Abstract
Background:
There is insufficient real-world data on the current status of Japanese patients with venous thromboembolism (VTE) or its treatment and prevention with rivaroxaban.
Methods and Results:
In this multicenter, prospective, observational study conducted in Japan, 1,039 patients with acute symptomatic/asymptomatic deep vein thrombosis (DVT) and pulmonary embolism (PE) with or without DVT prescribed rivaroxaban were enrolled at 152 institutions and observed for a median of 21.3 months. Mean age was 68.0±14.7 years, mean body weight was 60.3±14.1 kg, 59.0% were females, and 19.0% had active cancer. Incidences of recurrence or aggravation of symptomatic VTE (primary effectiveness outcome) and major bleeding (principal safety outcome) were 2.6% and 2.9% per patient-year, respectively. These outcomes did not differ between patients with DVT and those with PE (primary effectiveness outcome: 2.6% vs. 2.5% per patient-year, P=0.810; principal safety outcome: 3.5% vs. 2.4% per patient-year, P=0.394). The incidence of composite clinically relevant events, including recurrence or aggravation of symptomatic VTE, acute coronary syndrome, ischemic stroke, all-cause death, or major bleeding events, was 9.2% per patient-year. Multivariate analysis revealed that male sex, being underweight, having active cancer, chronic heart and lung disease, and previous stroke were independent determinants for composite clinically relevant events.
Conclusions:
In Japanese clinical practice, a single-drug approach with rivaroxaban was demonstrated to be a valuable treatment for a broad range of VTE patients.
Venous thromboembolism (VTE) is a common acute cardiovascular disease,1
and includes deep vein thrombosis (DVT) and pulmonary embolism (PE).2
According to a questionnaire-based Japanese epidemiologic study conducted in 2006, the annual numbers of newly diagnosed patients with DVT and PE were 14,674 and 7,864, respectively.3
In a more recent epidemiologic study in Japan, the numbers of patients newly diagnosed with DVT and PE in 2011 were 24,538 and 16,096, with overall annual incidences of 19.2 and 12.6 per 100,000 population, respectively.2,4
In several Asian countries, including Japan, as well as in Western countries, PE has been reported to show greater severity in the acute phase, higher mortality, and higher recurrence rate than DVT.5–10
Rivaroxaban is a direct oral anticoagulant (DOAC) that inhibits factor Xa. Rivaroxaban has several advantages over vitamin K antagonist (VKA), including no need for parenteral treatment, a predictable anticoagulant effect without a requirement for routine coagulation monitoring, and lower risk of food-drug interactions.11–13
The EINSTEIN-DVT and EINSTEIN-PE trials evaluated the concept of single-anticoagulant therapy with rivaroxaban as an alternative to heparin and VKA for acute DVT and PE, respectively.14–16
In these trials, the single-drug approach with a high dose of rivaroxaban for 3 weeks was shown to be non-inferior to VKA and had a lower incidence of major bleeding. Because Japanese patients were not enrolled in these trials, the J-EINSTEIN DVT and PE trial with Japanese patients with acute VTE showed consistent findings with the global trials on the efficacy and safety of rivaroxaban.17
However, this trial was conducted under treatment settings unique to Japan and used lower doses of rivaroxaban than those used in Western countries based on pharmacokinetic differences, and a lower number of patients (only 100 patients) were enrolled in the trial.
Treatment options for patients with VTE in Japan differ from those in other countries.18,19
Low molecular weight heparin, which does not require dose adjustment, still has no insurance coverage for the treatment of VTE in Japan, although it is used as the standard treatment in other countries. Although anticoagulation therapy with VKA is effective for preventing VTE recurrence, there is little available data regarding the treatment and prevention of VTE in Japanese patients based on prospective studies.10,20,21
Therefore, in the J’xactly Study (Japanese registry of rivaroXAban effectiveness and safety for the prevention of reCurrence in patients with deep vein Thrombosis and puLmonarY embolism), we aimed to investigate the effectiveness and safety of rivaroxaban in Japanese patients with VTE in a real-world setting.22
In particular, we aimed to clarify whether the outcomes differed between patients with DVT and patients with PE administered rivaroxaban for treatment and prevention.
Methods
Study Design and Oversight
The J’xactly Study was a multicenter, prospective, observational cohort study designed to obtain real-world evidence of clinical outcomes in Japanese patients with acute symptomatic/asymptomatic DVT, PE, or both. Patients were treated with rivaroxaban in real-world clinical practice, and followed up for a minimum of 18 months until 3 years after enrollment. The details of this study were described previously.22
The J’xactly Study was designed and led by an executive steering committee (Supplementary Appendix 1).
The J’xactly Study was conducted in accordance with the principles of the Declaration of Helsinki, the Ethical Guidelines for Clinical Studies from the Japanese Ministry of Health, Labour and Welfare, and all applicable laws in Japan. The protocol of the present study was reviewed and approved by the Institutional Review Board of Nihon University Itabashi Hospital, along with the Institutional Review Boards of all participating institutions. Data were reviewed by an independent data and safety monitoring committee.
Patients
Patients who were diagnosed with acute symptomatic/asymptomatic DVT, PE, or both and prescribed rivaroxaban for the treatment and prevention of VTE were enrolled. Patients who met any of the following criteria were excluded: patients with contraindications to rivaroxaban; patients with chronic thromboembolic pulmonary hypertension (CTEPH) other than those with CTEPH plus acute PE or DVT; and those with active bleeding. All patients provided written informed consent.
From December 2016 to April 2018, a total of 1,039 patients were enrolled at 152 institutions (Supplementary Appendix 2). All patients were enrolled in the study within 3 weeks of starting a rivaroxaban prescription and were followed until the end of the follow-up period whenever possible, regardless of whether rivaroxaban was continued, discontinued, or terminated according to patient preference or physician discretion. A flow diagram showing how the final population was derived from patients enrolled in the study is shown in
Figure 1. Patients were followed up until November 2019, and the median follow-up period was 21.3 months (interquartile range, 18.1–24.2 months). The severity of PE was stratified according to the Japanese guideline2
as follows: cardiac arrest or collapse; acute PE with cardiac arrest or collapse; massive, acute PE with sustained hypotension; submassive acute PE with stable hemodynamics accompanied by right ventricular overload determined by echocardiography; and non-massive PE other than those described above. DVT was classified by localization of the thrombus, and defined as proximal if the thrombus was located on the central side including the popliteal vein, and distal if the thrombus was located below the popliteal vein.

The choice of doses and duration of treatment with rivaroxaban was at the discretion of the treating physicians. The standard regimen for the single-drug approach was rivaroxaban 15 mg twice daily for 3 weeks after the diagnosis of DVT or PE, and 15 mg once daily thereafter. During the follow-up period, the investigator could suspend, discontinue, or terminate rivaroxaban or switch to other treatments at their discretion, and data on treatment status during the follow-up period were collected.
Outcome Assessments
The primary effectiveness outcome was the recurrence or aggravation of symptomatic VTE, according to established diagnostic criteria during the follow-up period.23–25
Recurrence or aggravation of symptomatic VTE was considered to have occurred if recurrent or new PE or DVT was documented objectively, if an event of PE or DVT was suspected but without objective diagnosis despite anticoagulation at a therapeutic dose for ≥48 h, or if there was a death in which PE was a contributing cause or could not be ruled out. The principal safety outcome was major bleeding that occurred during the treatment period and up to 2 days after discontinuation of rivaroxaban. Major bleeding was defined according to the criteria of the International Society on Thrombosis and Haemostasis (ISTH).26
The secondary outcomes included recurrence or aggravation of symptomatic PE and DVT, death from any cause, death related to VTE and cardiovascular disease, vascular event (acute coronary syndrome or ischemic stroke), and non-major bleeding. The composite of clinically relevant events were evaluated as a composite of recurrent VTE, acute coronary syndrome, ischemic stroke, all-cause death, or major bleeding events, which were all weighted equally. Blinded adjudication of outcomes was conducted by an independent clinical events committee.
Statistical Analysis
The primary and secondary effectiveness outcomes were performed in a modified intention-to-treat population, which was defined as all patients except those with an ethical violation or a technical reason for not participating in the study, and included all effectiveness outcome occurrences from the time of first treatment with rivaroxaban to the end of the follow-up period, regardless of whether the patient was administered rivaroxaban at the time of the event. The principal safety outcome was performed in the on-treatment population, which was defined as all patients who received at least one dose of rivaroxaban, and included all principal safety outcome occurrences from the time of first treatment with rivaroxaban to 2 days after the last treatment with rivaroxaban. The cumulative incidence and its 95% confidence interval (CI) were determined by using the Kaplan-Meier method. A multivariate Cox hazard model analysis was conducted to identify the major determinants for occurrence of composite clinically relevant events. Variables with a value of P<0.1, determined by using an unpaired test, were entered into the multivariate model. Numerical data were expressed as mean±standard deviation and categorical data were reported as number and percentage of patients. Subanalyses of the incidences of clinical events were performed according to VTE diagnosis at baseline. All statistical analyses were performed using SAS software, version 9.4 for Windows (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics and Treatment Status of VTE
The patient characteristics at baseline are shown in
Table 1. The mean age was 68.0±14.7 years, 59.0% of patients were female, 41.5% of those were outpatients, and 19.0% of those had active cancer. At baseline, the median D-dimer was 7.6 μg/mL (interquartile range, 3.5–15.1) and the mean creatinine clearance (CrCl) was 77.9±36.3 mL/min. A total of 597 patients (58.8%) were diagnosed with DVT only (DVT group), whereas 419 patients (41.2%) were diagnosed with PE with or without DVT (PE group). A history of VTE was present in 83 patients (8.2%), and there was no significant difference in the history of VTE between the DVT group and the PE group. No differences were noted in comorbidities such as hypertension and diabetes mellitus, except for chronic cardiopulmonary disease. CrCl was significantly lower and non-steroidal anti-inflammatory drugs (NSAIDs) were less frequently taken in the DVT group compared with the PE group. In the DVT group, the thrombus localization was proximal in 51.8% and distal in 48.2%. In the PE group, the clinical severity classification was cardiac arrest or collapse in 1.7%, massive in 3.8%, submassive in 29.4%, non-massive in 59.2%, and unknown in 6.0%. A total of 334 patients (32.9%) were treated for VTE prior to administration of rivaroxaban. The main pre-treatments were anticoagulation, inferior vena cava filtering, and thrombolysis in both the DVT and the PE groups. With respect to the initial dose of rivaroxaban, 30 mg/day was the most frequent dose (667/1,016 patients [65.6%]), followed by 15 mg/day (282/1,016 patients [27.8%]), and there were some patients who received initial doses of 20 mg/day or 10 mg/day. In the DVT group, the initial dose of rivaroxaban 30 mg/day was administered to 326/597 patients (54.6%), compared with 341/419 patients (81.4%) in the PE group.
Table 1.
Patient Characteristics at Baseline
| |
Overall
(N=1,016) |
DVT only
(N=597) |
PE with or without
DVT (N=419) |
P value† |
| Characteristics |
| Age (years) |
68.0±14.7 |
69.7±14.6 |
65.7±14.6 |
<0.001 |
| ≥75 |
390 (38.4) |
253 (42.4) |
137 (32.7) |
0.002 |
| Female sex |
599 (59.0) |
379 (63.5) |
220 (52.5) |
<0.001 |
| Body weight (kg) |
60.3±14.1 |
58.3±13.1 |
63.0±14.9 |
<0.001 |
| <50 |
222/981 (22.6) |
142/564 (25.2) |
80/417 (19.2) |
0.031 |
| Body mass index (kg/m2) |
23.8±4.2 |
23.5±4.0 |
24.2±4.4 |
0.011 |
| Outpatient |
422 (41.5) |
310 (51.9) |
112 (26.7) |
<0.001 |
| Hypertension |
380 (37.4) |
211 (35.3) |
169 (40.3) |
0.114 |
| Diabetes mellitus |
118 (11.6) |
64 (10.7) |
54 (12.9) |
0.320 |
| Heart failure |
34 (3.3) |
16 (2.7) |
18 (4.3) |
0.162 |
| Atrial fibrillation |
29 (2.9) |
13 (2.2) |
16 (3.8) |
0.130 |
| Coronary artery disease |
45 (4.4) |
26 (4.4) |
19 (4.5) |
0.878 |
| Chronic heart and lung disease |
47 (4.6) |
20 (3.4) |
27 (6.4) |
0.023 |
| Active cancer |
193 (19.0) |
105 (17.6) |
88 (21.0) |
0.194 |
| Previous stroke |
73 (7.2) |
45 (7.5) |
28 (6.7) |
0.624 |
| Previous VTE |
83 (8.2) |
55 (9.2) |
28 (6.7) |
0.163 |
| CrCl (mL/min) |
77.9±36.3 |
74.8±35.0 |
82.1±37.6 |
0.002 |
| <50 |
219/976 (22.4) |
148/559 (26.5) |
71/417 (17.0) |
<0.001 |
| D-dimer (μg/mL) median (IQR) |
7.6 (3.5–15.1) |
5.9 (2.7–12.6) |
10.0 (5.5–19.0) |
<0.001 |
| Concomitant medications |
| Antiplatelets |
104 (10.2) |
66 (11.1) |
38 (9.1) |
0.344 |
| Estrogen preparation |
23 (2.3) |
16 (2.7) |
7 (1.7) |
0.392 |
| Anticancer agents |
88 (8.7) |
45 (7.5) |
43 (10.3) |
0.141 |
| NSAIDs |
196 (19.3) |
135 (22.6) |
61 (14.6) |
0.002 |
| Presentation |
| DVT |
917 (90.3) |
597 (100.0) |
320 (76.4) |
|
| Proximal |
547/917 (59.7) |
309/597 (51.8) |
238/320 (74.4) |
<0.001 |
| Distal |
370/917 (40.3) |
288/597 (48.2) |
82/320 (25.6) |
|
| Symptoms of DVT |
599/917 (65.3) |
394/597 (66.0) |
205/320 (64.1) |
0.561 |
| PE |
419 (41.2) |
– |
419 (100) |
– |
| Cardiac arrest or collapse |
7/419 (1.7) |
– |
7/419 (1.7) |
– |
| Massive |
16/419 (3.8) |
– |
16/419 (3.8) |
– |
| Submassive |
123/419 (29.4) |
– |
123/419 (29.4) |
– |
| Non-massive |
248/419 (59.2) |
– |
248/419 (59.2) |
– |
| Unknown |
25/419 (6.0) |
– |
25/419 (6.0) |
– |
| Symptoms of PE |
222/419 (53.0) |
– |
222/419 (53.0) |
– |
| Prior treatment |
334 (32.9) |
131 (21.9) |
203 (48.4) |
<0.001 |
| Anticoagulation therapy |
264/334 (79.0) |
93/131 (71.0) |
171/203 (84.2) |
<0.001 |
| Inferior vena cava filter |
87/334 (26.0) |
47/131 (35.9) |
40/203 (19.7) |
0.364 |
| Thrombolytic therapy |
47/334 (14.1) |
14/131 (10.7) |
33/203 (16.3) |
<0.001 |
| Catheterization |
12/334 (3.6) |
8 /131 (6.1) |
4/203 (2.0) |
0.770 |
| Pulmonary thrombus removal |
1/334 (0.3) |
0/131 (0.0) |
1/203 (0.5) |
0.412 |
| PCPS |
3/334 (0.9) |
0/131 (0.0) |
3/203 (1.5) |
0.070 |
| Other |
26/334 (7.8) |
12/131 (9.2) |
14/203 (6.9) |
0.226 |
| Initial treatment of rivaroxaban |
| Dose (mg/day) |
| 30 |
667 (65.6) |
326 (54.6) |
341 (81.4) |
<0.001 |
| 20 |
22 (2.2) |
16 (2.7) |
6 (1.4) |
|
| 15 |
282 (27.8) |
218 (36.5) |
64 (15.3) |
|
| 10 |
45 (4.4) |
37 (6.2) |
8 (1.9) |
|
| Duration of 30 mg/day (21 days or less) |
540/667 (81.0) |
258/326 (79.1) |
282/341 (82.7) |
<0.001 |
Numerical data are expressed as mean±standard deviation and categorical data as number (percentage) of patients unless otherwise stated. †Comparisons of patients with DVT only and PE with or without DVT by using the Student’s t-test or Fisher’s exact test, as appropriate. CrCl, creatinine clearance; DVT, deep vein thrombosis; IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; PCPS, percutaneous cardiopulmonary support; PE, pulmonary embolism; VTE, venous thromboembolism.
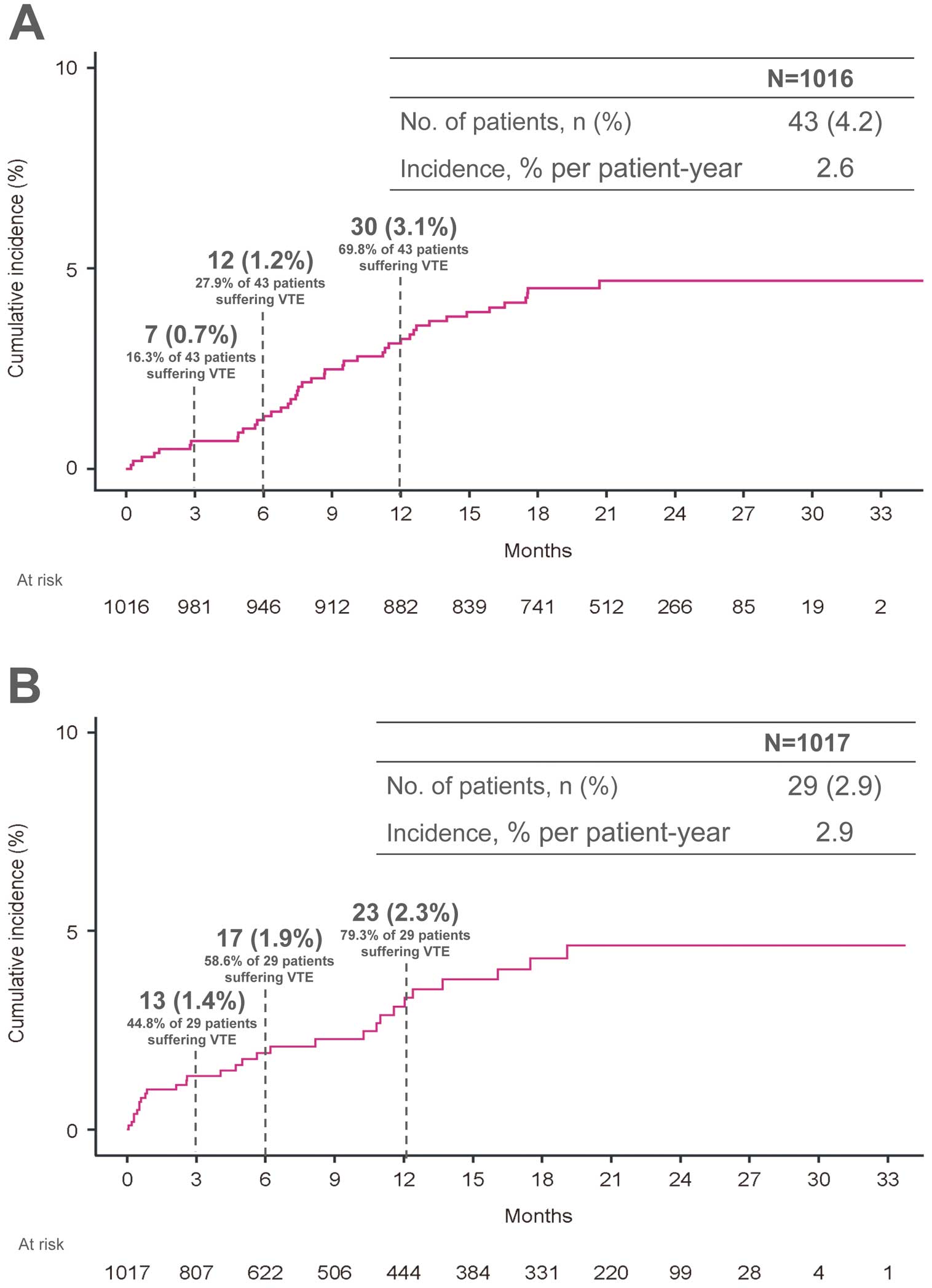
In the modified intention-to-treat population, the primary effectiveness outcome, recurrence or aggravation of symptomatic VTE occurred in 43 patients (4.2%), corresponding to an incidence rate of 2.6% per patient-year (95% CI, 1.8–3.3%) (Figure 2A). The cumulative numbers of patients with symptomatic recurrence or aggravation of VTE at 3, 6, and 12 months after enrollment were 7/43 patients (16.3%), 12/43 patients (27.9%), and 30/43 patients (69.8%), respectively. The incidence of major bleeding, according to the ISTH criteria, occurred in 29 patients (2.9%), corresponding to an incidence rate of 2.9% per patient-year (95% CI, 1.9–4.0%) (Figure 2B). The cumulative numbers of patients who experienced major bleeding at 3, 6, and 12 months after enrollment were 13/29 patients (44.8%), 17/29 patients (58.6%), and 23/29 patients (79.3%), respectively.
The incidences of the secondary effectiveness and safety outcomes are provided in
Table 2. All-cause mortality was 5.5% per patient-year (95% CI, 4.4–6.6%; 94/1,016 patients [9.3%]). Of these, VTE-related deaths and cardiovascular deaths accounted for 0.5% per patient-year (95% CI, 0.1–0.8%; 8/1,016 patients [0.8%]) and 0.8% per patient-year (95% CI, 0.3–1.2%; 13/1,016 patients [1.3%]), respectively. Fatal bleeding events occurred in 3/1,017 patients (0.3%), with a rate of 0.3% per patient-year (95% CI, 0.0–0.6%). Bleeding at a critical site occurred in 10 patients (1.0%), with intracranial hemorrhage in 6 patients (0.6%). Acute coronary syndrome during treatment occurred in 3/1,016 patients (0.3%) and ischemic stroke occurred in 8/1,016 patients (0.8%), resulting in rates of 0.2% and 0.5% per patient-year (95% CI, 0.0–0.4% and 0.1–0.8%), respectively. The composite outcome of clinically relevant events occurred in 151/1,016 patients (14.9%), resulting in a rate of 9.2% per patient-year (95% CI, 7.7–10.7%).
Table 2.
Clinical Outcomes for Patients
| |
No. of patients
(%) |
% per patient-year
(95% CI) |
| Primary effectiveness outcome |
| Recurrence or aggravation of symptomatic VTE |
43 (4.2) |
2.6 (1.8–3.3) |
| Secondary effectiveness outcome |
| Recurrence or aggravation of symptomatic PE |
20 (2.0) |
1.2 (0.7–1.7) |
| Recurrence or aggravation of symptomatic DVT |
26 (2.6) |
1.5 (1.0–2.1) |
| Acute coronary syndrome |
3 (0.3) |
0.2 (0.0–0.4) |
| Ischemic stroke |
8 (0.8) |
0.5 (0.1–0.8) |
| Death from any cause |
94 (9.3) |
5.5 (4.4–6.6) |
| Death related to VTE |
8 (0.8) |
0.5 (0.1–0.8) |
| Death related to cardiovascular disease |
13 (1.3) |
0.8 (0.3–1.2) |
| Principal safety outcome |
| Major bleeding |
29 (2.9) |
2.9 (1.9–4.0) |
| Fall in hemoglobin ≥2 g/dL |
14 (1.4) |
1.4 (0.7–2.2) |
| Transfusion of ≥2 units of packed red blood cells or whole blood |
12 (1.2) |
1.2 (0.5–1.9) |
| Critical site* |
10 (1.0) |
1.0 (0.4–1.6) |
| Fatal outcome |
3 (0.3) |
0.3 (0.0–0.6) |
| Secondary safety outcome |
| Non-major bleeding |
80 (7.9) |
8.4 (6.6–10.3) |
| Composite of clinically relevant events |
151 (14.9) |
9.2 (7.7–10.7) |
*A critical site was defined as follows: intracranial, intraarticular, intraocular/retinal, intramuscular with compartment syndrome, intrathecal, intrapericardial, or retroperitoneal bleeding. CI, confidence interval. Other abbreviations as in Table 1.
The incidences of clinical outcomes per VTE diagnosis are shown in
Figure 3. Recurrence or aggravation of symptomatic VTE occurred in 26/597 patients (4.4%) in the DVT group and 17/419 patients (4.1%) in the PE group, resulting in rates of 2.6% and 2.5% per patient-year (95% CI, 1.6–3.6% and 1.3–3.7%), respectively, with no significant difference observed (P=0.810 by log-rank test) (Figure 3A). Here, there were 4/26 patients (15.4%) in the DVT group and 3/17 patients (17.6%) in the PE group with recurrence or aggravation of symptomatic VTE recurrence within 3 months. In terms of major bleeding events, these occurred in 18/597 patients (3.0%) in the DVT group and 11/419 patients (2.6%) in the PE group, resulting in rates of 3.5% and 2.4% per patient-year (95% CI, 1.9–5.1% and 1.0–3.8%), respectively, with no significant difference (P=0.394 by log-rank test) (Figure 3B). Here, there were 6/18 patients (33.3%) in the DVT group and 4/11 patients (36.4%) in the PE group who experienced major bleeding within 3 months.

The patient characteristics associated with the composite of clinically relevant events are shown in
Table 3. Patients with clinically relevant events were older, had lower body weight, and had lower renal function. And those patients had a higher prevalence of chronic heart and lung disease, active cancer, and previous stroke. The composite of clinically relevant events did not differ significantly between the DVT group and the PE group. Multivariate Cox hazard model analysis revealed that male sex, low body weight (<50 kg), chronic heart and lung disease, active cancer, and previous stroke were independent determinants for the composite of clinically relevant events (Table 4).
Table 3.
Summary of Baseline Patient Characteristics Associated With the Composite of Clinically Relevant Events
| |
Overall
(N=1,016) |
Composite of clinically relevant events |
P value† |
| Yes (N=151) |
No (N=865) |
| Characteristics |
| Age (years) |
68.0±14.7 |
70.5±12.8 |
67.6±15.0 |
0.028 |
| ≥75 |
390 (38.4) |
64 (42.4) |
326 (37.7) |
0.278 |
| Female sex |
599 (59.0) |
79 (52.3) |
520 (60.1) |
0.074 |
| Body weight (kg) |
60.3±14.1 |
57.2±13.5 |
60.9±14.1 |
0.003 |
| <50 |
222/981 (22.6) |
48/148 (32.4) |
174/833 (20.9) |
0.003 |
| Body mass index (kg/m2) |
23.8±4.2 |
22.6±4.0 |
24.0±4.2 |
<0.001 |
| Outpatient |
422 (41.5) |
63 (41.7) |
359 (41.5) |
1 |
| Hypertension |
380 (37.4) |
55 (36.4) |
325 (37.6) |
0.855 |
| Diabetes mellitus |
118 (11.6) |
15 (9.9) |
103 (11.9) |
0.582 |
| Heart failure |
34 (3.3) |
5 (3.3) |
29 (3.4) |
1 |
| Atrial fibrillation |
29 (2.9) |
4 (2.6) |
25 (2.9) |
1 |
| Coronary artery disease |
45 (4.4) |
8 (5.3) |
37 (4.3) |
0.524 |
| Chronic heart and lung disease |
47 (4.6) |
13 (8.6) |
34 (3.9) |
0.019 |
| Active cancer |
193 (19.0) |
68 (45.0) |
125 (14.5) |
<0.001 |
| Previous stroke |
73 (7.2) |
21 (13.9) |
52 (6.0) |
0.002 |
| Previous VTE |
83 (8.2) |
11 (7.3) |
72 (8.3) |
0.750 |
| CrCl (mL/min) |
77.9±36.3 |
71.9±34.9 |
79.0±36.4 |
0.030 |
| <50 |
219/976 (22.4) |
47/146 (32.2) |
172/830 (20.7) |
0.004 |
| D-dimer (μg/mL), median (IQR) |
7.6 (3.5–15.1) |
9.9 (4.3–17.5) |
7.3 (3.4–14.7) |
0.180 |
| Concomitant medications |
| Antiplatelets |
104 (10.2) |
18 (11.9) |
86 (9.9) |
0.467 |
| Estrogen preparation |
23 (2.3) |
3 (2.0) |
20 (2.3) |
1 |
| Anticancer agents |
88 (8.7) |
42 (27.8) |
46 (5.3) |
<0.001 |
| NSAIDs |
196 (19.3) |
35 (23.2) |
161 (18.6) |
0.218 |
Numerical data are expressed as mean±standard deviation and categorical data as number (percentage) of patients unless otherwise stated. †Comparisons of patients with and without composite clinically relevant events by using the Student’s t-test or Fisher’s exact test, as appropriate. Abbreviations as in Table 1.
Table 4.
Patient-Related Determinants of a Composite Clinically Relevant Event
| Variable |
Hazard ratio (95% CI) |
P value* |
| Age (years)† |
1.01 (0.99–1.02) |
0.465 |
| Male sex |
1.68 (1.17–2.40) |
0.005 |
| Body weight <50 kg |
1.77 (1.18–2.66) |
0.006 |
| CrCl <50 mL/min |
1.28 (0.84–1.93) |
0.249 |
| Chronic heart and lung disease |
1.88 (1.05–3.36) |
0.034 |
| Active cancer |
4.15 (2.99–5.77) |
<0.001 |
| Previous stroke |
2.13 (1.32–3.45) |
0.002 |
*Pearson’s chi-squared test. †Continuous variable. CI, confidence interval; CrCl, creatinine clearance.
Discussion
The J’xactly Study is the largest real-world observational study for VTE treatment and prevention in Japanese patients with acute symptomatic/asymptomatic DVT, PE, or both. The study provides important insights into the effectiveness and safety of rivaroxaban for the treatment of VTE and the prognosis of VTE patients. The major findings are as follows: (1) a variety of Japanese patients, including those with low body weight, reduced renal function, and active cancer, were treated with rivaroxaban; (2) rivaroxaban was administered to patients without prior treatment for VTE and to outpatients, and a single-drug approach with rivaroxaban has recently been used in clinical practice for the management of VTE; and (3) the effectiveness and safety of rivaroxaban in real-world clinical practice were consistent with the findings in clinical trials, suggesting that rivaroxaban is a valuable treatment option for VTE in a broad range of patients.
There is an absence of data on the epidemiology, treatment, and prognosis of VTE in Japan, and there are limited data on the use of DOACs in patients with VTE in clinical practice.4
The patients in the J’xactly Study had similar demographic and clinical characteristics to those in 2 previous registry studies conducted in Japan; the JAVA study10
and the COMMAND VTE registry,20
but the present study participants had a higher prevalence of body weight <50 kg, CrCl <50 mL/min, and active cancer than those in studies from Western countries.27–29
In a pooled analysis of the global EINSTEIN-DVT and EINSTEIN-PE randomized trials,16
only 42 patients with body weight <50 kg were included in the rivaroxaban group, and thus there are few data to support the efficacy and safety of rivaroxaban 20 mg once daily for low-weight European VTE patients. For Japanese VTE patients, the J-EINSTEIN study demonstrated the efficacy and safety of rivaroxaban treatment, and the results of this study results were consistent with those of the EINSTEIN-DVT and EINSTEIN-PE studies.14–16
However, the J-EINSTEIN study included only 100 patients.17
Therefore, the results of the J’xactly Study may provide clinical insights into furthering our understanding of VTE treatment in not only Japanese VTE patients, but also some Western and Asian VTE patients with low body weight.
The J’xactly Study provides real-world data from routine clinical practice, which may aid current management strategies and treatment outcomes for patients with VTE. The inclusion of patients who did not receive anticoagulation therapy prior to receiving rivaroxaban suggests that these patients were managed by a single-drug approach with rivaroxaban from initial therapy through to maintenance therapy. In addition, the J’xactly Study included patients with characteristics that were not fully explored in previous randomized controlled trials in terms of efficacy and safety of rivaroxaban for VTE patients,14–17
such as low body weight (<50 kg), asymptomatic VTE, and distal DVT. Although the population in the J’xactly Study comprised a variety of patients who would be unique to a real-world clinical setting, the results of the study were consistent with the findings of the clinical trials. Recurrence of VTE within 12 months was observed in 30/1,016 patients (3.1%) in the J’xactly Study compared with 86/4,150 patients (2.1%) in the pooled analysis of the EINSTEIN-DVT and EINSTEIN-PE trials16
and in 1/71 patients (1.4%) in the J-EINSTEIN DVT and PE trial.17
Furthermore, real-world clinical studies investigating the effectiveness and safety of rivaroxaban in patients with VTE have mainly been conducted in Western countries.28–33
The results of the J’xactly Study were similar to the findings in these previous studies, although simple comparisons cannot be performed because of the differences in numbers and VTE details of the enrolled patients and follow-up periods. With respect to major bleeding within 12 months, the incidence rate was 23/1,017 patients (2.3%) in the J’xactly Study, 40/4,150 patients (1.0%) in the pooled analysis of the EINSTEIN-DVT and EINSTEIN-PE trials,20
and 0/71 patients in the J-EINSTEIN DVT and PE trial.17
These differences may be influenced by the inclusion of patients with a high risk of bleeding, such as elderly or tumor-bearing patients, in the J’xactly Study compared with the other 2 studies (mean age was 68.0 years in the J’xactly study, 56.9 years in the EINSTEIN-DVT and EINSTEIN-PE trials, and 65.7 years in the J-EINSTEIN DVT and PE trial, and active cancer prevalences were 19.0%, 5.2%, and 7.2%, respectively).
Patients with PE had a worse prognosis than patients with DVT, which is similar to the finding that patients with PE had a 2.5-fold higher rate of VTE recurrence than patients with DVT (7.0% vs. 2.8% per patient-year) in a study before DOAC treatment was approved for VTE.10
However, the rates of VTE recurrence in the J’xactly Study using rivaroxaban did not differ significantly between patients with PE and patients with DVT (2.5% vs. 2.6% per patient-year). In a previous study, DVT patients with residual thrombus had a higher recurrence rate than patients with thrombus resolution,34
whereas the J-EINSTEIN DVT and PE trial found that the thrombus resolution rate in patients with DVT or PE after initial reinforcement therapy was 31% in patients treated with rivaroxaban and 16% in patients treated with VKA. In terms of patients with PE, there were fewer patients with high disease severity, such as cardiac arrest and massive, in the J’xactly Study than in a previous survey conducted in Japan.1
Considering these facts, the results of the J’xactly Study may arise from younger patient age, lower prevalence of comorbidities, reduced severity of PE, and inclusion of patients with distal DVT and asymptomatic VTE. In addition, such a variety of patient characteristics might be related to the high prevalence of patients not complying with package insert of rivaroxaban for the initial dose, might be associated with the results of this study. Nevertheless, the single-drug rivaroxaban approach may be able to overcome the disadvantages of VKA treatment, such as more frequent bleeding. The present study findings will be useful for further standardization and optimization of VTE treatment in Japan.
The evaluation of composite clinically relevant events can more clearly represent the overall benefit–risk balance of treatment by analyzing efficacy and safety as aggregate outcomes. We defined composite clinically relevant events as a composite of recurrent VTE, cardiovascular disease (acute coronary syndrome and ischemic stroke), death from any cause, and major hemorrhage, and found an incidence of 9.2% per patient-year. In the multivariate Cox hazard analysis, male sex, underweight, active cancer, chronic heart and lung disease, and previous stroke were strongly associated with the incidence of composite clinically relevant events, whereas age and renal impairment were not retained as independent factors. These results are in line with previous reports that found that decreased renal function increases the risk of major bleeding in VTE patients treated with enoxaparin/VKA, but not with rivaroxaban.35
Depending on the severity of renal impairment and the presence of active cancer, rivaroxaban may be an attractive treatment option for a broad range of patients than VKA therapy.
Study Limitations
This study has several limitations. First, the study was a prospective observational study. The decisions regarding doses and duration of anticoagulation therapy during the follow-up period were at the discretion of the investigators. In addition, further detailed analyses, including subgroup analyses by patient characteristics, are needed because the patients in the study had a variety of characteristics. Second, there was selection bias because the patients enrolled in the study were administered rivaroxaban at the discretion of the attending physicians. Further, how to diagnose DVT or whether the presence of DVT was aggressively evaluated during the postoperative period is dependent on the physicians’ or institutions’ discretion, which might have also biased our outcome measurements. Nonetheless, diagnosis of DVT was generally done with ultrasound, as well as by measuring whether there was an increased level of D-dimer by the experienced physicians in this study. Third, the results of the study cannot be directly compared with the results of other treatments, because the J’xactly Study was a single-arm observational study. Finally, there were some patients who could not be included in the analysis because of issues such as withdrawal of consent, protocol violations, and duplicate enrollment. However, the excluded patients comprised ∼2% of the total number of patients and these exclusions may not have affected the results.
Conclusions
The J’xactly Study provides an important real-world observational study of VTE prognosis and treatment by rivaroxaban in Japanese patients with acute symptomatic/asymptomatic DVT and/or PE. The study findings support rivaroxaban treatment in specific Japanese patients with acute symptomatic/asymptomatic DVT and/or PE.
Acknowledgments
The authors wish to thank all of the centers that participated in this study and all of the patients who provided their consent to participate. We thank Serina Nakamoto and other members of Mebix for their assistance in the management of data collection, storage, and analysis. We also thank Masahiro Takita of Mebix for encouragement, and assistance with the reporting of our findings and writing the manuscript in English.
Funding
This study is financially supported by Bayer Yakuhin.
Disclosures
Y.O. has received lecture fees, research funding, scholarship funds, and donations from Bayer Yakuhin; lecture fees, scholarship funds, and donations from Daiichi-Sankyo; research funding from Bristol-Myers Squibb; scholarship funds and donations from Johnson & Johnson; and is associated with endowed departments sponsored by Boston Scientific Japan, Abbott Medical Japan, Medtronic Japan, Nihon Kohden, and Japan Lifeline. I.F. has received lecture fees from Bayer Yakuhin, Pfizer Japan, and Daiichi-Sankyo and research funding from Bayer Yakuhin and Pfizer Japan. M.N. has received lecture fees from Daiichi-Sankyo. N.Y. has received lecture fees from Bayer Yakuhin, Pfizer Japan, and Daiichi-Sankyo. T. Yamashita has received lecture fees, manuscript fees, and research funding from Daiichi-Sankyo, Bristol-Myers Squibb, and Bayer Yakuhin and lecture fees from Ono Pharmaceutical, Toa Eiyo, and Nippon Boehringer Ingelheim. T.I. has received lecture fees from Bayer Yakuhin, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim, and research funding from Daiichi-Sankyo. M.M. has received lecture fees from Bayer Yakuhin. T.K. has received lecture fees from Nippon Covidien. H. Matsuo has received lecture fees from Daiichi-Sankyo and Bristol-Myers Squibb. H.Y. has received lecture fees from Takeda Pharmaceutical, Otsuka Pharmaceutical, Amgen, and Bayer Yakuhin; and lecture fees, scholarship funds and donations from Daiichi-Sankyo. M.K. has received lecture fees from Bayer Yakuhin. A.H. has received lecture fees from Toa Eiyo, Nippon Boehringer Ingelheim, Sumitomo Dainippon Pharma, Amgen Astellas BioPharma, and AstraZeneca; lecture fees, scholarship funds, and donations from Bayer Yakuhin, Sanofi, Astellas Pharma, Bristol-Myers Squibb, and Daiichi-Sankyo; scholarship funds, donations, and is associated with endowed departments sponsored by Boston Scientific Japan and Otsuka Pharmaceutical; scholarship funds and donations from Takeda Pharmaceutical and Nihon Medi-Physics; and is associated with endowed departments sponsored by Fukuda Denshi, Abbott Medical Japan, Japan Lifeline, and Medtronic Japan. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. T. Yamashita, T.I., and A.H. are members of
Circulation Journal
’ Editorial Team.
IRB Information
The Institutional Review Board was the Nihon University Itabashi Hospital, Clinical Research Judging Committee, RK-160913-4.
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0636
References
- 1.
Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: A major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014; 34: 2363–2371.
- 2.
JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009): Digest version. Circ J 2011; 75: 1258–1281.
- 3.
Sakuma M, Nakamura M, Yamada N, Ota S, Shirato K, Nakano T, et al. Venous thromboembolism: Deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J 2009; 73: 305–309.
- 4.
Ota S, Matsuda A, Ogihara Y, Yamada N, Nakamura M, Mori T, et al. Incidence, characteristics and management of venous thromboembolism in Japan during 2011. Circ J 2018; 82: 555–560.
- 5.
Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost 2002; 88: 407–414.
- 6.
Kearon C. Natural history of venous thromboembolism. Circulation 2003; 107: I22–I30.
- 7.
Stein PD, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: A systematic review. Am J Med 2010; 123: 426–431.
- 8.
Baglin T, Douketis J, Tosetto A, Marcucci M, Cushman M, Kyrle P, et al. Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence?: A patient-level meta-analysis. J Thromb Haemost 2010; 8: 2436–2442.
- 9.
Lee CH, Cheng CL, Lin LJ, Tsai LM, Yang YH. Epidemiology and predictors of short-term mortality in symptomatic venous thromboembolism. Circ J 2011; 75: 1998–2004.
- 10.
Nakamura M, Miyata T, Ozeki Y, Takayama M, Komori K, Yamada N, et al. Current venous thromboembolism management and outcomes in Japan. Circ J 2014; 78: 708–717.
- 11.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352.
- 12.
Mavrakanas T, Bounameaux H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol Ther 2011; 130: 46–58.
- 13.
Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376: 1211–1222.
- 14.
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510.
- 15.
Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297.
- 16.
Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J 2013; 11: 21.
- 17.
Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MH, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism: The J-EINSTEIN DVT and PE program. Thromb J 2015; 13: 2.
- 18.
Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th edn. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e152S–e184S.
- 19.
Cardiovascular Disease Educational and Research Trust, Cyprus Cardiovascular Disease Educational and Research Trust, European Venous Forum, International Surgical Thrombosis Forum, International Union of Angiology, Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25: 101–161.
- 20.
Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, et al. Anticoagulation therapy for venous thromboembolism in the real world: From the COMMAND VTE Registry. Circ J 2018; 82: 1262–1270.
- 21.
Nakamura M, Yamada N, Asamura T, Shiosakai K, Uchino K. Safety and effectiveness of edoxaban in Japanese patients with venous thromboembolism: An interim analysis of data from a Japanese postmarketing observational study (ETNA-VTE-Japan). Circ J 2019; 83: 1394–1404.
- 22.
Okumura Y, Fukuda I, Nakamura M, Yamada N, Takayama M, Maeda H, et al. Design and rationale for the Japanese Registry of Rivaroxaban Effectiveness & Safety for the Prevention of Recurrence in Patients with Deep Vein Thrombosis and Pulmonary Embolism (J’xactly) study. BMJ Open 2018; 8: e020286.
- 23.
Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003; 349: 1695–1702.
- 24.
Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, Gent M, et al. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med 2007; 357: 1094–1104.
- 25.
Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, Gent M, et al. Extended prophylaxis of venous thromboembolism with idraparinux. N Engl J Med 2007; 357: 1105–1112.
- 26.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694.
- 27.
Ageno W, Haas S, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, et al. Characteristics and management of patients with venous thromboembolism: The GARFIELD-VTE Registry. Thromb Haemost 2019; 119: 319–327.
- 28.
Ageno W, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): An international, prospective, non-interventional study. Lancet Haematol 2016; 3: e12–e21.
- 29.
Kreutz R, Mantovani LG, Haas S, Monje D, Schneider J, Bugge JP, et al. XALIA-LEA: An observational study of venous thromboembolism treatment with rivaroxaban and standard anticoagulation in the Asia-Pacific, Eastern Europe, the Middle East, Africa and Latin America. Thromb Res 2019; 176: 125–132.
- 30.
Gaertner S, Cordeanu EM, Nouri S, Faller AM, Frantz AS, Mirea C, et al. Rivaroxaban versus standard anticoagulation for symptomatic venous thromboembolism (REMOTEV observational study): Analysis of 6-month outcomes. Int J Cardiol 2017; 226: 103–109.
- 31.
Kucher N, Aujesky D, Beer JH, Mazzolai L, Baldi T, Banyai M, et al. Rivaroxaban for the treatment of venous thromboembolism: The SWIss Venous ThromboEmbolism Registry (SWIVTER). Thromb Haemost 2016; 116: 472–479.
- 32.
Sindet-Pedersen C, Langtved Pallisgaard J, Staerk L, Gerds TA, Fosbol EL, Torp-Pedersen C, et al. Comparative safety and effectiveness of rivaroxaban versus VKAs in patients with venous thromboembolism: A Danish nationwide registry-based study. Thromb Haemost 2017; 117: 1182–1191.
- 33.
Larsen TB, Skjoth F, Kjaeldgaard JN, Lip GYH, Nielsen PB, Sogaard M. Effectiveness and safety of rivaroxaban and warfarin in patients with unprovoked venous thromboembolism: A propensity-matched nationwide cohort study. Lancet Haematol 2017; 4: e237–e244.
- 34.
Prandoni P, Lensing AW, Prins MH, Pesavento R, Piccioli A, Sartori MT, et al. The impact of residual thrombosis on the long-term outcome of patients with deep venous thrombosis treated with conventional anticoagulation. Semin Thromb Hemost 2015; 41: 133–140.
- 35.
Bauersachs RM, Lensing AW, Prins MH, Kubitza D, Pap AF, Decousus H, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J 2014; 12: 25.





