2023 年 87 巻 2 号 p. 345-347
2023 年 87 巻 2 号 p. 345-347
Although it is well known that an increased heart rate (HR) in patients with coronary artery disease (CAD) is associated with poor prognosis, data on the efficacy of β-blocker therapy in these patients are limited. In this issue of the Journal, Oba et al1 reconfirmed the worsening effect of increased HR in patients with overall CAD and report on the difference in effect of β-blockers in patients with CAD according to their clinical condition, including acute coronary syndrome (ACS) and chronic coronary syndrome (CCS), in a large Japanese population. We review the efficacy of β-blockers in patients with CAD, as stated by Oba et al, based on guidelines and previous reports (Figures 1,2).

Recommendation for β-blockers in stable coronary artery disease in JCS, ACC, and ESC guidelines.
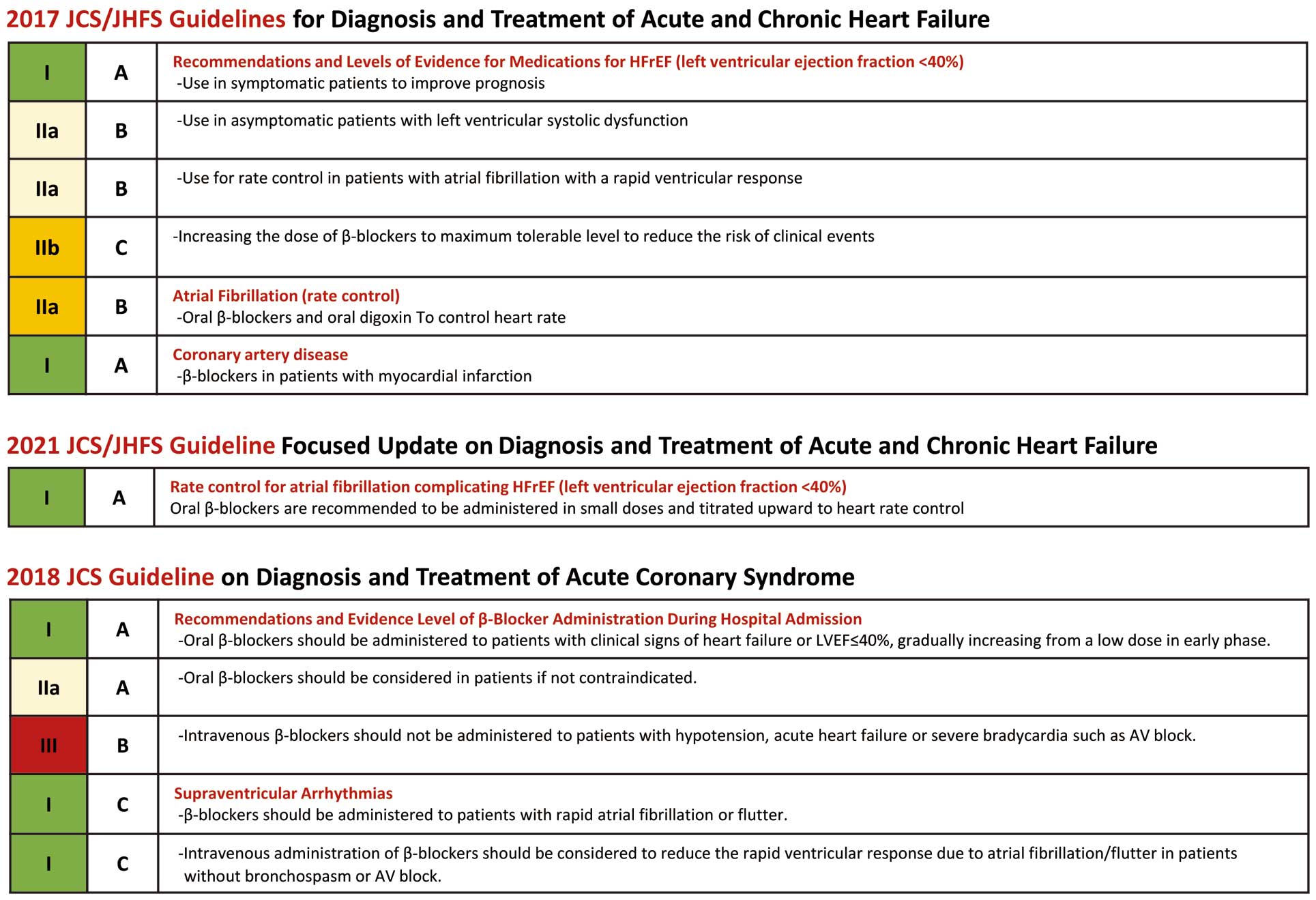
Recommendation for β-blockers in acute coronary syndrome, heart failure in JCS guidelines.
Article p 336
Impact of HR on Life ExpectancyThe total number of heartbeats in a lifetime remains fairly consistent across species, and there is an inverse relationship between resting HR and life expectancy.2 Epidemiological studies have shown that increased HR is related to cardiovascular death in otherwise healthy humans.3,4 Furthermore, an association between resting HR and death has been observed in patients with hypertension, metabolic syndrome, and stable CAD. Oba et al report that HR ≥75 beats/min was independently associated with major adverse cardiac and cerebral events (MACCE) in Japanese patients with CAD,1 in line with previous reports.2–4
How Does HR Affect Cardiovascular Events?A high HR leads to both greater myocardial oxygen consumption (MV̇O2) and decreased myocardial perfusion, the latter by shortening the duration of diastole, which can induce or exacerbate myocardial ischemia. In addition, the HR itself may contribute to the development of coronary atherosclerosis seen on coronary angiography,5 leading to an increased risk of coronary plaque destruction.6 In general, an accelerated HR is associated with cellular signaling events that lead to vascular oxidative stress, endothelial dysfunction, and accelerated atherogenesis. This hypothesis is supported by experimental data showing that a reduction in HR delays the progression of coronary atherosclerosis,7 and sinus node ablation reduces the development of coronary atherosclerosis in monkeys.8 Furthermore, Palatini9 reported that resting HR is a marker of autonomic nervous system tone and is significantly correlated with blood pressure, increased body mass index, and metabolic disturbances in large population studies. This association was particularly striking in patients with hypertension or diabetes, and cardiovascular morbidity related to a high HR depends mainly on the clustering of these risk factors. The prospective relationship between tachycardia and metabolic abnormalities found in longitudinal studies indicates that adrenergic overdrive is the cause rather than consequence of an insulin-resistant state.9
Does a Controlled HR Reduce Cardiovascular Events?On the therapeutic side, chronic administration of β-blockers leads to decreased HR and left ventricular reverse remodeling, effects that can be explained as follows: (1) reduced HR prolongs the diastolic time, increasing stroke volume according to the Frank-Starling law; (2) reduced HR increases coronary perfusion, improving exercise capacity; and (3) reduced HR decreases afterload and effective arterial elastance, resulting in increased stroke volume.10,11 Consequently, treatment with β-blockers reduces HR and improves life expectancy, particularly in patients post-myocardial infarction (MI) or with heart failure with reduced ejection fraction (HFrEF). Therefore, available evidence suggests that β-blocker use is beneficial in patients with acute MI (without impending shock or heart block) and may be effective in the short-to intermediate-term for patients after MI and those with chronic HF. The benefit of a higher dose of β-blockers is well established in patients with HFrEF.12,13 Kato et al showed that HR ≤71 beats/min and carvedilol ≥10 mg/day could be positive surrogate markers when titrating β-blockers in patients with HFrEF.12 Similar to these previous data, Oba et al report that ACS patients treated with a low dose of β-blockers had a significantly higher incidence of MACCE than those treated with a standard dose.1 Beta-blockers have more potential benefits in patients with ACS, given that these patients are usually at high risk of myocardial injury, reduced left ventricular contraction caused by ischemia, and fatal arrhythmias. Guidelines also strongly recommend the use of β-blockers for patients with EF <40%, but the present report did not address the relationship between the effect of β-blockers and HR based on cardiac function, which is an issue for future study.
However, current guidelines do not recommend the routine use of β-blockers in patients with CCS, after the results from the Reduction of Atherothrombosis for Continued Health (REACH) registry, which showed that in patients with either CAD risk factors only, known prior MI, or known CAD without MI, the use of β-blockers was not associated with a lower risk of composite cardiovascular events.14 The mechanism for the lack of efficacy of β-blockers includes decreased effectiveness at lowering central aortic pressure and adverse metabolic effects such as new diabetes and dyslipidemia. In addition, β-blockers are often not well tolerated and adherence may be suboptimal. In accordance with the REACH registry, Oba et al also did not find any association between β-blocker dose and MACCE incidence in patients with CCS.1 However, the fact that HR ≥75 beats/min was independently associated with MACCE in patients with CCS suggests that controlling HR to <75 beats/min is important and is associated with fewer cardiovascular events.
Although only theoretical, ivabradine may be an alternative for reducing HR because it does not affect cardiac contraction. However, because of limited data, the indication for ivabradine is limited to patients with HFrEF, as recommended by the JCS 2021 guideline.15 Therefore, only β-blockers are available for patients with CCS based on the guideline. In such cases, clinical outcomes with β-blockers may be better than expected if the goal is not to increase the dose of β-blockers but rather decrease the resting HR itself. Further dedicated studies regarding the correlation between controlled HR and β-blocker dose are necessary to determine the drugs’ effect on resting HR in patients with CCS.
ConclusionsIn the report by Oba et al,1 increased HR had a negative prognostic effect in Japanese patients with CAD, regardless of whether they had a history of ACS. In patients with ACS, β-blockers had enhanced efficacy in a dose-dependent manner, which is consistent with previous studies and guidelines. However, in patients with CCS, a controlled HR <75 beats/min may improve prognosis, regardless of β-blocker dose and use. Rate control may be of paramount importance for patients with CCS.
T.N. is an Associate Editor of Circulation Journal. K.T. has received lecture fees from Abbott Medical Japan Co., Ltd. and Edwards Life Sciences.