Abstract
Despite the early loss of glycemic differences between the original intensive therapy group and conventional treatment in the DCCT/EDIC and UKPDS 80 trials, a continued reduction in microvascular risk and risk reductions for emergency myocardial infarction and all-cause death were observed 10–30 years after the end of these trials. These observations demonstrated that so-called “metabolic memory” could cause chronic abnormalities in diabetic vessels that are not easily reversed, even by subsequent improvement in blood glucose levels, thus suggesting a long-term beneficial influence of early metabolic control; that is, legacy effects on the risk of vascular complications and death in patients with both type 1 and type 2 diabetes. Formation and accumulation of advanced glycation endproducts (AGEs) are known to progress at an accelerated rate under diabetes. Furthermore, AGEs are hardly degraded and remain for a long time in diabetic vessels even after glycemic control is improved. Therefore, AGEs could explain why former cumulative diabetic exposure could contribute to current progression of vascular complications in diabetes. Here, the clinical utility of measurement of serum and tissue accumulation levels of AGEs for evaluating the prevalence and severity of numerous types of cardiovascular disease is reviewed and novel therapeutic strategies that could target the AGE-RAGE axis in CVD are discussed.
Monosaccharides, such as glucose, fructose and glyceraldehyde, can react non-enzymatically with the amino groups of proteins, nucleic acids, and lipids to form reversible Schiff bases, and then Amadori products.1–5
Over the course of days to weeks, these early products undergo further complex reactions, including rearrangement, dehydration and condensation to constitute a heterogeneous group of irreversible adducts termed “advanced glycation endproducts” (AGEs) (Figure 1).1–5
Nonenzymatic glycation of macromolecules alter their structural integrity and physiological function, the process of which progresses under aging, diabetic or inflammatory conditions.1–5
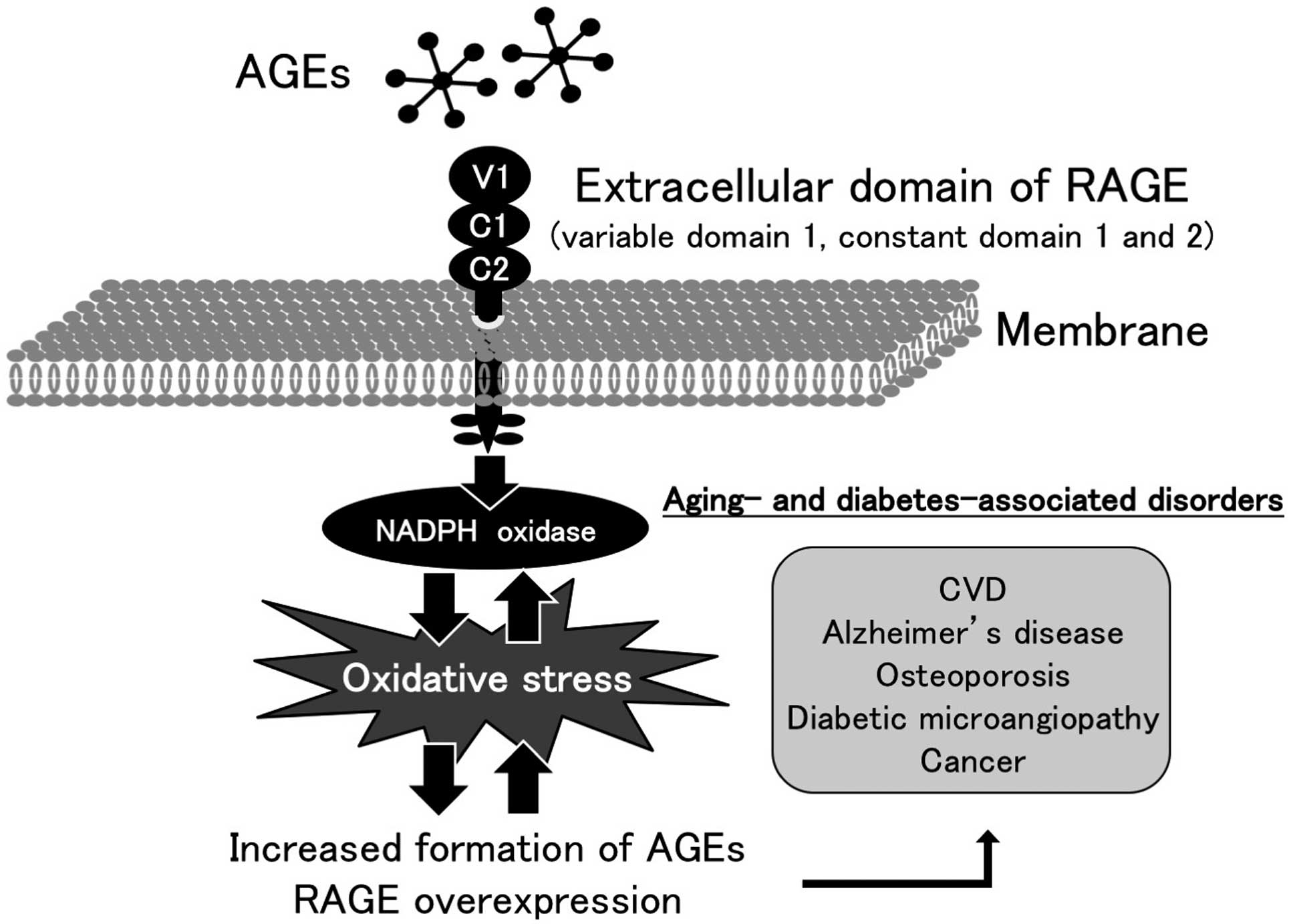
Moreover, AGEs are recognized by a cell surface receptor (RAGE) that belongs to the immunoglobulin superfamily, which could cause oxidative stress and resultantly evoke inflammatory and fibrotic reactions in numerous types of cells and tissues, thereby playing a crucial role in the development and progression of aging- and/or diabetes-associated disorders such as atherosclerotic cardiovascular disease (CVD) (Figure 2).6–16

There is accumulating evidence to show the pathophysiological involvement of “metabolic memory” in vascular complications in diabetes.17
Indeed, although intensive management of blood glucose for 6.5–10 years did not significantly reduce the risk of CVD or death in both type 1 and 2 diabetic patients, the 10–30-year follow-up studies of the Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS) revealed that the risk of CVD and all-cause death were significantly lower in the former intensive therapy group compared with conventional therapy, associated with continued risk reduction of microvascular complications.18–21
Moreover, in the Veterans Affairs Diabetes Trial, risk reduction of emergency CVD in diabetic patients originally assigned to intensive therapy was observed over a 10-year follow-up.22
These observations indicate that past cumulative diabetic exposure can persistently cause chronic abnormalities in diabetic vessels, kidneys, and hearts that are not easily reversed, even by subsequent, relatively good glycemic control.
AGEs are hardly degraded and remain for a long time in various types of diabetic tissues.1–5
Engagement of RAGE with AGEs can further promote the formation and accumulation of AGEs and induce RAGE expression via oxidative stress, thereby creating a positive feedback loop in an AGE-RAGE axis (Figure 2).6–16
In addition, skin levels of AGE-modified collagen at the close of the DCCT were reported to correlate with the progression of carotid artery intima-media thickness (IMT) during the follow-up period.23
These findings suggested that activation of the AGE-RAGE axis may partly explain the phenomenon of former diabetic exposure contributing to current progression of vascular complications in diabetes. Therefore, the clinical utility of measurement of serum and tissue accumulation levels of AGEs for evaluating the prevalence and severity of various kinds of CVD is reviewed and novel therapeutic strategies that could target the AGE-RAGE axis in CVD are discussed.
Tissue Levels of AGEs and CVD
Tissue accumulation of AGEs can be non-invasively measured as skin autofluorescence (SAF) by an AGE-Reader (Diagnoptics, Groningen, Netherlands).24,25
SAF is defined as the ratio of average autofluorescence over the entire 420–600-nm emission spectrum to that over 300–420 nm, which correlates with pentosidine and Nε-(carboxymethyl)lysine (CML), well-characterized fluorescent and non-fluorescent AGEs, respectively, in the skin.24,25
Although signals from skin fluorophores, such as NAD(P)H and FAD can affect the SAF values, skin AGEs have been shown to contribute 76% of the variance in the SAF signal from the AGE-Reader.26,27
Cross-linking modification of matrix proteins, such as collagen and elastin by AGEs, is involved in arterial and myocardial stiffness, which are associated with increased risk of CVD.5,25
SAF is independently associated with arterial stiffness evaluated by pulse wave velocity in type 1 diabetic patients without a history of CVD and in subjects with endstage renal disease (ESRD).28–30
Furthermore, SAF correlates with diastolic dysfunction and/or reduced aerobic capacity in diabetic patients with heart failure and in subjects on dialysis.31,32
SAF is independently associated with carotid IMT, a marker of atherosclerosis in non-diabetic patients without clinically manifest CVD.33
In addition, SAF significantly correlates with carotid IMT, high-sensitivity C-reactive protein, and plasma pentosidine, and is associated with the presence of CVD in hemodialysis subjects.34
Moreover, SAF is associated with low levels of circulating endothelial progenitor cells in patients with ESRD, suggesting the involvement of AGEs in impaired endothelial cell repair.35
SAF is also independently associated with macrovascular complications in patients with type 2 diabetes.36–38
SAF is significantly associated with coronary artery calcium score in patients with chronic kidney disease.39
Optical coherence tomography revealed that high SAF is associated with plaque vulnerability, such as thin-cap fibroatheroma, calcified plaques, and ruptured plaques in patients with CVD.40
SAF can predict future cardiovascular events in patients with ST-elevation myocardial infarction,41
and is also an independent predictor of graft loss in renal transplant recipients.42
SAF is significantly higher in patients with peripheral artery disease, which predicts future limb amputation, cardiovascular events and 5-year mortality independently of traditional risk factors.43–47
SAF is also higher in patients with chronic cerebral infarction or silent brain infarction compared with controls.48
SAF independently predicts overall and cardiovascular mortality risks in Caucasian patients on hemodialysis.49
In addition, SAF is associated with the prevalence of CVD and is an independent predictor of CVD death in Japanese subjects on hemodialysis.50,51
High SAF levels correlate with an increased risk of all-cause death in peritoneal dialysis patients; SAF values >3.61 arbitrary units predict death in these subjects.52,53
A systematic review revealed that SAF is significantly associated with a higher pooled risk estimate for death from cardiovascular causes and total mortality in high- risk patients such as those with ESRD.54
In a median 4-year follow-up study of more than 70,000 participants without diabetes or CVD, SAF was shown to predict the development of type 2 diabetes, CVD, and death, independently of classical risk factors.55
Accumulation of CML increases in the atrial appendage of patients with atrial fibrillation, which is associated with severity of fibrosis in both diabetic and non-diabetic subjects.56,57
Circulating Levels of AGEs and CVD
Serum levels of AGE levels correlate with the soluble form of RAGE, a marker that can reflect tissue RAGE expression in both non-diabetic and diabetic subjects.58–61
Furthermore, circulating levels of AGEs were independently associated with low-density lipoprotein cholesterol and thrombotic markers, such as plasminogen activator inhibitor-1 and fibrinogen, in a general population.62–64
In addition, AGEs levels correlate with inflammatory and/or endothelial cell damage biomarkers, including monocyte chemoattractant protein-1, soluble form of vascular cell adhesion molecule-1, and asymmetric dimethylarginine.60,61,65–67
AGEs not only correlate with vascular inflammation and endothelial dysfunction in high-risk patients for CVD,68,69
but are also associated with reduced number and migratory activity of endothelial progenitor cells.70
Serum levels of AGEs are reported to predict atherosclerotic plaque progression in patients with acute coronary syndrome (ACS).71
Serum levels of AGEs are elevated in patients with non-alcoholic steatohepatitis, and associated with insulin resistance irrespective of the presence or absence of non-alcoholic steatohepatitis.72–74
Indeed, AGEs positively correlate with serum levels of pigment epithelium-derived factor and dipeptidyl peptidase-4, markers of insulin resistance75,76
and inversely associated with adiponectin values.72
Circulating levels of AGEs are independently associated with inflammation in the visceral and subcutaneous adipose tissues.77
Circulating levels of AGEs, such as CML and methylglyoxal-derived hydroimidazolone, are reported to predict total mortality and/or CVD death in both type 1 and type 2 diabetic patients, subjects on hemodialysis, non-diabetic subjects, and patients with ACS.78–83
Increased levels of CML also predicted CVD death among older community-dwelling women.84
Moreover, non-diabetic older adults with plasma CML in the highest tertile had greater all-cause and CVD mortality compared with those in the lower 2 tertiles.85
Circulating levels of CML or pentosidine have also been reported to be an independent prognostic factor of heart failure.86,87
Therapeutic Intervention of the AGE-RAGE Axis
Diet and smoking are major environmental sources of pro-oxidative and pro-inflammatory AGEs.88–90
Fat-rich foods cooked at high temperature or ultraprocessed meat-derived products contain greater amounts of AGEs.91
On the other hand, foods prepared at lower temperature with more moisture, especially under acid conditions contain less AGEs.88–90
Because ≈7% of exogenously derived AGEs is considered to remain in the body,92,93
restriction of consumption of dietary AGEs is a novel therapeutic target that could block the AGE-RAGE axis.88–90,94–98
Indeed, a low-AGE diet improved renal function in overweight and obese individuals, which was associated with a reduction in inflammatory biomarkers, including serum levels of AGEs.95,96
In addition, restriction of dietary AGEs resulted in a significant decrease in the serum levels of C-reactive protein and plasminogen activator inhibitor-1 in patients with ESRD, which may contribute to cardiovascular protection.97
A recent meta-analysis of randomized controlled trials revealed that consumption of a low-AGE diet decreased the levels of total cholesterol, circulating AGEs, and inflammatory and oxidative stress biomarkers, such as tumor necrosis factor-α, soluble vascular cell adhesion molecule-1, and 8-isoprostanetotal, in patients with and without type 2 diabetes.98
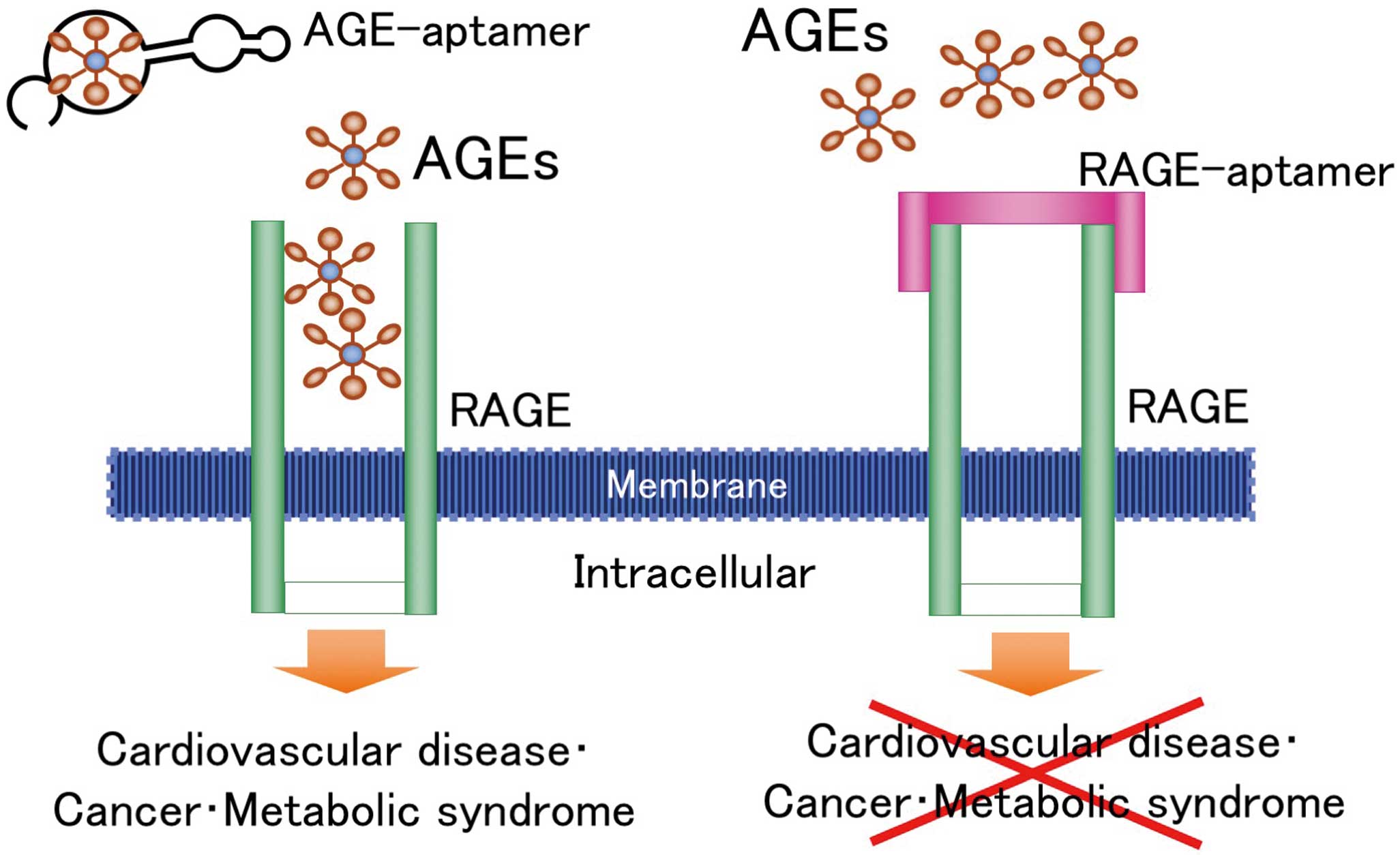
Aptamers are short, single-stranded RNA or DNA oligonucleotides that can bind to various proteins, peptides, and viruses with high specificity and affinity.99–101
DNA-aptamers directed against AGEs and RAGE (AGE-aptamers and RAGE-aptamers) that attenuate the interaction of AGEs with RAGE in vitro have been recently developed (Figure 3).102
The AGE-aptamer significantly attenuates neointimal formation after balloon angioplasty in rats through a reduction of platelet-derived growth factor-BB via suppression of the AGE-RAGE oxidative stress axis.103
Moreover, AGE-aptamers inhibited insulin resistance and adipose tissue remodeling in fructose-fed rats, progression of diabetic nephropathy in type 2 diabetic mice, development of diabetic retinopathy in type 1 diabetic rats, and growth of malignant melanoma in nude mice.104–107
Furthermore, RAGE-aptamers promoted regression of experimental diabetic nephropathy in type 1 diabetic animals, attenuated melanoma growth and metastasis in nude mice, and ameliorated renal injury in hypertensive mice.108–110
These findings suggest that blockade of the AGE-RAGE axis by AGE- or RAGE-aptamers may be a therapeutic target for CVD.
Perspectives
A growing body of evidence suggests the clinical utility of measuring tissue and circulating levels of AGEs for evaluating the severity and prognosis of various types of CVD. A recent pilot study revealed that switching dipeptidyl peptidase-4 inhibitors to an inhibitor of sodium-glucose cotransporter 2 ameliorated arterial stiffness in type 2 diabetic patients, which was associated with a reduction in the serum levels of AGEs.111
Further longitudinal study is needed to clarify whether a reduction in the burden of AGEs by sodium-glucose cotransporter 2 inhibitors or consumption of a low-AGE diet could contribute to CVD protection in patients with type 2 diabetes.
Sources of Funding
This study was supported in part by Grants-in-Aid for Scientific Research (no. 17K08968) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosures
The author received lecture fees from Sanofi K.K. and Eli Lilly, Japan.
References
- 1.
Vlassara H, Brownlee M, Cerami A. Accumulation of diabetic rat peripheral nerve myelin by macrophages increases with the presence of advanced glycosylation endproducts. J Exp Med 1984; 160: 197–207.
- 2.
Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol 1990; 45: B105–B111.
- 3.
Lee AT, Cerami A. Role of glycation in aging. Ann NY Acad Sci 1992; 663: 63–70.
- 4.
Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care 1992; 15: 1835–1843.
- 5.
Yamagishi S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res 2012; 15: 564–572.
- 6.
Vlassara H, Striker GE. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol Metab Clin North Am 2013; 42: 697–719.
- 7.
Schmidt AM, Stern D. Atherosclerosis and diabetes: The RAGE connection. Curr Atheroscler Rep 2000; 2: 430–436.
- 8.
Yamagishi S, Imaizumi T. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005; 11: 2279–2299.
- 9.
Ward MS, Fortheringham AK, Cooper ME, Forbes JM. Targeting advanced glycation endproducts and mitochondrial dysfunction in cardiovascular disease. Curr Opin Pharmacol 2013; 13: 654–661.
- 10.
Fukami K, Yamagishi S, Okuda S. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des 2014; 20: 2395–2402.
- 11.
Yamagishi SI, Matsui T. Role of ligands of receptor for advanced glycation end products (RAGE) in peripheral artery disease. Rejuvenation Res 2018; 21: 456–463.
- 12.
Jud P, Sourij H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res Clin Pract 2019; 148: 54–63.
- 13.
Yamagishi SI, Matsui T, Fukami K. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Res 2015; 18: 48–56.
- 14.
Takeuchi M, Yamagishi S. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des 2008; 14: 973–978.
- 15.
Yamagishi S, Matsui T. Role of receptor for advanced glycation end products (RAGE) in liver disease. Eur J Med Res 2015; 20: 15.
- 16.
Yamagishi S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets 2011; 12: 2096–2102.
- 17.
Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: A perspective on the concept of metabolic memory. J Diabetes 2017; 9: 141–148.
- 18.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653.
- 19.
Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: The DCCT/EDIC study 30-year follow-up. Diabetes Care 2016; 39: 686–693.
- 20.
Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015; 313: 45–53.
- 21.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589.
- 22.
Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197–2206.
- 23.
Monnier VM, Sun W, Gao X, Sell DR, Cleary PA, Lachin JM, et al. Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc Diabetol 2015; 14: 118.
- 24.
Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004; 47: 1324–1330.
- 25.
Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: A novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol 2015; 185: 263–268.
- 26.
Chen JH, Lin X, Bu C, Zhang X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr Metab (Lond) 2018; 15: 72.
- 27.
Yamagishi S. Are finger skin fluorophores other than advanced glycation end products (AGEs) associated with impaired musculoskeletal properties? J Gerontol A Biol Sci Med Sci, doi:10.1093/gerona/gly280.
- 28.
Llauradó G, Ceperuelo-Mallafré V, Vilardell C, Simó R, Gil P, Cano A, et al. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol 2014; 221: 405–413.
- 29.
Januszewski AS, Sachithanandan N, Karschimkus C, O’Neal DN, Yeung CK, Alkatib N, et al. Non-invasive measures of tissue autofluorescence are increased in Type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet Med 2012; 29: 726–733.
- 30.
Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, et al. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 2008; 57: 1452–1457.
- 31.
Willemsen S, Hartog JW, Hummel YM, van Ruijven MH, van der Horst IC, van Veldhuisen DJ, et al. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail 2011; 13: 76–82.
- 32.
Hartog JW, Hummel YM, Voors AA, Schalkwijk CG, Miyata T, Huisman RM, et al. Skin-autofluorescence, a measure of tissue advanced glycation end-products (AGEs), is related to diastolic function in dialysis patients. J Card Fail 2008; 14: 596–602.
- 33.
Lutgers HL, Graaff R, de Vries R, Smit AJ, Dullaart RP. Carotid artery intima media thickness associates with skin autofluoresence in non-diabetic subjects without clinically manifest cardiovascular disease. Eur J Clin Invest 2010; 40: 812–817.
- 34.
Tanaka K, Katoh T, Asai J, Nemoto F, Suzuki H, Asahi K, et al. Relationship of skin autofluorescence to cardiovascular disease in Japanese hemodialysis patients. Ther Apher Dial 2010; 14: 334–340.
- 35.
Ueno H, Koyama H, Fukumoto S, Tanaka S, Shoji T, Shoji T, et al. Advanced glycation end products, carotid atherosclerosis, and circulating endothelial progenitor cells in patients with end-stage renal disease. Metabolism 2011; 60: 453–459.
- 36.
Rigalleau V, Cougnard-Gregoire A, Nov S, Gonzalez C, Maury E, Lorrain S, et al. Association of advanced glycation end products and chronic kidney disease with macroangiopathy in type 2 diabetes. J Diabetes Complications 2015; 29: 270–274.
- 37.
Noordzij MJ, Mulder DJ, Oomen PH, Brouwer T, Jager J, Castro Cabezas M, et al. Skin autofluorescence and risk of micro- and macrovascular complications in patients with Type 2 diabetes mellitus: A multi-centre study. Diabet Med 2012; 29: 1556–1561.
- 38.
Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, et al. Skin autofluorescence is associated with severity of vascular complications in Japanese patients with Type 2 diabetes. Diabet Med 2012; 29: 492–500.
- 39.
Wang AY, Wong CK, Yau YY, Wong S, Chan IH, Lam CW. Skin autofluorescence associates with vascular calcification in chronic kidney disease. Arterioscler Thromb Vasc Biol 2014; 34: 1784–1790.
- 40.
Fujino Y, Attizzani GF, Tahara S, Wang W, Takagi K, Naganuma T, et al. Association of skin autofluorescence with plaque vulnerability evaluated by optical coherence tomography in patients with cardiovascular disease. Atherosclerosis 2018; 274: 47–53.
- 41.
Mulder DJ, van Haelst PL, Graaff R, Gans RO, Zijlstra F, Smit AJ. Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one-year incidence of major adverse cardiac events. Neth Heart J 2009; 17: 162–168.
- 42.
Hartog JW, Gross S, Oterdoom LH, van Ree RM, de Vries AP, Smit AJ, et al. Skin-autofluorescence is an independent predictor of graft loss in renal transplant recipients. Transplantation 2009; 87: 1069–1077.
- 43.
de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, et al. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol 2013; 33: 131–138.
- 44.
den Dekker MA, Zwiers M, van den Heuvel ER, de Vos LC, Smit AJ, Zeebregts CJ, et al. Skin autofluorescence, a non-invasive marker for AGE accumulation, is associated with the degree of atherosclerosis. PLoS One 2013; 8: e83084.
- 45.
de Vos LC, Boersema J, Mulder DJ, Smit AJ, Zeebregts CJ, Lefrandt JD. Skin autofluorescence as a measure of advanced glycation end products deposition predicts 5-year amputation in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol 2015; 35: 1532–1537.
- 46.
de Vos LC, Mulder DJ, Smit AJ, Dullaart RP, Kleefstra N, Lijfering WM, et al. Skin autofluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol 2014; 34: 933–938.
- 47.
Schmidt AM. Skin autofluorescence, 5-year mortality, and cardiovascular events in peripheral arterial disease: All that glitters is surely not gold. Arterioscler Thromb Vasc Biol 2014; 34: 697–699.
- 48.
Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Intern Med 2009; 48: 587–591.
- 49.
Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3687–3693.
- 50.
Kimura H, Tanaka K, Kanno M, Watanabe K, Hayashi Y, Asahi K, et al. Skin autofluorescence predicts cardiovascular mortality in patients on chronic hemodialysis. Ther Apher Dial 2014; 18: 461–467.
- 51.
Furuya F, Shimura H, Takahashi K, Akiyama D, Motosugi A, Ikegishi Y, et al. Skin autofluorescence is a predictor of cardiovascular disease in chronic kidney disease patients. Ther Apher Dial 2015; 19: 40–44.
- 52.
Mácsai E, Benke A, Kiss I. Skin autofluorescence and mortality in patients on peritoneal dialysis. Medicine (Baltimore) 2015; 94: e1933.
- 53.
Siriopol D, Hogas S, Veisa G, Mititiuc I, Volovat C, Apetrii M, et al. Tissue advanced glycation end products (AGEs), measured by skin autofluorescence, predict mortality in peritoneal dialysis. Int Urol Nephrol 2015; 47: 563–569.
- 54.
Cavero-Redondo I, Soriano-Cano A, Álvarez-Bueno C, Cunha PG, Martínez-Hortelano JA, Garrido-Miguel M, et al. Skin autofluorescence-indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: A systematic review and meta-analysis. J Am Heart Assoc 2018; 7: e009833.
- 55.
van Waateringe RP, Fokkens BT, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, et al. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia 2019; 62: 269–280.
- 56.
Begieneman MP, Rijvers L, Kubat B, Paulus WJ, Vonk AB, van Rossum AC, et al. Atrial fibrillation coincides with the advanced glycation end product N(ε)-(carboxymethyl)lysine in the atrium. Am J Pathol 2015; 185: 2096–2104.
- 57.
Yamagishi SI, Sotokawauchi A, Matsui T. Pathological role of advanced glycation end products (AGEs) and their receptor axis in atrial fibrillation. Mini Rev Med Chem 2019; 19: 1040–1048.
- 58.
Yamagishi S, Adachi H, Nakamura K, Matsui T, Jinnouchi Y, Takenaka K, et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism 2006; 55: 1227–1231.
- 59.
Nakamura K, Yamagishi SI, Matsui T, Adachi H, Takeuchi M, Imaizumi T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are correlated with AGEs in both diabetic and non-diabetic subjects. Clin Exp Med 2007; 7: 188–190.
- 60.
Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, et al. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc Res 2008; 76: 52–56.
- 61.
Yanagisawa K, Ashihara J, Obara S, Wada N, Takeuchi M, Nishino Y, et al. Switching to multiple daily injection therapy with glulisine improves glycaemic control, vascular damage and treatment satisfaction in basal insulin glargine-injected diabetic patients. Diabetes Metab Res Rev 2014; 30: 693–700.
- 62.
Enomoto M, Adachi H, Yamagishi S, Takeuchi M, Furuki K, Hino A, et al. Positive association of serum levels of advanced glycation end products with thrombogenic markers in humans. Metabolism 2006; 55: 912–917.
- 63.
Yamagishi S, Adachi H, Takeuchi M, Enomoto M, Furuki K, Matsui T, et al. Serum level of advanced glycation end-products (AGEs) is an independent determinant of plasminogen activator inhibitor-1 (PAI-1) in nondiabetic general population. Horm Metab Res 2007; 39: 845–848.
- 64.
Yamagishi S, Adachi H, Matsui T, Nakamura K, Takeuchi M, Enomoto M, et al. Low-density lipoprotein levels are one of the independent determinants of circulating levels of advanced glycation end products in nondiabetic subjects. Clin Cardiol 2009; 32: E12–E15.
- 65.
Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, et al. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev 2008; 24: 109–114.
- 66.
Ando R, Ueda S, Yamagishi S, Miyazaki H, Kaida Y, Kaifu K, et al. Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diab Vasc Dis Res 2013; 10: 436–441.
- 67.
Tahara N, Kojima R, Yoshida R, Bekki M, Sugiyama Y, Tahara A, et al. Serum levels of protein-bound methylglyoxal-derived hydroimidazolone-1 are independently CORRELATED with asymmetric dimethylarginine. Rejuvenation Res, doi:10.1089/rej.2018.2152.
- 68.
Tahara N, Yamagishi S, Takeuchi M, Honda A, Tahara A, Nitta Y, et al. Positive association between serum level of glyceraldehyde-derived advanced glycation end products and vascular inflammation evaluated by [(18)F]fluorodeoxyglucose positron emission tomography. Diabetes Care 2012; 35: 2618–2625.
- 69.
Kajikawa M, Nakashima A, Fujimura N, Maruhashi T, Iwamoto Y, Iwamoto A, et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015; 38: 119–125.
- 70.
Ueda S, Yamagishi S, Matsui T, Noda Y, Ueda S, Jinnouchi Y, et al. Serum levels of advanced glycation end products (AGEs) are inversely associated with the number and migratory activity of circulating endothelial progenitor cells in apparently healthy subjects. Cardiovasc Ther 2012; 30: 249–254.
- 71.
Fukushima Y, Daida H, Morimoto T, Kasai T, Miyauchi K, Yamagishi S, et al. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: The JAPAN-ACS sub-study. Cardiovasc Diabetol 2013; 12: 5.
- 72.
Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 2007; 22: 1112–1119.
- 73.
Tahara N, Imaizumi T, Takeuchi M, Yamagishi SI. Insulin resistance is an independent correlate of high serum levels of advanced glycation end products (AGEs) and low testosterone in non-diabetic men. Oxid Med Cell Longev 2010; 3: 262–265.
- 74.
Tahara N, Yamagishi S, Matsui T, Takeuchi M, Nitta Y, Kodama N, et al. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovasc Ther 2012; 30: 42–48.
- 75.
Yamagishi S, Matsui T, Adachi H, Takeuchi M. Positive association of circulating levels of advanced glycation end products (AGEs) with pigment epithelium-derived factor (PEDF) in a general population. Pharmacol Res 2010; 61: 103–107.
- 76.
Tahara N, Yamagishi S, Takeuchi M, Tahara A, Kaifu K, Ueda S, et al. Serum levels of advanced glycation end products (AGEs) are independently correlated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Clin Biochem 2013; 46: 300–303.
- 77.
Tahara N, Yamagishi SI, Kodama N, Tahara A, Honda A, Nitta Y, et al. Clinical and biochemical factors associated with area and metabolic activity in the visceral and subcutaneous adipose tissues by FDG-PET/CT. J Clin Endocrinol Metab 2015; 100: E739–E747.
- 78.
Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia 2007; 50: 1409–1417.
- 79.
Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, et al. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011; 34: 442–447.
- 80.
Wagner Z, Molnár M, Molnár GA, Tamaskó M, Laczy B, Wagner L, et al. Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis 2006; 47: 294–300.
- 81.
Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Birkeland KI, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: A population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 2005; 25: 815–820.
- 82.
Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, et al. Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis 2009; 205: 590–694.
- 83.
Raposeiras-Roubín S, Rodino-Janeiro BK, Paradela-Dobarro B, Almansour H, Grigorian-Shamagian L, Reino-Maceiras MV, et al. Advanced glycation end-products as long-term predictors of death and reinfarction after an acute coronary syndrome. Biomark Med 2015; 9: 209–216.
- 84.
Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, et al. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res 2009; 21: 182–190.
- 85.
Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc 2009; 57: 1874–1880.
- 86.
Hartog JW, Voors AA, Schalkwijk CG, Scheijen J, Smilde TD, Damman K, et al. Clinical and prognostic value of advanced glycation end-products in chronic heart failure. Eur Heart J 2007; 28: 2879–2885.
- 87.
Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, et al. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail 2007; 13: 199–206.
- 88.
Vlassara H. Advanced glycation in health and disease: Role of the modern environment. Ann NY Acad Sci 2005; 1043: 452–460.
- 89.
Yamagishi S, Ueda S, Okuda S. Food-derived advanced glycation end products (AGEs): A novel therapeutic target for various disorders. Curr Pharm Des 2007; 13: 2832–2836.
- 90.
Yamagishi S, Matsui T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 2016; 32: 157–165.
- 91.
Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci 2005; 1043: 461–466.
- 92.
Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, et al. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 1997; 94: 6474–6479.
- 93.
He C, Sabol J, Mitsuhashi T, Vlassara H. Dietary glycotoxins: inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999; 48: 1308–1315.
- 94.
Isami F, West BJ, Nakajima S, Yamagishi SI. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese population. J Int Med Res 2018; 46: 1043–1051.
- 95.
Harcourt BE, Sourris KC, Coughlan MT, Walker KZ, Dougherty SL, Andrikopoulos S, et al. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int 2011; 80: 190–198.
- 96.
Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab 2009; 94: 4483–4491.
- 97.
Peppa M, Uribarri J, Cai W, Lu M, Vlassara H. Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis 2004; 43: 690–695.
- 98.
Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci Rep 2017; 7: 2266.
- 99.
Osborne SE, Matsumura I, Ellington AD. Aptamers as therapeutic and diagnostic reagents: Problems and prospects. Curr Opin Chem Biol 1997; 1: 5–9.
- 100.
Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov 2010; 9: 537–550.
- 101.
Yamagishi S, Taguchi K, Fukami K. DNA-aptamers raised against AGEs as a blocker of various aging-related disorders. Glycoconj J 2016; 33: 683–690.
- 102.
Yamagishi SI, Matsui T. Therapeutic potential of DNA-aptamers raised against AGE-RAGE axis in diabetes-related complications. Curr Pharm Des 2018; 24: 2802–2809.
- 103.
Ojima A, Oda E, Higashimoto Y, Matsui T, Yamagishi S. DNA aptamer raised against advanced glycation end products inhibits neointimal hyperplasia in balloon-injured rat carotid arteries. Int J Cardiol 2014; 171: 443–446.
- 104.
Ojima A, Matsui T, Nakamura N, Higashimoto Y, Ueda S, Fukami K, et al. DNA aptamer raised against advanced glycation end products (AGEs) improves glycemic control and decreases adipocyte size in fructose-fed rats by suppressing AGE-RAGE axis. Horm Metab Res 2015; 47: 253–258.
- 105.
Kaida Y, Fukami K, Matsui T, Higashimoto Y, Nishino Y, Obara N, et al. DNA aptamer raised against AGEs blocks the progression of experimental diabetic nephropathy. Diabetes 2013; 62: 3241–3250.
- 106.
Maeda S, Matsui T, Ojima A, Suematsu M, Kaseda K, Higashimoto Y, et al. DNA aptamer raised against advanced glycation end products prevents abnormalities in electroretinograms of experimental diabetic retinopathy. Ophthalmic Res 2015; 54: 175–180.
- 107.
Ojima A, Matsui T, Maeda S, Takeuchi M, Inoue H, Higashimoto Y, et al. DNA aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab Invest 2014; 94: 422–429.
- 108.
Matsui T, Higashimoto Y, Nishino Y, Nakamura N, Fukami K, Yamagishi SI. RAGE-aptamer blocks the development and progression of experimental diabetic nephropathy. Diabetes 2017; 66: 1683–1695.
- 109.
Nakamura N, Matsui T, Ishibashi Y, Sotokawauchi A, Fukami K, Higashimoto Y, et al. RAGE-aptamer attenuates the growth and liver metastasis of malignant melanoma in nude mice. Mol Med 2017; 23: 295–306.
- 110.
Taguchi K, Yamagishi SI, Yokoro M, Ito S, Kodama G, Kaida Y, et al. RAGE-aptamer attenuates deoxycorticosterone acetate/salt-induced renal injury in mice. Sci Rep 2018; 8: 2686.
- 111.
Bekki M, Tahara N, Tahara A, Igata S, Honda A, Sugiyama Y, et al. switching dipeptidyl peptidase-4 inhibitors to tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2 improve arterial stiffness evaluated by cardio-ankle vascular index in patients with type 2 diabetes: A pilot study. Curr Vasc Pharmacol 2019; 17: 411–420.


