Abstract
Background:
Data on the clinical outcomes of percutaneous coronary intervention (PCI) for unprotected left main coronary artery (LMCA) in patients with acute coronary syndrome (ACS) are limited. Therefore, this study aimed to assess the clinical outcome of patients with ACS who underwent PCI for LMCA culprit lesion.
Methods and Results:
Of 1,809 patients enrolled in the Assessing Optimal Percutaneous Coronary Intervention for the LMCA (AOI-LMCA) registry (a retrospective 6-center registry of consecutive patients undergoing LMCA stenting in Japan), the current study population consisited of 1,500 patients with unprotected LMCA stenting for LMCA ACS (ACS with shock: 115 patients, ACS without shock: 281 patients) and stable CAD (1,104 patients). The cumulative 180-day incidence of death was markedly higher in the ACS with shock group than in the other groups (49.5%, 8.6%, and 3.3%, respectively; P<0.0001), but mortality beyond 180-day was not significantly different among the 3 groups (30.2%, 20.4%, and 19.5%, respectively; P=0.65). In the ACS with shock group, the initial TIMI flow grade did not affect 5-year mortality (57.1% and 62.2%, P=0.99), but in the ACS without shock group, 5-year mortality was significantly higher in patients with initial TIMI flow grade ≤1 than in patients with TIMI flow grade ≥2 (44.4% and 23.7%, respectively; P=0.008).
Conclusions:
In patients with LMCA ACS, survival correlates with baseline hemodynamic and coronary flow status.
Acute coronary syndrome (ACS) caused by left main coronary artery (LMCA) disease often presents as cardiogenic shock and is associated with a subsequent high mortality rate.1–4
Acute occlusion involving the LMCA is a clinically severe adverse event causing cardiogenic shock, and fatal arrhythmias because of the large area of myocardium in jeopardy. The current clinical guidelines for ST-elevation myocardial infarction (MI) and non-ST-elevation ACS recommend primary percutaneous coronary intervention (PCI) in patients with cardiogenic shock,5,6
because emergency coronary reperfusion is the current evidence-based therapeutic intervention;7,8
however, data on the long-term clinical outcomes of PCI for unprotected LMCA in patients with ACS are limited. This study aimed to evaluate the long-term clinical outcomes of patients with ACS who underwent PCI for LMCA culprit lesion.
Methods
Study Design and Patient Population
The Assessing Optimal Percutaneous Coronary Intervention for the LMCA (AOI-LMCA) registry is a physician-directed, non-company-sponsored and retrospective multicenter registry that enrolls consecutive patients undergoing PCI using coronary stents, either drug-eluting (DES) or bare metal (BMS) for LMCA disease in Japan. From November 2004 to December 2012, 1,809 patients underwent LMCA stenting for the first time at 6 tertiary hospitals in Japan. The study design, patient enrollment, and main outcomes from the AOI-LMCA registry have been reported elsewhere.9
All procedures, including stent choice, stenting technique, and the duration of dual antiplatelet therapy, were left to the discretion of individual centers.
In the present analysis, we compared the baseline characteristics and clinical outcomes among 3 groups of patients undergoing unprotected LMCA stenting (ACS with shock, ACS without shock and stable coronary artery disease [CAD]). The diagnosis of shock was made at hospital admission. The study population consisited of 1,500 patients (ACS with shock: 115 patients, ACS without shock: 281 patients, stable CAD: 1,104 patients), after excluding 190 patients with protected LMCA disease, 6 patients who underwent LMCA PCI without stent use, 106 ACS patients with non-LMCA culprit lesion, 6 patients with missing information on ACS, and 1 patients with shock vital at admission without ACS (Figure 1).

The research protocol was approved by the local ethics committee of all 6 participating medical centers, which waived the requirement of obtaining informed consent from patients because of the retrospective nature of the study. However, we excluded those patients who declined to participate in this study when contacted for follow-up. All patient records were anonymized and de-identified before the analysis.
Definitions and Clinical Outcomes
We collected demographic, angiographic, and procedural data from hospital charts based on prespecified definitions. The details of the definitions used in this study were reported previously.9
We defined unprotected LMCA disease as angiographically significant stenosis of the LMCA without patent surgical grafts to the left coronary artery system. We defined cardiogenic shock as Killip class 4 at presentation, indicating systolic blood pressure <90 mmHg and manifestations of hypoperfusion such as oliguria, cyanosis and diaphoresis. ACS includes ST-segment elevation MI, non-ST-segment elevation MI on the basis of myocardial enzyme elevation, and unstable angina, which was defined as myocardial ischemia at rest or on minimal exertion in the absence of cardiomyocyte necrosis.
The primary outcome measure in this study was all-cause death. The secondary outcome measures included cardiac death, sudden death, MI, definite stent thrombosis, target lesion revascularization (TLR), any coronary revascularization, and major bleeding. We defined stent thrombosis according to the Academic Research Consortium definition.10
In addition, TLR was defined as either PCI or coronary artery bypass grafting (CABG) for restenosis or thrombosis of the target lesion, which involved the proximal and distal edge segments as well as the ostium of any side branches originating from the LMCA. Major bleeding was defined as Bleeding Academic Research Consortium categorization types 3 and 5.11
Statistical Analysis
We show continuous variables as the mean and standard deviation unless otherwise noted, and they were compared using Student’s t-test or the Wilcoxon rank-sum test based on their distributions. Categorical variables are presented as numbers and percentages, and were compared using the χ2
or Fisher’s exact test, as appropriate. Cumulative incidences of clinical events were estimated using the Kaplan-Meier method, and compared with log-rank test. We estimated the adjusted risks of the ACS with and without shock groups relative to the stable CAD group for individual endpoints using multivariable Cox proporional hazard models. We used 20 clinically relevant factors as the risk-adjusting variables (Table 1). Furthermore, we also conducted a landmark analysis evaluating the risks within and beyond 180 days after PCI. We also evaluated the influence of initial Thrombolysis in Myocardial Infarction (TIMI) flow grade on mortality in the 2 ACS groups with and without shock.
Table 1.
Baseline Characteristics of the ACS Patients
| Variable |
ACS with shock
(n=115) |
ACS without shock
(n=281) |
Stable CAD
(n=1,104) |
P value |
| Age (years) |
70.2±11.7 |
72.0±11.1 |
72.0±9.8 |
0.18 |
| ≥80* |
26 (23%) |
74 (26%) |
263 (24%) |
0.61 |
| Male |
89 (77%) |
205 (73%) |
834 (76%) |
0.63 |
| Hypertension |
62 (54%) |
194 (69%) |
853 (77%) |
<0.0001 |
| Dyslipidemia |
37 (33%) |
122 (43%) |
631 (57%) |
<0.0001 |
| Current smoker |
31 (27%) |
68 (24%) |
151 (14%) |
<0.0001 |
| Diabetes mellitus* |
39 (34%) |
104 (37%) |
478 (43%) |
0.03 |
| Treated with insulin |
7 (6.1%) |
24 (8.6%) |
121 (11%) |
0.14 |
| Familial history of CAD |
19 (18%) |
36 (13%) |
179 (18%) |
0.16 |
| eGFR ≤30 without HD* |
15 (13%) |
19 (6.8%) |
49 (4.4%) |
0.0004 |
| ESRD on HD* |
6 (5.2%) |
11 (3.9%) |
69 (6.3%) |
0.28 |
| Atrial fibrillation |
11 (9.6%) |
19 (6.8%) |
85 (7.7%) |
0.66 |
| Prior PCI* |
19 (17%) |
76 (27%) |
555 (50%) |
<0.0001 |
| Prior CABG |
0 |
2 (0.7%) |
52 (4.7%) |
<0.0001 |
| Prior MI |
16 (14%) |
51 (18%) |
350 (32%) |
<0.0001 |
| Prior stroke* |
13 (11%) |
38 (14%) |
144 (13%) |
0.81 |
| Prior HF* |
15 (13%) |
38 (14%) |
140 (13%) |
0.89 |
| COPD |
3 (2.6%) |
6 (2.1%) |
36 (3.3%) |
0.57 |
| Malignancy* |
14 (12%) |
24 (8.5%) |
109 (9.9%) |
0.56 |
| Aortic disease |
5 (4.4%) |
11 (3.9%) |
82 (7.4%) |
0.047 |
| PAD* |
14 (12%) |
26 (9.3%) |
158 (14%) |
0.06 |
| LVEF (%)† |
42.3±14.6 |
54.5±13.8 |
58.1±12.9 |
<0.0001 |
| EURO SCORE |
10.9±4.01 |
7.9±3.6 |
4.7±2.8 |
<0.0001 |
| EURO SCORE >6.0 |
96 (84%) |
173 (62%) |
267 (24%) |
<0.0001 |
| Clinical presentation of ACS |
|
|
|
<0.0001 |
| UA/NSTEMI |
28 (24%) |
226 (80%) |
|
|
| STEMI |
87 (76%) |
55 (20%) |
|
|
| Laboratory data |
| WBC (×103/μL) |
10.5±5.9 (n=102) |
8.2±3.9 (n=273) |
6.1±1.8 (n=1,073) |
<0.0001 |
| Hemoglobin (g/dL) |
12.9±2.9 (n=102) |
12.8±2.5 (n=273) |
12.7±4.3 (n=1,073) |
0.84 |
| Creatinine (mg/dL) |
1.52±1.44 (n=104) |
1.38±1.67 (n=274) |
1.38±1.79 (n=1,087) |
0.74 |
| LDL-C (mg/dL) |
112.6±39.0 (n=37) |
105.8±30.1(n=97) |
110.2±39.0 (n=691) |
0.99 |
| HbA1c (%) |
5.8±0.96 (n=28) |
6.0±1.2 (n=77) |
6.0±1.02 (n=650) |
0.70 |
| BNP (pg/mL) |
554.7±1,713.1 (n=65) |
375.6±534.0 (n=136) |
247.8±497.4 (n=261) |
0.02 |
| Albumin (g/dL) |
3.5±0.68 (n=101) |
3.8±0.5 (n=269) |
3.9±0.46 (n=947) |
<0.0001 |
| CRP (mg/L) |
1.4±3.0 (n=102) |
1.5±3.0 (n=250) |
0.59±1.3 (n=806) |
<0.0001 |
| Max CPK after PCI |
10,225±13,640 (n=86) |
2,539±4,490 (n=180) |
|
<0.0001 |
| Medications at discharge |
| Aspirin |
66 (96%, n=69) |
261 (98%, n=266) |
1,084 (99%, n=1,095) |
<0.0001 |
| Thienopyridine |
63 (91%, n=69) |
256 (96%, n=266) |
1,076 (98%, n=1,095) |
<0.0001 |
| Warfarin |
12 (17%, n=69) |
25 (9.4%, n=266) |
101 (9.2%, n=1,095) |
0.76 |
| Statins |
38 (55%, n=69) |
191 (72%, n=266) |
699 (64%, n=1,095) |
<0.0001 |
| β-blockers |
24 (35%, n=69) |
84 (32%, n=266) |
301 (28%, n=1,095) |
0.23 |
| ACEI/ARB |
41 (59%, n=69) |
167 (63%, n=266) |
599 (55%, n=1,095) |
0.003 |
| PPI |
42 (61%, n=69) |
148 (56%, n=266) |
423 (39%, n=1,095) |
<0.0001 |
Data are presented as number (%) or mean±SD unless otherwise noted. *Risk-adjusting variables selected for Cox proportional hazards models. †Calculated in 883 patients in the stable CAD group, in 64 patients in the ACS with shock group, and 217 patients in the ACS without shock group. ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic polypeptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPK, creatine phosphokinase; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; HD, hemodialysis; HF, heart failure; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; NSTEMI, non-ST-segment elevation MI; STEMI, ST-segment elevation MI; UA, unstable angina; WBC, white blood cell.
The statistical analysis in this study was conducted by a physician (H.H.), using JMP10.0 (SAS Institute Inc., Cary, NC, USA) software. All statistical analyses were two-tailed, and we considered P<0.05 as statistically significant. The authors had full access to and take full responsibility for the integrity of the data.
Results
Baseline Characteristics
The study cohort mainly comprised elderly patients without any significant differences among the 3 groups. We observed some significant differences among the groups in the baseline clinical characteristics. The prevalence of hypertension, dyslipidemia, diabetes mellitus, prior PCI, and prior CABG were significantly lower in the ACS groups than in the stable CAD group. In contrast, the Euro Score was higher in the ACS with shock group than others (Table 1).
Regarding lesion characteristics, isolated LMCA disease was significantly more common in the ACS with shock group than in the other groups. Total occlusion of the LMCA was more often observed in the ACS with shock (21.9%) and ACS without shock groups (3.2%) than in the stable CAD group. Intra-aortic balloon pumping (IABP) and percutaneous cardiopulmonary support (PCPS) were significantly more frequently used in the ACS groups than in the stable CAD group. In the ACS with shock group, IABP and PCPS were used in 85% and 26% of patients, respectively. There were no significant differences among the 3 groups in the stenting strategies, such as a 2-stenting strategy for LMCA bifurcation lesion, final kissing technique, post-optimization technique, using intravascular ultrasound (IVUS), and approach site. Furthermore, BMS were more frequently used in the ACS groups, especially in the ACS with shock group. No-reflow or slow flow of the left coronary artery during the procedure was more frequent in the ACS with shock (27.0%) and ACS without shock groups (6.1%) than in the stable CAD group (2.7%) (Table 2).
Table 2.
Lesion and Procedural Characteristics
| Variable |
ACS with shock
(n=115) |
ACS without shock
(n=281) |
Stable CAD
(n=1,104) |
P value |
| Extent of CAD |
|
|
|
0.03 |
| Left main only |
23 (20%) |
36 (13%) |
115 (10%) |
|
| 1-vessel disease |
27 (24%) |
70 (25%) |
347 (31%) |
|
| 2-vessel disease* |
43 (37%) |
107 (38%) |
428 (39%) |
|
| 3-vessel disease* |
22 (19%) |
68 (24%) |
214 (19%) |
|
| CTO |
25 (22%) |
44 (16%) |
191 (17%) |
0.42 |
| RCA-CTO |
10 (8.7%) |
10 (3.6%) |
75 (6.8%) |
0.06 |
| Stenosis (%) |
|
|
|
<0.0001 |
| Total occlusion |
25 (22%) |
9 (3.2%) |
11 (1.0%) |
|
| Non-occlusive stenosis |
90 (78%) |
271 (97%) |
1,093 (99%) |
|
| TIMI grade |
|
|
|
<0.0001 |
| ≤1 |
25 (22%) |
9 (3.2%) |
11 (1.0%) |
|
| 2 |
32 (28%) |
38 (14%) |
29 (2.6%) |
|
| 3 |
58 (50%) |
234 (83%) |
1,064 (96%) |
|
| AHA/ACC classification (B2/C lesion) |
92 (80%) |
223 (83%) |
997 (91%) |
|
| Emergency PCI |
114 (99%) |
264 (94%) |
20 (1.8%) |
|
| De novo lesion |
113 (98%) |
269 (96%) |
1,071 (97%) |
0.34 |
| Moderate/severe calcification* |
16 (14%) |
41 (15%) |
167 (15%) |
0.91 |
| Trifurcation |
12 (11%) |
29 (10%) |
131 (12%) |
0.74 |
| 2-stent procedure |
|
|
|
0.34 |
| Cullote |
18/23 (78%) |
45/62 (73%) |
131/189 (69%) |
|
| T-stenting |
3/23 (13%) |
16/62 (26%) |
52/189 (28%) |
|
| V-stenting |
0/23 (0%) |
0/62 (0%) |
1/189 (0.5%) |
|
| Crush |
0/23 (0%) |
2/62 (0%) |
3/189 (1.6%) |
|
| Mini-crush |
2 (8.7%) |
1 (1.6%) |
2/189 (1.1%) |
|
| Stenting ostial LMCA |
92 (80%) |
231 (82%) |
899 (81%) |
0.83 |
| POT |
12 (10%) |
39 (14%) |
143 (13%) |
0.62 |
| Final kissing balloon technique |
71 (62%) |
204 (73%) |
737 (67%) |
0.10 |
| Stent type* |
|
|
|
<0.0001 |
| BMS |
60 (52%) |
76 (27%) |
150 (14%) |
|
| G1-DES |
22 (19%) |
117 (42%) |
576 (52%) |
|
| G2-DES |
33 (29%) |
88 (31%) |
378 (34%) |
|
| No. of implanted stents |
1.25±0.05 |
1.21±0.03 |
1.26±0.016 |
0.43 |
| Stent length (main branch) (mm) |
22.4±1.16 |
22.9±0.74 |
23.9±0.37 |
0.27 |
| Stent diameter (main branch) (mm) |
3.31±0.03 |
3.28±0.02 |
3.28±0.01 |
0.69 |
| Stent length (side branch) (mm) |
19.6±1.41 |
18.4±0.87 |
20.7±0.50 |
0.07 |
| Stent diameter (side branch) (mm) |
2.88±0.08 |
2.93±0.05 |
2.88±0.03 |
0.07 |
| Stent length (main branch) ≥30 mm* |
21 (18%) |
56 (20%) |
223 (20%) |
0.86 |
| Stent diameter (main branch) ≥3.5 mm* |
75 (65%) |
158 (56%) |
689 (62%) |
0.14 |
| IVUS* |
69 (60%) |
192 (68%) |
731 (66%) |
0.32 |
| Rotablator |
3 (2.6%) |
6 (2.1%) |
89 (8.1%) |
<0.0001 |
| IABP |
98 (85%) |
67 (24%) |
30 (2.7%) |
<0.0001 |
| PCPS |
30 (26%) |
5 (1.8%) |
3 (0.3%) |
<0.0001 |
| No-reflow/slow flow during procedure |
31 (27%) |
17 (6.1%) |
30 (2.7%) |
<0.0001 |
| Approach site |
|
|
|
0.73 |
| Radial artery |
18 (16%) |
55 (20%) |
209 (19%) |
|
| Brachial artery |
11 (9.6%) |
32 (11%) |
133 (12%) |
|
| Femoral artery |
86 (75%) |
194 (69%) |
759 (69%) |
|
Data are presented as number (%) or mean±SD unless otherwise noted. *Potential independent risk-adjusting variables selected for Cox proportional hazards models. ACC, American College of Cardiology; AHA, American Heart Association; BMS, bare metal stent; CTO, chronic total occlusion; G1-DES, 1st-generation drug-eluting stent; G2-DES, 2nd-generation drug-eluting stent; IABP, intra-aortic balloon pumping; IVUS, intravascular ultrasound; PCPS, percutaneous cardiopulmonary support; POT, proximal optimization technique; RCA, right coronary artery. Other abbreviations as in Table 1.
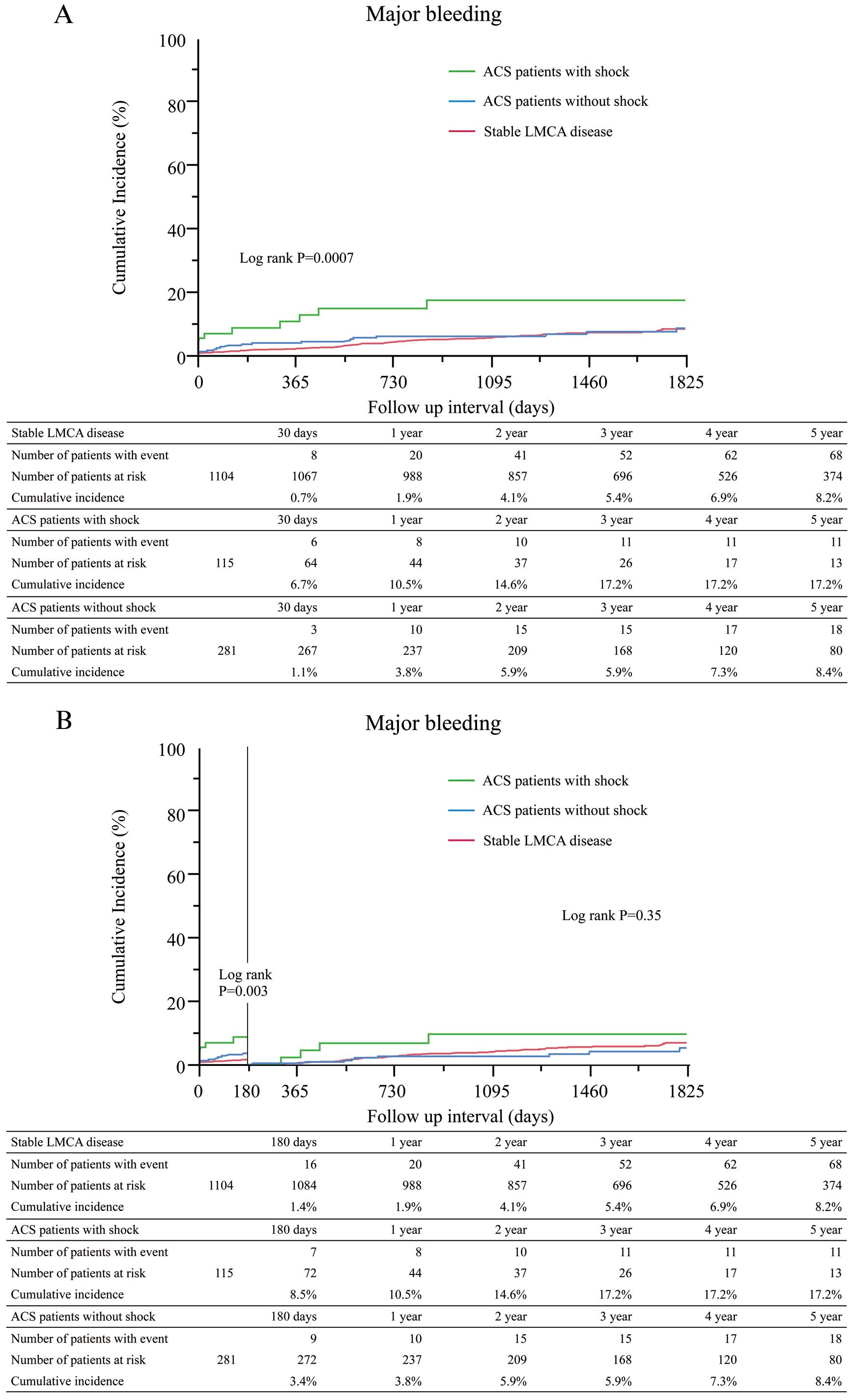
The median follow-up duration was 1,467 (IQR: 851–2,222) days in the stable CAD group, 95 (IQR: 13–1,117) days in the ACS with shock group, and 1,319 (IQR: 784–1,942) days in the ACS without shock group. The cumulative 5-year incidence of death was significantly higher in the ACS with shock group than in the other groups (Table 3, Figure 2A). The cumulative incidence of death at 180-day was markedly higher in the ACS with shock group than in the other groups, but the cumulative incidence of death beyond 180 days was not significantly different among the 3 groups (Figure 2B). After adjusting confounders, the excess mortality risk of the ACS with shock group relative to the stable CAD group remained significant, while the mortality risk of the ACS without shock group relative to the stable CAD group was not significant (Table 4). Regarding the 180-day incidence of in-hospital death, the ACS with shock group had a significantly higher incidence than the other groups (41% in ACS witho shock group, 5.4% in ACS without shock group, 0.8% in stable CAD group, P<0.0001) (Figure S1). The cumulative 5-year incidences of cardiac death, MI, stent thrombosis, and major bleeding were also significantly higher in the ACS with shock group than in the other groups (Table 3, Figure 3A). The cumulative incidence of major bleeding at 180 days was markedly higher in the ACS with shock group than in the other groups, but the cumulative incidence of major bleeding beyond 180 days was not significantly different among the 3 groups (Table 3, Figure 3B).
Table 3.
Crude Clinical Outcomes
| |
No. of patients with event (cumulative incidence, %) |
P value |
ACS with shock
(n=115) |
ACS without shock
(n=281) |
Stable CAD
(n=1,104) |
| 30-day |
| All-cause death |
42 (36.6%) |
8 (2.9%) |
13 (1.2%) |
|
| Cardiac death |
38 (33.3%) |
13 (5.4%) |
12 (1.1%) |
|
| Sudden death |
1 (1.0%) |
0 |
3 (0.3%) |
|
| MI |
1 (1.1%) |
2 (0.7%) |
8 (0.7%) |
|
| Definite ST |
3 (3.2%) |
3 (1.1%) |
6 (0.5%) |
|
| TLR |
3 (3.2%) |
6 (2.2%) |
5 (0.5%) |
|
| Any CR |
5 (6.0%) |
15 (5.5%) |
12 (1.1%) |
|
| Major bleeding |
6 (6.7%) |
3 (1.1%) |
8 (0.7%) |
|
| 180-day |
| All-cause death |
54 (48.5%) |
17 (8.6%) |
36 (3.3%) |
|
| Cardiac death |
49 (44.1%) |
7 (2.5%) |
18 (1.6%) |
|
| Sudden death |
1 (1.0%) |
2 (0.8%) |
7 (0.6%) |
|
| MI |
3 (4.4%) |
3 (1.1%) |
10 (0.9%) |
|
| Definite ST |
4 (5.0%) |
3 (1.1%) |
8 (0.7%) |
|
| TLR |
5 (6.9%) |
24 (9.2%) |
40 (3.7%) |
|
| Any CR |
10 (14.8%) |
47 (18%) |
83 (7.8%) |
|
| Major bleeding |
7 (8.5%) |
9 (3.4%) |
16 (1.4%) |
|
| 5-year |
| All-cause death |
65 (61.3%) |
55 (24.4%) |
179 (20.3%) |
<0.0001 |
| Cardiac death |
51 (46.4%) |
3 (9.5%) |
55 (6.5%) |
<0.0001 |
| Sudden death |
2 (3.0%) |
3 (1.2%) |
19 (2.2%) |
0.63 |
| MI |
4 (9.0%) |
9 (4.7%) |
23 (2.8%) |
0.04 |
| Definite ST |
5 (7.1%) |
3 (1.1%) |
8 (0.73%) |
<0.0001 |
| TLR |
8 (12.9%) |
38 (16.2%) |
140 (15.0%) |
0.58 |
| Any CR |
17 (29.6%) |
89 (41.2%) |
304 (32.5%) |
0.08 |
| Major bleeding |
11 (17.2%) |
18 (8.4%) |
68 (8.2%) |
0.0007 |
CI, confidence interval; CR, coronary revascularization; HR, hazard ratio; ST, stent thrombosis; TLR, target lesion revascularization. Other abbreviations as in Table 1.

Table 4.
Adjusted Clinical Outcomes: Overall Results and Landmark Analysis
| |
ACS with shock vs. Stable CAD |
ACS without shock vs. Stable CAD |
No. of patient
with event (crude
incidence rate) |
Adjusted HR
(95% CI) |
P value |
No. of patient
with event (crude
incidence rate) |
Adjusted HR
(95% CI) |
P value |
| Entire follow-up period |
| All-cause death |
66 (57.4%) vs.
217 (19.7%) |
5.29
(3.85–7.19) |
<0.0001 |
68 (24.2%) vs.
217 (19.7%) |
0.98
(0.48–1.77) |
0.95 |
| Cardiac death |
51 (44.3%) vs.
71 (6.4%) |
12.16
(7.86–18.80) |
<0.0001 |
30 (10.7%) vs.
71 (6.4%) |
1.20
(0.36–2.95) |
0.74 |
| Sudden death |
2 (1.7%) vs.
25 (2.3%) |
NA |
|
4 (1.4%) vs.
25 (2.3%) |
NA |
|
| MI |
5 (4.3%) vs.
31 (2.8%) |
5.15
(1.58–14.33) |
0.009 |
10 (3.6%) vs.
31 (2.8%) |
2.39
(0.56–6.99) |
0.21 |
| Definite ST |
5 (4.3%) vs.
8 (0.7%) |
NA |
|
4 (1.4%) vs.
8 (0.7%) |
NA |
|
| TLR |
9 (7.8%) vs.
155 (10.4%) |
0.98
(0.45–1.87) |
0.95 |
41 (14.6%) vs.
155 (10.4%) |
0.63
(0.22–1.42) |
0.30 |
| Any CR |
17 (14.8%) vs.
335 (30.3%) |
1.19
(0.70–1.91) |
0.50 |
92 (32.7%) vs.
335 (30.3%) |
1.14
(0.67–1.81) |
0.62 |
| Major bleeding |
12 (10.4%) vs.
82 (7.4%) |
3.32
(1.62–6.33) |
0.0017 |
23 (8.2%) vs.
82 (7.4%) |
1.26
(0.44–2.87) |
0.64 |
| Within 180 days |
| All-cause death |
56 (448.7%) vs.
36 (3.3%) |
11.13
(6.90–18.20) |
<0.0001 |
26 (9.3%) vs.
36 (3.3%) |
1.62
(0.39–4.56) |
0.46 |
| Cardiac death |
49 (42.6%) vs.
18 (1.6%) |
30.99
(16.78–59.56) |
<0.0001 |
7 (2.5%) vs.
18 (1.6%) |
1.79
(0.28–6.40) |
0.47 |
| Sudden death |
1 (0.9%) vs.
7 (0.6%) |
NA |
|
2 (0.7%) vs.
7 (0.6%) |
NA |
|
| MI |
3 (2.6%) vs.
10 (0.9%) |
4.43
(1.37–12.04) |
0.015 |
3 (1.1%) vs.
10 (0.9%) |
1.80
(0.28–6.42) |
0.47 |
| Definite ST |
4 (3.5%) vs.
8 (0.7%) |
9.73
(2.03–42.68) |
0.0058 |
3 (1.1%) vs.
8 (0.7%) |
NA |
|
| TLR |
5 (4.3%) vs.
35 (3.2%) |
1.45
(0.48–3.62) |
0.48 |
24 (8.5%) vs.
35 (3.2%) |
0.92
(0.22–2.57) |
0.89 |
| Any CR |
10 (8.7%) vs.
81 (7.3%) |
1.58
(0.74–3.06) |
0.22 |
47 (16.7%) vs.
81 (7.3%) |
1.80
(0.83–3.44) |
0.13 |
| Major bleeding |
7 (6.1%) vs.
16 (1.4%) |
6.13
(2.87–12.64) |
<0.0001 |
9 (3.2%) vs.
16 (1.4%) |
0.90
(0.05–4.34) |
0.91 |
| Beyond 180 days |
| All-cause death |
10 (17.9%) vs.
181 (17.2%) |
1.30
(0.63–2.39) |
0.45 |
42 (16.7%) vs.
181 (17.2%) |
0.81
(0.34–1.61) |
0.57 |
| Cardiac death |
2 (4.1%) vs.
53 (5.0%) |
1.11
(0.18–3.80) |
0.89 |
23 (9.2%) vs.
53 (5.0%) |
0.77
(0.12–2.61) |
0.72 |
| Sudden death |
1 (2.3%) vs.
18 (1.9%) |
NA |
|
2 (1.0%) vs.
18 (1.9%) |
NA |
|
| MI |
2 (3.9%) vs.
21 (2.0%) |
4.31
(0.61–19.06) |
0.13 |
7 (2.8%) vs.
21 (2.0%) |
2.42
(0.37–9.0) |
0.3 |
| Definite ST |
1 (2.0%) vs.
0 |
NA |
|
1 (0.4%) vs.
0 |
NA |
|
| TLR |
4 (8.0%) vs.
120 (11.8%) |
0.74
(0.22–1.82) |
0.55 |
17 (7.4%) vs.
120 (11.8%) |
0.43
(0.07–1.37) |
0.18 |
| Any CR |
7 (15.9%) vs.
254 (26.1%) |
0.83
(0.35–1.67) |
0.63 |
45 (22.1%) vs.
254 (26.1%) |
0.87
(0.39–1.65) |
0.69 |
| Major bleeding |
5 (6.9%) vs.
66 (6.1%) |
2.07
(0.69–5.05) |
0.18 |
34 (12.5%) vs.
66 (6.1%) |
1.19
(0.36–2.97) |
0.75 |
Abbreviations as in Tables 1,3.
In the ACS with shock group, 56 of 115 patients died within 180 days of LMCA stenting; of these, 75% died of refractory heart failure, which was the most frequent cause of death, 7% of bleeding, and 7% of infection. In contrast, 26 of 281 patients died within 180 days in the ACS without shock group; of these, 50% died because of refractory heart failure, 15% of arrhythmia, and 12% of infection.
Initial TIMI flow grade was ≤1 in 22% of patients in the ACS with shock group and in 3.2% of patients in the ACS without shock group (Table 2). In the ACS with shock group, the initial TIMI flow grade did not affect mortality, but in the ACS without shock group, the cumulative incidence of death was significantly higher in patients with initial TIMI flow grade ≤1 than in patients with TIMI flow grade ≥2 (Figure 4).
Discussion
The primary findings of this study were as follows. (1) Among the study patients undergoing LMCA stenting, ACS patients with shock had significantly higher mortality at 180 days than either ACS patients without shock or stable CAD patients, but mortality beyond 180 days was comparable across the 3 groups of patients. (2) In ACS patients with shock, the initial TIMI flow grade did not affect mortality, but in ACS patients without shock, initial TIMI flow grade ≤1 was associated with significantly higher mortality than TIMI flow grade ≥2.
Patients with ACS caused by LMCA disease have increased mortality and worse clinical outcomes compared with patients with stable CAD.12,13
Previous reports reported an early hazard after PCI in patients with ACS, with the in-hospital mortality rate being higher than the rate for scheduled PCI of LMCA disease.3,14–17
In particular, patients with ACS and cardiogenic shock exhibited 40% in-hospital mortality after PCI.1,9
In this study, the 180-day mortality of patients with ACS with shock was 49.5%, which corroborated previous reports.1
However, the 180-day mortality of the ACS without shock group was 8.6%, which was much lower than in the ACS with shock group. Furthermore, no significant differences were observed in all-cause death and cardiac death beyond 180 days among the 3 groups, suggesting that preventing short-term mortality is crucial in patients with ACS and culprit LMCA disease. This study suggested that the cause of most deaths within 180 days of the index PCI for LMCA in patients with ACS was refractory heart failure. In particular, 42 of 115 patients in the ACS with shock group died within 180 days from heart failure, suggesting that initial myocardial damage could be directly connected to short-term mortality.
The current clinical guidelines for ST-segment elevation MI and non-ST-segment elevation ACS recommend primary PCI in hemodynamically unstable patients,5,6
because emergency coronary reperfusion is the current evidence-based therapeutic intervention for patients with cardiogenic shock.7,8
In the ACS without shock group, patients with initial TIMI flow grade ≤1 had a similarly high mortality rate to patients in the ACS with shock group. We classified the hemodynamic status only at admission and not at time of PCI. It is plausible that for patients with initial TIMI flow grade ≤1 in the ACS without shock group their hemodynamics had deteriorated by the time of PCI. It seems crucial to achieve adequate revascularization by PCI before cardiogenic shock develops. The hemodynamics of patients with LMCA ACS can deteriorate rapidly, so in patients with LMCA ACS, PCI should be performed immediately to prevent cardiogenic shock even in patients initially not presenting with shock and without flow limitation in the LMCA.
The main reason for the increased early mortality in the ACS with shock group was refractory heart failure. In the present study, hemodynamic support devices such as IABP and PCPS were used in 85% and 26% of patients, respectively, in the ACS with shock group. The role of these conventional hemodynamic support devices has not been established.18–20
We also do not know the role of newer hemodynamic support devices such as IMPELLA.21–23
Nevertheless, all catheterization laboratory staff, including operators, should be well prepared for instituting hemodynamic support devices when treating LMCA ACS patients. In this study, the incidence of major bleeding within 180 days was significantly higher in the ACS with shock group than in the stable CAD group. The higher bleeding risk of the ACS with shock group could be explained by the higher prevalence of IABP and PCPS use, and the anticoagulant therapy associated with use of these hemodynamic support devices.
Study Limitations
First, there could be several unmeasured differences in patient and lesion backgrounds, making it difficult to draw definitive conclusions on the comparison across the 3 groups. In particular, regarding the prognosis of ACS, the door-to-balloon time, onset-to-balloon time and Rentrop grade may severely affect the short- and long-term clinical outcomes.24,25
However, we did not have these data. Second, this study did not have data of patients with LMCA disease who underwent CABG as a comparison group. Third, as the rate of BMS use was relatively high in the ACS groups, we could not assess the efficacy of DES for ACS with LMCA culprit lesion. Finally, the multivariable adjustment was not incorporated for assessment of the mortality rate according to the degree of stenosis of the LMCA culprit lesion because of the small number of patients with ACS. Also, we did not evaluate the hemodynamic status at the time of PCI.
In conclusion, in patients LMCA ACS, survival correlates with baseline hemodynamic and coronary flow status.
Disclosures
T.K. serves as an advisory board member for Abbott Vascular. The other authors report no conflicts of interest with regard to this manuscript.
Acknowledgments
We thank the staff of the catheterization laboratories and physicians at the participating centers.
Conflict of Interest Statement
None of the authors has any potential conflict of interest to declare with respect to this study.
Supplementary Files
Supplementary File 1
Figure S1.
Kaplan-Meier curves for in-hospital death.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-0896
References
- 1.
Montalescot G, Brieger D, Eagle KA, Anderson FA, Fitzgerald G, Lee MS, et al. Unprotected left main revascularization in patients with acute coronary syndromes. Eur Heart J 2009; 30: 2308–2317.
- 2.
Pedrazzini GB, Radovanovic D, Vassalli G, Sürder D, Moccetti T, Eberli F, et al. Primary percutaneous coronary intervention for unprotected left main disease in patients with acute ST-segment elevation myocardial infarction: The AMIS (Acute Myocardial Infarction in Switzerland) plus registry experience. JACC Cardiovasc Interv 2011; 4: 627–633.
- 3.
Sim DS, Ahn Y, Jeong MH, Kim YJ, Chae SC, Hong TJ, et al. Clinical outcome of unprotected left main coronary artery disease in patients with acute myocardial infarction. Int Heart J 2013; 54: 185–191.
- 4.
Patel N, De Maria GL, Kassimis G, Rahimi K, Bennett D, Ludman P, et al. Outcomes after emergency percutaneous coronary intervention in patients with unprotected left main stem occlusion: The BCIS national audit of percutaneous coronary intervention 6-year experience. JACC Cardiovasc Interv 2014; 7: 969–980.
- 5.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–e228.
- 6.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013; 61: e78–e140.
- 7.
Holmes DR Jr, Berger PB, Hochman JS, Granger CB, Thompson TD, Califf RM, et al. Cardiogenic shock in patients with acute ischemic syndromes with and without ST-segment elevation. Circulation 1999; 100: 2067–2073.
- 8.
Van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: A Scientific Statement from the American Heart Association. Circulation 2017; 136: e232–e268.
- 9.
Ohya M, Kadota K, Toyofuku M, Morimoto T, Higami H, Fuku Y, et al. Long-term outcomes after stent implantation for left main coronary artery (from the Multicenter Assessing Optimal Percutaneous Coronary Intervention for Left Main Coronary Artery Stenting Registry). Am J Cardiol 2017; 119: 355–364.
- 10.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 11.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the bleeding academic research consortium. Circulation 2011; 123: 2736–2747.
- 12.
Lee MS, Sillano D, Latib A, Chieffo A, Zoccai GB, Bhatia R, et al. Multicenter international registry of unprotected left main coronary artery percutaneous coronary intervention with drug-eluting stents in patients with myocardial infarction. Catheter Cardiovasc Interv 2009; 73: 15–21.
- 13.
Prasad SB, Whitbourn R, Malaiapan Y, Ahmar W, MacIsaac A, Meredith IT. Primary percutaneous coronary intervention for acute myocardial infarction caused by unprotected left main stem thrombosis. Catheter Cardiovasc Interv 2009; 73: 301–307.
- 14.
Rodés-Cabau J, DeBlois J, Bertrand OF, Mohammadi S, Courtis J, Larose E, et al. Nonrandomized comparison of coronary artery bypass surgery and percutaneous coronary intervention for the treatment of unprotected left main coronary artery disease in octogenarians. Circulation 2008; 118: 2374–2381.
- 15.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360: 961–972.
- 16.
Shemin RJ. Coronary artery bypass grafting versus stenting for unprotected left main coronary artery disease: Where lies the body of proof? Circulation 2008; 118: 2326–2329.
- 17.
Almudarra SS, Gale CP, Baxter PD, Fleming SJ, Brogan RA, Ludman PF, et al. Comparative outcomes after unprotected left main stem percutaneous coronary intervention: A national linked cohort study of 5,065 acute and elective cases from the BCIS registry (British Cardiovascular Intervention Society). JACC Cardiovasc Interv 2014; 7: 717–730.
- 18.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296.
- 19.
Prondzinsky R, Unverzagt S, Russ M, Lemm H, Swyter M, Wegener N, et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: The prospective, randomized IABPshock trial. Shock 2012; 37: 378–384.
- 20.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013; 382: 1638–1645.
- 21.
Ouweneel DM, Engstrom AE, Sjauw KD, Hirsch A, Hill JM, Gockel B, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABPin STEMI patients with cardiogenic pre-shock: Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol 2016; 202: 894–896.
- 22.
Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287.
- 23.
Engström AE, Cocchieri R, Driessen AH, Sjauw KD, Vis MM, Baan J, et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: The Academic Medical Center intensive care unit experience. Crit Care Med 2011; 39: 2072–2079.
- 24.
Shiomi H, Nakagawa Y, Morimoto T, Furukawa Y, Nakano A, Shirai S, et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: Observational study. BMJ 2012; 344: e3257.
- 25.
Brodie BR, Gersh BJ, Stuckey T, Witzenbichler B, Guagliumi G, Peruga JZ, et al. When is door-to-balloon time critical?: Analysis from the HORIZONS-AMI (Harmonizing outcomes with revascularization and stents in acute myocardial infarction) and CADILLAC (Controlled abciximab and device investigation to lower late angioplasty complications) trials. J Am Coll Cardiol 2010; 56: 407–413.





