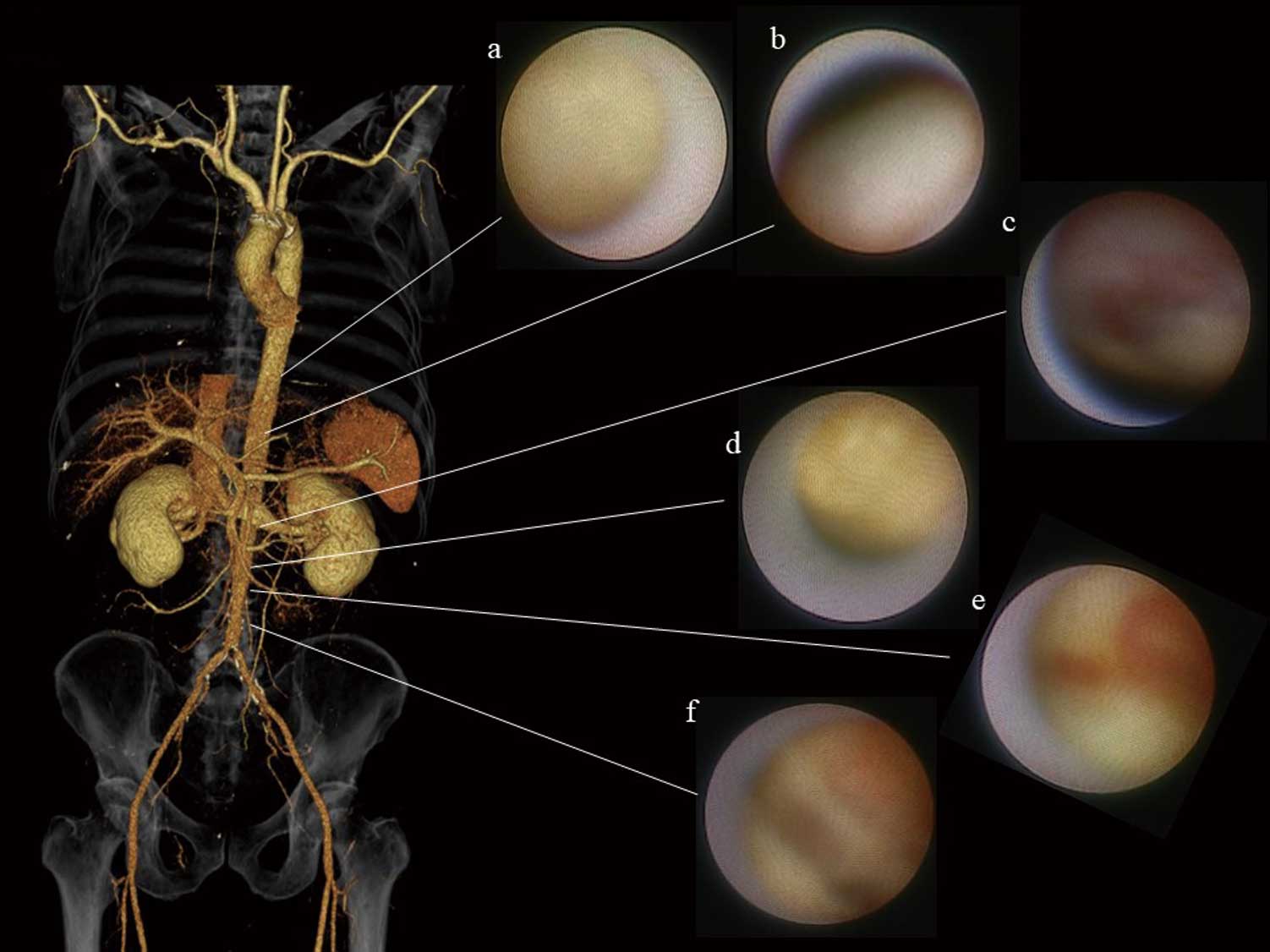
Representative angioscopy of the aorta in a 79-year-old man. Highly vulnerable plaques are observed in the infrarenal aorta but the vulnerability was lower in the suprarenal aorta. (a) Smooth plaque with yellowness grade 2; (b) smooth plaque with yellowness grade 1; (c) erosion; (d) chandelier appearance; (e) subintimal bleeding; (f) fissure.13
To treat the patients with CAD, comprehensive management of coronary risk factors is essential. The lowering of low-density lipoprotein cholesterol (LDL-C) by statins directly prevents plaque rupture. In patients in whom treatment with statins alone is insufficient to achieve the target LDL-C levels, additional prescription of other LDL-C-lowering agents such as ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitor is available. For appropriate assessment of patient risk and of drug effectiveness, the observation of plaque vulnerability on imaging such as IVUS, OCT and/or angioscopy is crucial. The current study by Bryniarski et al provides information on the 3-D evaluation of fibrous cap extent and TCFA distribution as a landmark of plaque vulnerability.3 Future studies showing that this information leads to prediction of outcome and to prevention of cardiovascular events are expected.