Methods
Experimental Model
All animal experiments were approved by the National Animal Experiment Board and conform to National Institutes of Health guidelines. Domestic pigs (a hybrid of Finnish-Norwegian Landrace swine) weighing 30–35 kg (n=28) were used in the study. After atropine (0.05 mg/kg i.m., Leiras, Helsinki, Finland), animals were sedated with azaperone (8 mg/kg i.m., Stresnil, Jansen, Titusville, NJ, USA) prior to anesthesia with propofol (15 mg/kg/h i.v., Propofol-Lipuro, Braun Medical, Melsungen, Germany). The animals were intubated and mechanically ventilated. Fentanyl (10 μg/kg/h i.v., Janssen), buprenorphine (0.3 mg i.m., Temgesic, BR Pharmaceuticals, Leeds, UK) and carprofen (4 mg/kg i.m. Rimadyl, Pfizer, New York, NY, USA) were used as analgesics. The animals received 100 mg aspirin (ASA Ratiopharm, Ratiopharm, Ulm, Germany) and 2.5 mg bisoprolol (Bisoprolol Orion, Orion, Espoo, Finland) starting 3 days before scaffold implantation until the end of the experiment. Clopidogrel (Plavix, Sanofi, Gentilly, France) was used with a loading dose of 150 mg before scaffold implantation and a dose of 75 mg daily until the end of the experiment. The animals received 30 mg enoxaparine (Klexane, Sanofi, Gentilly, France) daily after scaffold implantation to prevent scaffold thrombosis caused by the comparatively active coagulation system of the pigs. Enoxaparine was given i.v. before catheterization procedures (30 mg i.v.).
Animals were catheterized via the right femoral artery with a 6-F introducer sheath (Cordis, Bridgewater, NJ, USA) using the standard Seldinger technique. Angiography was performed using a GE Innova 3100IQ system (GE Healthcare, Waukesha, WI, USA). Amplatz-type guiding catheters were used to access and image the coronary arteries (left anterior descending artery [LAD], left circumflex artery [LCX] and right coronary artery [RCA]). Animals received 1 ABSORB BVS 1.1. or bare metal stent (BMS; ICROS, amg, Winsen-Luhe, Germany) either in the LAD, LCX or RCA. ABSORB scaffolds and ICROS stents were implanted in normal (non-atherosclerotic) vessels according to instructions to accommodate a device to artery ratio of 1.1:1. The target vessels underwent OCT immediately after device implantation. OCT was performed using a C7-XR intravascular imaging system and Dragonfly catheter (St. Jude Medical, St. Paul, MN, USA) with a pullback speed of 20 mm/s. Angiography and OCT were repeated 7 days, 1 month, 3 months, 6 months and 12 months after the index procedure.
Quantitative Coronary Angiography (QCA)
QCA was performed using a GE Advanced Workstation by an experienced blinded analyzer (J.H.). Measurements of minimum lumen diameter (MLD), reference lumen diameter, diameter stenosis (DS) and late lumen loss (LLL) at each time point (before, after the procedure, 7 days, 1, 3, 6, 12 months after implantation) were made. Reference lumen diameter was measured as mean lumen diameter at 5 mm proximal to the device segment. LLL was calculated as the difference in minimum lumen diameter between post-procedure and follow-up assessments. MLD, DS and LLL were measured on in-scaffold/stent analysis.
OCT
For OCT, quantitative measurements were performed across device segments and peri-device segments (≤5 mm proximal and distal to the device edge) at 1-mm intervals according to previously published methods.13
Parameters were measured using QCU-CMS version 4.69 (Leiden University Medical Center, Leiden, The Netherlands) by 2 experienced observers (T.A. and P.C.): mean and minimum lumen area, mean and minimum device area, neointimal area and device expansion index.8,14
Device and neointimal area were measured based on endoluminal scaffold/stent contour. The circularity of the lumen was evaluated using the eccentricity index, calculated as the ratio of minimum and the maximum lumen diameter per cross-section.15
The longitudinal variance in lumen diameter was assessed using the asymmetry index (1−minimum lumen diameter/maximum lumen diameter).15
Neointimal volume obstruction was calculated as mean endoluminal neointimal volume divided by mean endoluminal scaffold/stent volume.
Histology
The coronary arteries were harvested after perfusion fixation of the hearts with 1% paraformaldehyde and further fixed in 4% paraformaldehyde at 4℃ overnight, which were subsequently dehydrated and processed into methacrylate-based mold and sectioned for histology. Histological sections were stained with hematoxylin and eosin. Morphological measurements were performed for the cross-sectional area of external elastic lamina (EEL), internal elastic lamina (IEL), lumen, neointimal thickness and percent stenosis. The percent stenosis was calculated as (1−[lumen area/IEL area])×100. Neointimal thickness was defined as the distance from the inner surface of each strut to the luminal border. The degrees of vessel injury and neointimal inflammation were scored in accordance with the methods previously reported.16
Statistical Analysis
Data are expressed as mean±SD or median (IQR). Group means for continuous variables with normal and non-normal distributions were compared using Student’s t-test and Mann-Whitney U-test, respectively. Categorical variables were compared using the Pearson’s chi-squared test or Fischer’s exact test, as appropriate. Paired Student’s t-test or Wilcoxon signed rank test was applied for paired serial comparisons of OCT parameters (e.g., at baseline vs. at 1 month), as appropriate. On serial OCT analysis, a mixed-effects model (random intercept and slope) liner regression analysis was performed to assess the difference in the rate of change (slope) in each parameter between the ABSORB and ICROS group.17
In this mixed-model analysis, the repeated observations were clustered within a subject. Statistical significance was assumed at P<0.05. All statistical analysis was performed with SPSS version 24.0.0 (IBM, New York, NY, USA).
Results
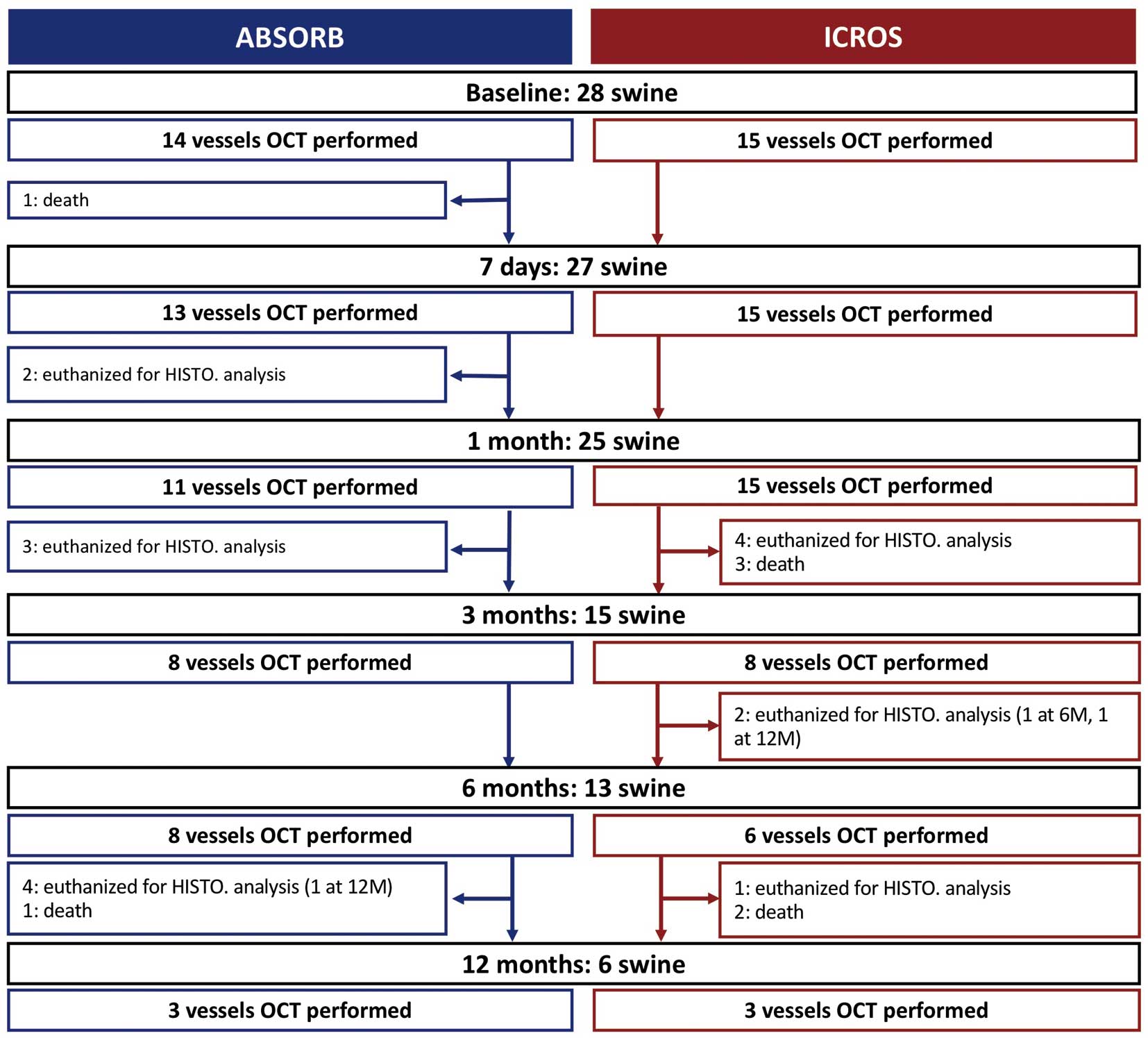
Twenty-eight swine with 29 vessels (14 vessels in the ABSORB group, 15 vessels in the ICROS group) were investigated on OCT analysis. Histological analysis was performed in 25 swine with 27 vessels (17 vessels in the ABSORB group, 10 vessels in the ICROS group), including 7 swine dedicated for histological analysis without OCT analysis. The flow chart of the current OCT study is presented in
Figure 1. During the study period, 2 swine died during the procedure and 19 swine were killed for the pathology analysis (Figure 1). During the course of the experiment (12 months), the swine grew from 30.4 kg median weight (IQR, 33.2–36.6 kg) to 270 kg median weight (IQR, 267–270 kg;
Figure 2).
Anatomical Site and Procedural Characteristics
The details of the anatomical site for device deployment are reported in
Table 1. No balloon dilatations were performed prior to device implantation. Mean nominal device diameter was numerically higher in the ABSORB groups (2.86±0.23 mm for ABSORB and 2.75±0.00 mm for ICROS, P=0.051). Post-balloon dilatation was performed after 11 implantations (78.6%) in the ABSORB group whereas 4 implantations (26.7%) were followed by post-balloon dilatation in the ICROS group (P=0.005;
Table 1).
Table 1.
Lesion and Procedure Characteristics
| |
ABSORB
(n=14) |
ICROS
(n=15) |
P-value |
| Vessel |
|
|
0.002 |
| LAD |
2 (14.3) |
7 (46.7) |
|
| LCX |
6 (42.9) |
5 (33.3) |
|
| RCA |
6 (42.9) |
3 (20) |
|
| Procedure |
| Pre-dilatation |
0 (0) |
0 (0) |
NA |
| Device implantation |
| Nominal device diameter (mm) |
2.86±0.23 |
2.75±0.0 |
0.051 |
| Device length (mm) |
18.0±0.0 |
18.0±0.0 |
1.000 |
| Maximum inflation pressure (atm) |
9.64±2.73 |
12.07±3.31 |
0.057 |
| Post-dilatation |
11 (78.6) |
4 (26.7) |
0.005 |
| Nominal balloon diameter (mm) |
3.05±0.35 |
3.13±0.14 |
0.661 |
| Balloon length (mm) |
15.0±0.0 |
15.0±0.0 |
1.000 |
| Maximum inflation pressure (atm) |
12.82±5.17 |
15.75±2.87 |
0.343 |
Data given as n (%) or mean±SD. LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
The results of QCA are summarized in
Supplementary Table 1. Post-procedural MLD was similar between the 2 groups (ABSORB, 2.60±0.36 mm vs. ICROS, 2.52±0.24 mm; P=0.400). At 1-month follow-up, MLD in the ABSORB group was significantly lower than in the ICROS group (1.78±0.40 mm vs. 2.32±0.28 mm, P=0.001). DS and LLL were significantly higher in the ABSORB group 1 month after the index procedure (DS, 38.3±11.7% vs. 13.5±7.9%, P<0.001; LLL, 0.75±0.36 mm vs. 0.20±0.24 mm, P<0.001). After 1-month follow-up, in the ABSORB group, MLD increased and reached 3.63±0.40 mm at the 12-month follow-up whereas MLD remained stable in the ICROS arm (2.23±0.49 mm at 12-month follow-up).
OCT
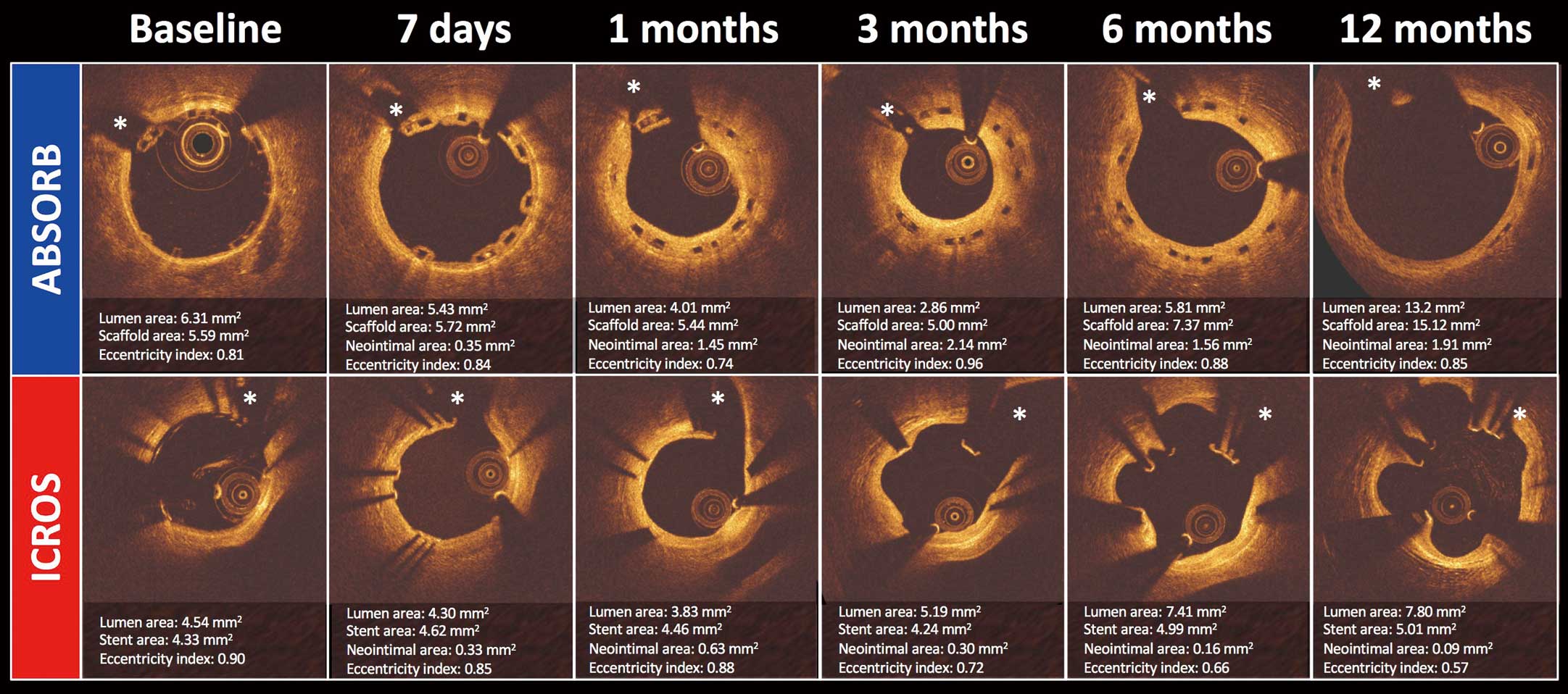
Representative serial OCT assessment in both groups is presented in
Figure 2. At 12 months, strut boxes of ABSORB were still clearly observed using OCT. The results of serial OCT are presented with body weight in
Table 2, Figure 3. In proportion to the swine growth, reference lumen area increased in both groups over 12 months (baseline, 5.46±1.82 for ABSORB vs. 5.52±0.82 mm2
for ICROS; 12 months, 10.78±1.68 for ABSORB vs. 9.12±1.83 mm2
for ICROS;
Figure 3A,3B).
Table 2.
Serial OCT and Body Weight
| |
Baseline |
7 days |
1 M |
3 M |
6 M |
12 M |
P-value‡ |
P-value for paired test |
| n |
Mean±SD |
n |
Mean±SD |
n |
Mean±SD |
n |
Mean±SD |
n |
Mean±SD |
n |
Mean±SD |
Baseline
vs. 7 days |
Baseline
vs. 1 M |
Baseline
vs. 3 M |
Baseline
vs. 6 M |
Baseline
vs. 12 M |
| Body weight (kg) |
| ABSORB |
14 |
31.59±3.55 |
13 |
35.93±4.42 |
11 |
53.02±18.35 |
8 |
107.68±26.65 |
8 |
168.25±41.81 |
3 |
269±1.730 |
0.988 |
0.001 |
0.003 |
0.012 |
0.012 |
0.109 |
| ICROS |
15 |
35.34±3.96 |
15 |
38.82±4.20 |
15 |
50.45±4.48 |
8 |
106.73±11.11 |
6 |
158.28±7.81 |
3 |
280±17.32 |
0.001 |
0.001 |
0.012 |
0.028 |
0.109 |
| P-value |
0.016 |
0.098 |
0.54 |
0.382 |
0.414 |
0.400 |
|
|
|
|
|
|
| Reference lumen area (mm2) |
| ABSORB |
14 |
5.46±1.84 |
13 |
4.84±1.24 |
11 |
5.58±2.10 |
8 |
6.58±1.95 |
8 |
7.93±1.89 |
3 |
10.78±1.68 |
0.240 |
0.011 |
0.657 |
0.069 |
0.012 |
0.109 |
| ICROS |
15 |
5.52±0.82 |
15 |
5.09±0.70 |
15 |
5.93±1.79 |
8 |
7.22±1.43 |
6 |
8.22±1.47 |
3 |
9.12±1.83 |
0.191 |
0.112 |
0.012 |
0.028 |
0.109 |
| P-value |
0.847 |
0.413 |
0.443 |
0.645 |
0.852 |
0.400 |
|
|
|
|
|
|
| Minimum lumen area (mm2) |
| ABSORB |
14 |
5.56±1.33 |
13 |
4.59±1.00 |
11 |
2.82±1.47 |
8 |
3.16±0.99 |
8 |
4.35±1.25 |
3 |
8.01±2.48 |
0.513 |
0.002 |
0.003 |
0.012 |
0.017 |
0.109 |
| ICROS |
15 |
5.11±0.80 |
15 |
4.42±0.88 |
15 |
3.68±1.20 |
8 |
3.86±1.18 |
6 |
4.85±1.93 |
3 |
3.94±1.72 |
0.002 |
0.001 |
0.036 |
0.833 |
1.000 |
| P-value |
0.290 |
0.717 |
0.109 |
0.234 |
0.852 |
0.200 |
|
|
|
|
|
|
| Mean lumen area (mm2) |
| ABSORB |
14 |
6.25±1.37 |
13 |
5.33±1.05 |
11 |
3.80±1.47 |
8 |
3.63±1.00 |
8 |
5.11±1.49 |
3 |
9.24±3.01 |
0.285 |
0.002 |
0.003 |
0.012 |
0.017 |
0.109 |
| ICROS |
15 |
5.79±0.91 |
15 |
4.91±0.88 |
15 |
4.53±1.18 |
8 |
4.72±1.40 |
6 |
6.27±2.39 |
3 |
6.76±3.85 |
0.001 |
0.003 |
0.050 |
0.600 |
0.593 |
| P-value |
0.27 |
0.274 |
0.217 |
0.083 |
0.491 |
0.400 |
|
|
|
|
|
|
| Minimum device area (mm2) |
| ABSORB |
14 |
5.22±1.15 |
13 |
4.81±0.98 |
11 |
4.74±1.13 |
8 |
4.52±0.82 |
8 |
5.70±1.27 |
3 |
10.22±2.83 |
0.006 |
0.011 |
0.008 |
0.050 |
0.036 |
0.109 |
| ICROS |
15 |
4.75±0.74 |
15 |
4.55±0.98 |
15 |
4.90±0.91 |
8 |
4.68±0.89 |
6 |
4.97±1.26 |
3 |
4.57±0.12 |
0.112 |
0.156 |
0.263 |
0.345 |
0.593 |
| P-value |
0.172 |
0.467 |
0.646 |
0.878 |
0.491 |
0.100 |
|
|
|
|
|
|
| Mean device area (mm2) |
| ABSORB |
14 |
5.81±1.25 |
13 |
5.37±0.97 |
11 |
5.26±1.21 |
8 |
5.09±0.84 |
8 |
6.63±1.61 |
3 |
11.58±3.48 |
0.003 |
0.023 |
0.006 |
0.036 |
0.017 |
0.109 |
| ICROS |
15 |
5.32±0.89 |
15 |
5.03±0.96 |
15 |
5.30±0.98 |
8 |
5.15±1.02 |
6 |
5.49±1.25 |
3 |
4.88±0.100 |
0.061 |
0.532 |
1.000 |
0.463 |
1.000 |
| P-value |
0.234 |
0.363 |
0.878 |
0.959 |
0.345 |
0.100 |
|
|
|
|
|
|
| Mean neointimal thickness (mm) |
| ABSORB |
|
|
13 |
0.03±0.01 |
11 |
0.21±0.10 |
8 |
0.20±0.08 |
8 |
0.18±0.05 |
3 |
0.20±0.02 |
0.121 |
NA |
NA |
NA |
NA |
NA |
| ICROS |
|
|
15 |
0.03±0.02 |
15 |
0.11±0.09 |
8 |
0.10±0.15 |
6 |
0.07±0.13 |
3 |
0.13±0.21 |
NA |
NA |
NA |
NA |
NA |
| P-value |
|
|
0.170 |
0.009 |
0.028 |
0.043 |
0.700 |
|
|
|
|
|
|
| Mean neointimal area (mm2) |
| ABSORB |
|
|
13 |
0.28±0.06 |
11 |
1.49±0.59 |
8 |
1.46±0.55 |
8 |
1.52±0.43 |
3 |
2.34±0.52 |
0.014 |
NA |
NA |
NA |
NA |
NA |
| ICROS |
|
|
15 |
0.23±0.14 |
15 |
0.87±0.64 |
8 |
0.76±1.01 |
6 |
0.51±0.93 |
3 |
0.86±1.42 |
NA |
NA |
NA |
NA |
NA |
| P-value |
|
|
0.156 |
0.013 |
0.028 |
0.043 |
0.400 |
|
|
|
|
|
|
| Neointimal volume (mm3) |
| ABSORB |
|
|
13 |
5.08±0.96 |
11 |
25.53±10.15 |
8 |
25.21±11.15 |
8 |
27.74±7.53 |
3 |
41.32±22.35 |
0.005 |
NA |
NA |
NA |
NA |
NA |
| ICROS |
|
|
15 |
3.49±1.89 |
15 |
13.42±9.52 |
8 |
9.91±10.64 |
6 |
10.67±21.98 |
3 |
8.08±12.47 |
NA |
NA |
NA |
NA |
NA |
| P-value |
|
|
0.008 |
0.004 |
0.021 |
0.043 |
0.200 |
|
|
|
|
|
|
| Neointimal volume obstruction (%) |
| ABSORB |
|
|
13 |
5.47±1.52 |
11 |
30.05±14.08 |
8 |
29.49±10.99 |
8 |
23.87±8.34 |
3 |
20.67±2.77 |
0.246 |
NA |
NA |
NA |
NA |
NA |
| ICROS |
|
|
15 |
4.43±2.32 |
15 |
16.53±11.85 |
8 |
14.32±19.81 |
6 |
9.82±18.28 |
3 |
17.46±28.61 |
NA |
NA |
NA |
NA |
NA |
| P-value |
|
|
0.201 |
0.015 |
0.028 |
0.043 |
0.700 |
|
|
|
|
|
|
‡Difference in the rate of change (slope) (baseline-12 months) between ABSORB and ICROS. M, month(s).
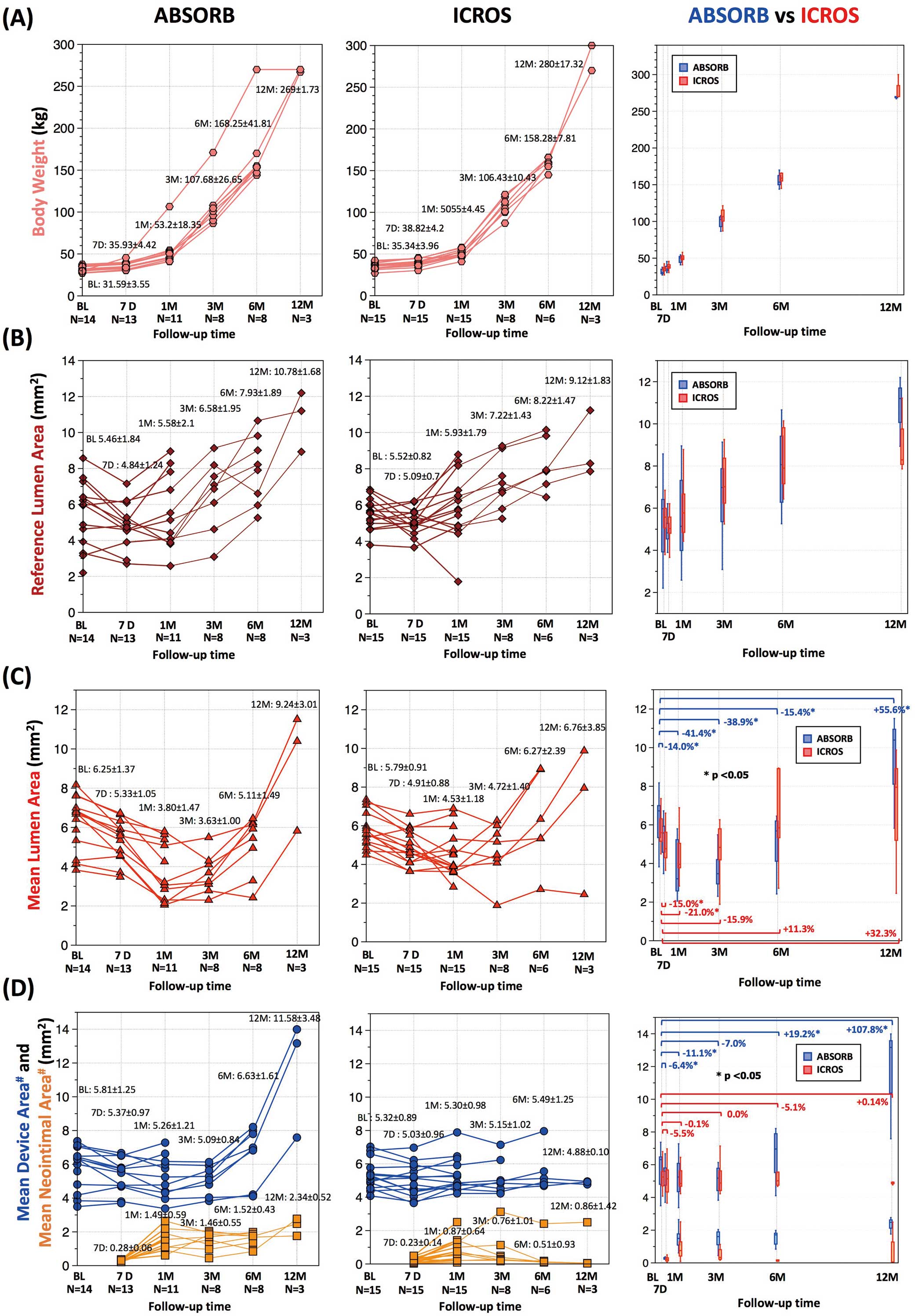
In the ABSORB group, mean lumen area decreased at 1 month (baseline, 6.25±1.37 mm2; 1 month, 3.80±1.47 mm2; relative change from baseline, −41.4±15.6%; P=0.003) and subsequently increased especially between 3 and 12 months (12 months, 9.24±3.01 mm2; relative change from baseline, +55.6±22.4%; P=0.109;
Figure 3C). In the ICROS group, lumen area decreased at 1 month (baseline, 5.79±0.91 mm2; 1 month, 4.53±1.18 mm2; relative change from baseline, −20.9±18.6%; P=0.003) and later increased by 12 months (12 months, 6.76±3.85 mm2; relative change from baseline, +32.3±83.6%; P=0.593;
Figure 3C). The mechanisms of lumen increase are fundamentally different: in the ABSORB group, a homogeneous concentric lumen increase was observed, whereas the increase in lumen area in the ICROS group was the result of evagination of the vessel wall between the struts, as observed in the representative case (Figure 2). The serial change in lumen eccentricity index in the ICROS group reflected the evagination process (Supplementary Figure 1;
Supplementary Table 2).
In the ABSORB group, mean device area decreased at 1 month (baseline, 5.81±1.25 mm2; 1 month, 5.26±1.21 mm2; relative change from baseline, −11.1±9.4%; P=0.006) and later increased especially between 3 and 12 months (12 months, 11.58±3.48 mm2; relative change from baseline, +107.8±25.7%; P=0.109). Scaffold discontinuity was observed as early as 1 month after implantation in 1 vessel. In that case, relative change in scaffold area at 1 month was −38.8%. In contrast, in the ICROS group, mean device area remained stable over time (baseline, 5.32±0.89 mm2; 12 months, 4.88±0.10 mm2; relative change from baseline, +0.14±7.95%; P=1.00;
Figure 3D).
Figure 3
shows the regression lines between time and mean lumen area (Figure 4A) or mean device area (Figure 4B) according to 2 time periods (baseline–1 month and 1–12 months). In this mixed-model linear regression analysis, the rate of decrease (negative slope) in mean lumen area was significantly steeper in the ABSORB group during the first 1 month (estimated mean difference in the slopes, −1.4 mm2
per month, 95% CI: −2.32 to −0.49, P=0.004), while there was no significant difference between the groups in the slopes, which were both positive, between 1 and 12 months (estimated mean difference in the slopes, 0.09 mm2
per month; 95% CI: −0.20 to 0.38, P=0.515;
Figure 4A).
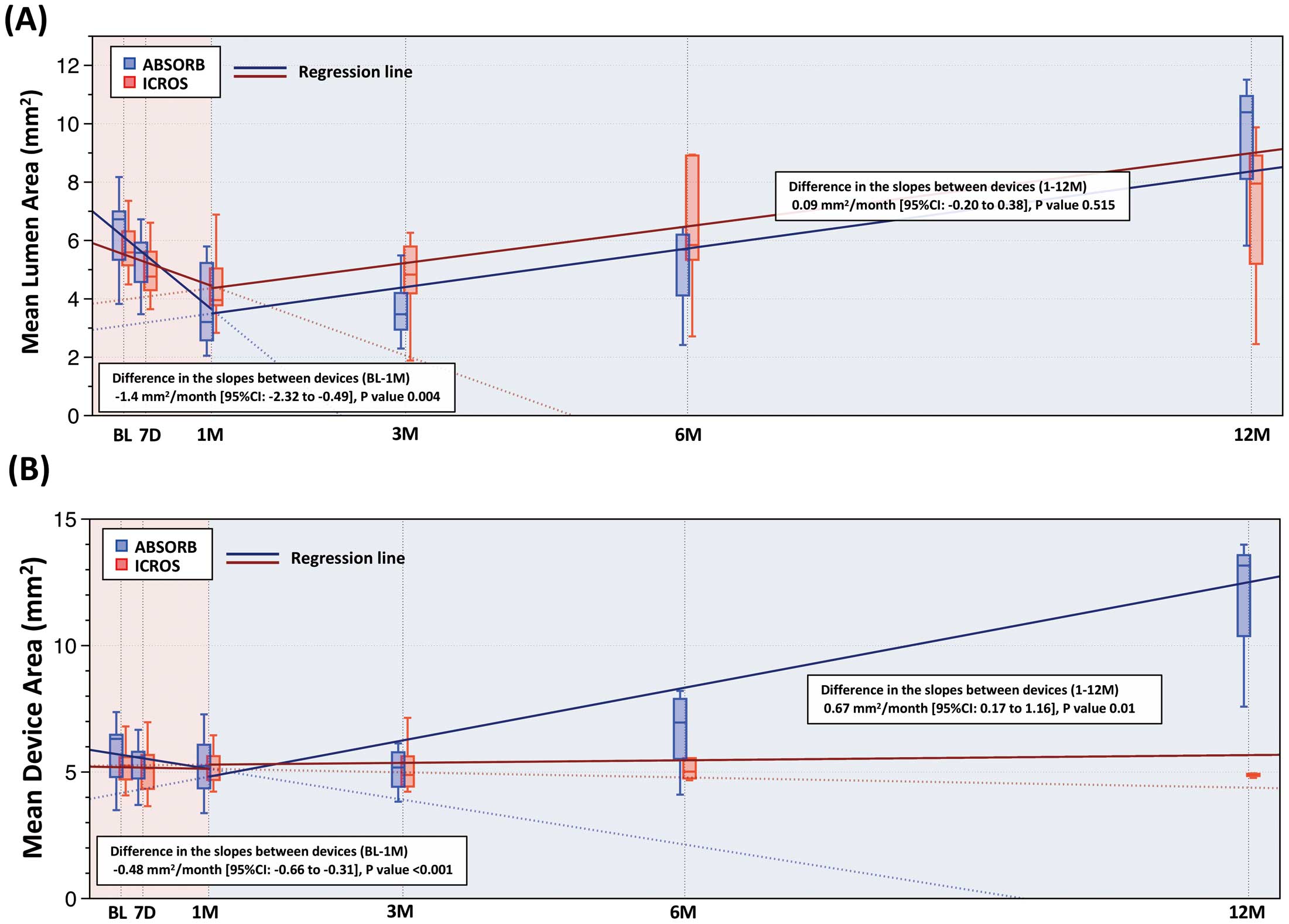
Similarly, the rate of decrease (negative slope) in mean device area was significantly steeper in the ABSORB group during the first 1 month (estimated mean difference in the slopes, −0.48 mm2
per month; 95% CI: −0.66 to −0.31, P<0.001). Device area in the ABSORB group increased while that in the ICROS group remained stable between 1 and 12 months (estimated mean difference in the slopes, 0.67 mm2
per month; 95% CI: 0.17 to 1.16, P=0.01;
Figure 4B).
Histology
Supplementary Table 3
lists the results of histological analysis. The inflammation scores were increased at 6 and 12 months in both arms (1 month, 0.42±0.38 in ABSORB vs. 0.39±0.05 in ICROS, P=0.63; 12 months, 1.11±0.07 in ABSORB vs. 0.80±0.28 in ICROS, P=0.10) whereas the injury scores were stable through 12 months.
Discussion
The major findings of this serial OCT study in a growing porcine model are as follows: (1) during the first 1 month, lumen area decreased in both the ABSORB and ICROS groups, and the rate of decrease in area was higher in the ABSORB group; (2) during the first 1 month, device area also decreased in the ABSORB group but remained stable in the ICROS group; (3) after the first month, lumen area increased in both groups due to growth of the animal; (4) thereafter, device area increased in the ABSORB group between 3 and 12 months but remained stable in the ICROS group. A schematic representation of the results is given in
Supplementary Figure 2.
Early-Phase Luminal Change
Lumen area reduction was observed in both groups in the early phase (the first 1 month) but the mechanism differed between the 2 groups. In the ICROS group, it appears that neointimal hyperplasia resulting from vessel injury after device implantation was mainly responsible for the lumen reduction, whereas in the ABSORB group, device recoil played an important role in the luminal reduction (Supplementary Figure 2).
The ABSORB BVS has a history of modification of its manufacturing process and strut design to achieve slower degradation and to enhance radial strength for the reduction of acute and late recoil. In the ABSORB Cohort A clinical trial, the first iteration of BVS 1.0 had a relatively high angiographic in-device LLL of 0.44 mm 6 months after implantation.18
In that trial, considerable “late recoil”, defined as reduction in stent area from after the procedure to the 6-month follow-up, was observed on intravascular ultrasound (IVUS; 11.8%). Based on these findings, the BVS was modified with improved performance, which was demonstrated in the ABSORB Cohort B clinical trial. In that trial, angiographic in-stent LLL of BVS model 1.1 was 0.19 mm 6 months after implantation, with the elimination of late recoil (relative scaffold area reduction on IVUS, 2.0%).19
Conversely, in the present animal study, we observed significant scaffold area reduction of the ABSORB BVS 1.1 at 1 month (11.1±9.4%, P=0.006), which played a major role in the greater luminal area reduction compared with the BMS (Figure 4). This discrepancy may be attributed to the difference in the cellular (smooth muscle cell etc.) and extracellular matrix (collagen contents etc.) reaction to vessel injury after device implantation between humans and the porcine model.20
The time frame over which constrictive remodeling develops also seems to differ between swine and human. In the porcine model, constrictive remodeling developed in ≤1 week after balloon angioplasty and peaked at 6 weeks while, in humans, constrictive remodeling progresses between 2 and 6 months after intervention.1,2,21,22
For future animal experiments for BRS, the differences in the remodeling process between human and animals should be taken into consideration.
Furthermore, in the current study, we observed 1 case of scaffold discontinuity at an early time point (1 month) with a major scaffold area reduction of 38.8%. This might be ascribed to an undetected disruption of scaffold created during the procedure. In that case, a 2.5 mm-sized scaffold was post-dilated by a 3.0-mm-sized balloon with a maximum pressure of 8 atm.
Late-Phase Luminal Change
In the current study, lumen enlargement was observed in both groups in the late phase (3–12 months). Vessel wall expansion has been previously attributed to shear stress or inflammation, although the current study showed the impact of the healthy growth process. In addition to growth, neointimal maturation and regression could have played a role in the ICROS group, a phenomenon explained by the replacement of water-trapping proteoglycans (hyaluronan and versican) by decorin and type I collagen and observed in several BMS studies.23,24
In the present study, however, the impact of this phenomenon on luminal enlargement was difficult to evaluate due to the evagination process (Figure 2).
In accordance with swine growth, vessel size gradually increased, as demonstrated by the increase in reference lumen area on OCT (5.46±1.84 mm2 at post-procedure assessment and 10.78±1.68 mm2 12 months after implantation). In the present growing animal model, ABSORB showed substantial adaptability to vessel enlargement. Device area in the ABSORB group began to increase following vessel enlargement between 3 months and 6 months after implantation. This resulted in the maintenance of the lumen circularity. Conversely, device area in the ICROS group remained stable over time despite the increasing luminal area. This phenomenon worsened lumen eccentricity due to coronary evagination, which was caused by the outward expansion of the vessel wall despite tethering by the fixed metallic struts hampering outward growth of the lumen (Figure 2, Supplementary Figure 2).25
In the current porcine study, scaffold area increased especially between 6 and 12 months following the growth of coronary artery, suggesting that the structural integrity of the device was outperformed by the expansive force of the growth process. In several reports, along with biodegradation, the radial support of ABSORB, which acts to counteract the constrictive force generated by barotrauma of the vessel, begins to decrease 6–12 months after implantation.7,26
Likewise, it would be expected that the structural integrity of ABSORB vs. the expansive force of the growth process decreases at the same time.
Supplementary Figure 3
shows a representative case with 2-D spread-out maps of ABSORB derived from OCT pullback.27
In
Supplementary Figure 3, along with the vessel growth, the scaffold was expanded with straightening of the crown of struts at 12 months, although motion artifact by heart beat precluded precise longitudinal assessment. Discontinuation of struts due to scaffold expansion was not clearly identified because the accuracy of the longitudinal assessment was limited.
Current Role of the ABSORB Scaffold and Future Implications
Despite the fact that the ABSORB scaffold was associated with decreased long-term efficacy and safety compared with current drug-eluting stents, this device can still be used as a benchmark for other devices when it comes to mechanical properties, especially in the early phase. ABSORB 1.1 has been developed to achieve substantial radial force to prevent recoil and constrictive remodeling in humans.19
Nevertheless, in the porcine model, considerable scaffold recoil (11.1±9.4%) was observed 1 month after implantation. This extent of recoil in the porcine model may be acceptable for human usage considering the difference in injury response of the vessel after device implantation between human and swine. One should bear in mind this difference for future device experiments in the porcine model.
In the ABSORB II trial, on IVUS 3 years after implantation, expansive vessel wall remodeling was more frequent and intense with the ABSORB BVS than the metallic everolimus-eluting stent.28
Assuming that the biodegradation process of ABSORB in the porcine model does not differ from that in humans, the late-phase adaptability of ABSORB observed in the current study may play an important role in the expansive remodeling of human coronary arteries with atherosclerosis.
In the present growing animal model, ABSORB demonstrated substantial growth conformity. In keeping with this observation, a magnesium scaffold (AMS; Biotronik, Bülach, Switzerland) was implanted in the left pulmonary artery of a preterm baby (1.7 kg) born at 26 weeks of gestation, which achieved left lung reperfusion that persisted throughout the 5-month follow-up period without complications.5
And the case of a 3-year-old child treated with ABSORB was reported with postmortem histology following non-cardiac death 3 months after implantation. On histology, substantial strut coverage with neointima was seen.29
Taken together these data suggest that the concept of BRS – full bioresorption after fulfilling the role of scaffolding – is potentially applicable for the treatment of pediatric patients.30
It should be noted, however, that the application of BRS technology in pediatric patients will be acceptable once the concerns of long-term safety are eliminated and the applicability for a small vessel is confirmed. On histology in the present study, ABSORB had increased inflammation scores 6 and 12 months after implantation. These results are in line with the report by Otsuka et al in a porcine model.16
In that report, the increased inflammation, which could cause instability of neointima, was presumably associated with the scaffold degradation process.
Study Limitations
There are several limitations in the current study. The current study was performed using a non-atherosclerotic porcine model. The potential difference in the biological vascular reactions and the growth rate after device implantation between the current model and human atherosclerotic coronary artery should be considered. The difference in procedural characteristics may influence the present results even though OCT measurements (lumen area, device area etc.) after the procedure were similar between the 2 arms. QCA and OCT analysts could not be blinded to the device type. Biomarker assessment for swine growth using insulin-like growth factor (IGF) or IGF-binding protein 3 was not performed, although somatic growth was obviously observed.31