Abstract
Background: Pulmonary vein (PV) stenosis after atrial fibrillation (AF) ablation is rare; however, it remains a serious complication. PV angioplasty is reportedly an effective therapy; however, a dedicated device for PV angioplasty has not been developed, and the detailed procedural methods remain undetermined. This study describes the symptoms, indications, treatment strategies, and long-term outcomes for PV stenosis after AF ablation.
Methods and Results: This study retrospectively analyzed 7 patients with PV stenosis after catheter ablation for AF and who had undergone PV angioplasty at our hospital during 2015–2021. PV stenosis occurred in the left superior (5 patients) and left inferior (2 patients) PV. Six patients had hemoptysis, chest pain, and dyspnea. Seven de novo lesions were treated using balloon angioplasty (BA) (3 patients), a bare metal stent (BMS) (3 patients), and a drug-coated balloon (DCB) (1 patient). The restenosis rate was 42.9% (n=3; 2 patients in the BA group and 1 patient in the DCB group). The repeat treatment rate was 28.6% (2 patients in the BA group). Stenting was performed as repeat treatment. One patient with subsequent repeat restenosis development underwent BA. Ten PV angioplasties were performed; there were no major complications.
Conclusions: Regarding PV angioplasty after ablation therapy for AF, stenting showed superior long-term PV patency than BA alone; therefore, it should be considered as a standard first-line approach.
Abnormal excitation from the pulmonary veins (PVs) has been recognized as a primary cause of the development of atrial fibrillation (AF).1 Catheter ablation, which electrically isolates the PVs, has been utilized as a less invasive therapy for the treatment of AF. Initially, mainstream therapy is aimed at isolating and cauterizing the affected PVs using radiofrequency; however, the combined use of a three-dimensional (3D) mapping system and an ablation catheter with a contact force sensor has been introduced recently, resulting in major improvements in treatment quality and safety.2 Balloon technology, including cryoballoon, hot balloon, and laser balloon catheters, has been introduced to provide electrical isolation through the application of individual balloons into the PVs. These new modalities have several important advantages, including reduced procedure and exposure times compared with radiofrequency (RF) catheter ablation, and have resulted in the further expansion of the indications for catheter ablation. However, even with these new treatment modalities, the risk of complications, especially PV stenosis, remains a major concern. PV stenosis is usually recognized 3–5 months after catheter intervention,3,4
and it is more likely to occur distal to the ablation point. This complication with RF ablation has been reported to have an incidence rate of 3.4–42.4%.5,6
However, recently, the incidence rate of PV stenosis has significantly decreased to 0.7% because of the more proximal atrial side of PV isolation.7 Although symptomatic PV stenosis was not observed during the FIRE AND ICE study,8 which was a prospective, randomized study comparing the efficacy and safety of RF ablation compared to cryoballoon ablation, other studies have reported an approximately 1% incidence rate of PV stenosis after cryoballoon ablation.9 This risk is not reduced compared with that of RF ablation. Moreover, a significant number of patients with severe PV stenosis resulting in morbid events, including blood sputum and pneumonia, and who required therapeutic intervention, have been identified.10 Therefore, we aimed to describe typical symptoms, indications, treatment strategies, and long-term outcomes for patients with PV stenosis after catheter ablation for AF. Although this is a report of a single institution, it is the first report of consecutive cases in Japan. This work provides additional details of the treatment technique.
Methods
This work reports on experiments involving human subjects. The procedures were performed in accordance with the Declaration of Helsinki and the ethical standards of the committee (institutional or regional) in charge of human subject experimentation. This study was approved by the Ethics Committee of Jikei University School of Medicine (approval number: 33-034 [10644]), and it complied with the standard ethical regulations of our institution. During this study, among a total of 2,314 AF ablation procedures (RF for 1,611; balloon system including the cryoballoon/hot balloon/laser balloon for 703) performed between January 2014 and December 2020 at our institution, 7 (0.3%) patients with severe PV stenosis (>75%) requiring angioplasty between 2014 and 2021 were included.
The treatment strategy for PV stenosis at our institution was as follows: under the guidance of intracardiac echocardiography (ICE), an 8.5-Fr SL0 sheath (Abbott Medical, Chicago, IL, USA) was inserted through the femoral vein and guided to the left atrium (LA) using a Brockenbrough needle. After the target PV was selected, it was contrasted under rapid pacing (214 beats/min) from the right ventricular apex. In the case of difficulty performing PV venography, we used an 8.5-Fr Agilis NxT Stealable Sheath (Abbott Medical) instead of the SL0 sheath. Then, the lesion was selected using a 190-cm guidewire (GW) such as the Cruise® (Asahi Intec, Seto, Japan) or the Chevalier® (FMD, Shibuya, Japan). For dilatation, the 190-cm GW was replaced with a 300-cm GW using a microcatheter because the balloon type was over the wire. In some cases, when the lesion was completely occluded, GWs such as a Chevalier tapered 3® (FMD) or a Gradius® (Asahi Intec) were used. In such cases, the GWs were carefully selected with reference to previous PV angiography findings and 3D computed tomography (CT) images because of a lack of peripheral contrast findings. Using fluoroscopic imaging, the anterior posterior view was used to visualize the left superior PV (LSPV) and the left anterior oblique view was used to visualize the left inferior PV (LIPV) to correctly identify the ostium of the PV. After GW selection, an intravascular ultrasound (IVUS) evaluation was performed for all 7 patients. Based on the IVUS findings, the size of the pre-dilatation balloon was determined with reference to the distal lumen diameter for safety. After pre-dilatation, the patients were reevaluated using IVUS, and additional dilatation was performed using a larger balloon. A high-pressure balloon was selected for additional dilatation. For patients with significant recoil or inadequate lumen gain size after balloon dilatation, stenting was performed in the absence of obvious anatomic problems such as vessel diameter. When the PV is dilated to a certain extent, it is challenging to visualize the PV from the LA using conventional angiography because of increased forward flow from the PV. Therefore, angiography with rapid pacing is recommended; however, special attention must be given because the position of the catheter may become shifted during pacing. Q-Angio XA version 7.3 software (Medis Medical Imaging Systems BV, Leiden, Netherlands) was used for the quantitative PV angiography analysis, and VISICUBE (Terumo, Tokyo, Japan) was used for IVUS measurements.
Results
From 2015 to 2021, 7 patients with PV stenosis development after catheter ablation for AF at our institution required treatment with angioplasty. All patients had severe PV stenosis according to CT, and hypoperfusion according to pulmonary blood flow scintigraphy. Their varying symptoms are listed in Table 1.
Table 1. Patient Background, Catheter Ablation Method, Postoperative CT Results, Presence of Symptoms, and Other Laboratory Results
| Case |
Site |
Age
(years) |
Sex |
ABL date
1st |
ABL date
2nd |
CT at 3
months |
CT at 6
months |
CT at 9
months |
CT at 12
months |
Perfusion
lung
SPECT |
Symptoms |
Days* |
Days** |
| 1 |
LSPV |
48 |
Male |
2014.
Cryo |
|
SS |
|
VSS |
|
Right 63.9%:
Left 36.1% |
None |
293 |
|
| 2 |
LIPV |
39 |
Male |
2017.
RF |
|
OC |
|
|
|
Right 66.2%:
Left 33.8% |
Hemoptysis /
CP |
170 |
|
| |
|
|
|
|
|
VSS |
|
|
|
|
CP |
|
133 |
| 3 |
LSPV |
49 |
Male |
2018.
HOT |
|
SS |
OC |
|
|
|
Hemoptysis /
CP |
203 |
|
| |
|
|
|
|
|
VSS |
|
|
|
Right 77.1%:
Left 22.9% |
None |
|
117 |
| |
|
|
|
|
|
SS |
VSS |
|
OC |
Right 73.4%:
Left 26.6% |
None |
|
441 |
| 4 |
LSPV |
65 |
Male |
2018.
Cryo |
2019.
RF |
VS |
|
|
|
Right 79.8%:
Left 20.2% |
CP |
203 |
|
| 5 |
LSPV |
59 |
Male |
2019.
Cryo |
|
|
|
OC |
|
|
Hemoptysis /
CP |
310 |
|
| 6 |
LSPV |
65 |
Male |
2019.
Cryo |
2020.
RF |
SS |
|
OC |
|
Right 72.9%:
Left 27.1% |
Dyspnea /
CP |
336 |
|
| 7 |
LIPV |
53 |
Female |
2020.
RF |
|
SS |
|
OC |
|
Right 65.6%:
Left 34.4% |
CP |
334 |
|
*Days, days from ablation treatment to PV treatment. **Days, days from PV treatment to PV repeat treatment. ABL, ablation; CP, chest pain; CT, computed tomography; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; OC, occlusion; SS, severe stenosis; VSS, very severe stenosis.
PV stenosis was defined as >75% stenosis or total obstruction. This occurred in 5 patients in the LSPV and in 2 patients in the LIPV. Of these 7 patients, 6 had symptoms such as hemoptysis, chest pain, and dyspnea. A follow-up CT scan at 3 months after ablation showed severe stenosis (>75%) or obstruction in 6 patients (Figure 1). Severe stenosis was observed within 3–6 months. Pulmonary blood flow scintigraphy was performed for 6 patients, and decreased pulmonary blood flow was observed in all 6 patients. Table 1 shows the patients’ background characteristics, catheter ablation methods, postoperative CT results, symptoms, and main laboratory test results. The treatment method, device used, inflation pressure, initial results, and antithrombotic therapy are shown in Table 2.

Table 2. Treatment Method, Device Used, Inflation Pressure, Initial Results, and Antithrombotic Therapy
| Case |
Date
(year) |
State |
Denovo/reste |
Lesion morphology |
Site |
Device 1 |
Max IP
(atm) |
Device 2 |
Max IP
(atm) |
Device 3 |
Max IP
(atm) |
Result |
Final
procedure |
Anti thrombotic
therapy |
| 1 |
2015 |
Elective |
Denovo |
Stenosis |
LSPV |
NSE PTA 4.0×40 mm |
8 |
Sterling 8.0×20 mm |
10 |
|
|
Success |
BA |
DOAC + SAPT |
| 2 |
2018 |
Elective |
Denovo |
Stenosis |
LIPV |
NSE PTA 4.0×40 mm |
12 |
Sterling 8.0×20 mm |
8 |
|
|
Success |
BA |
DOAC + DAPT |
| |
2018 |
Elective |
Reste |
Stenosis |
|
Sterling 4.0×20 mm |
6 |
Express SD 6.0×18 mm |
9 |
NC Emerge 6.0×12 mm |
18 |
Success |
BMS |
DOAC + SAPT |
| 3 |
2018 |
Emergency |
Denovo |
Stenosis 99% |
LSPV |
NSE PTA 4.0×20 mm |
8 |
Sterling 8.0×20 mm |
6 |
|
|
Success |
BA |
DOAC |
| |
2019 |
Elective |
Reste |
Occlusion 100% |
|
NSE PTA 4.0×20 mm |
14 |
Sterling 6.0×20 mm |
14 |
Express SD 6.0×18 mm |
14 |
Success |
BMS |
DAPT |
| |
2020 |
Elective |
ISR |
Stenosis |
|
DCA (L) |
|
Peripheral CB 6.0×20 mm |
6 |
Sterling 8.0×20 mm |
12 |
Success |
BA |
DAPT |
| 4 |
2020 |
Elective |
Denovo |
Stenosis 99% |
LSPV |
NSE PTA 4.0×20 mm |
14 |
Express LD 8.0×17 mm |
12 |
|
|
Success |
BMS |
DOAC + DAPT |
| 5 |
2020 |
Elective |
Denovo |
Stenosis |
LSPV |
NSE PTA 4.0×20 mm |
14 |
Express LD 8.0×17 mm |
12 |
MUSTANG 10×20 mm |
12 |
Success |
BMS |
DAPT |
| 6 |
2021 |
Elective |
Denovo |
Stenosis |
LSPV |
NSE PTA 6.0×20 mm |
12 |
Express LD 8.0×17 mm |
12 |
Sterling 10×20 mm |
10 |
Success |
BMS |
DOAC + DAPT |
| 7 |
2021 |
Elective |
Denovo |
Occlusion 100% |
LIPV |
PCB 4.0×15 mm |
14 |
NSE PTA 6.0×20 mm |
4 |
Sequent Please 4.0×20 mm |
14 |
Success |
DCB |
DOAC + DAPT |
NSE PTA® (Goodman, Nagoya, Japan). DCA: ATHEROCUT® (Nipro, Osaka, Japan). PCB, peripheral cutting balloonTM
(Boston Scientific, Marlborough, MA, USA). SterlingTM
(Boston Scientific). Express SDTM
(Boston Scientific). Express LDTM
(Boston Scientific). NC EmergeTM
(Boston Scientific). MUSTANGTM
(Boston Scientific). Sequent Please® (B. Braun, Melsungen, Germany). BA, balloon angioplasty; BMS, bare metal stent; DAPT, dual antiplatelet therapy; DCB, drug-coated balloon; DOAC, direct oral anticoagulants; ISR, in-stent restenosis; max IP, maximum inflation pressure; SAPT, single antiplatelet therapy. Other abbreviations as in Table 1.
Seven de-novo lesions were selected for treatment. Balloon angioplasty (BA) was used for 3 patients (Figure 2A–D), a bare metal stent (BMS) was used for 3 patients (Figure 2E–J), and a drug-coated balloon (DCB) was used for 1 patient (Figure 3A–E). All patients were evaluated using IVUS before pre-dilatation. IVUS findings indicated a soft tissue-like hypoechoic lesion that was very short. A scoring balloon was selected for the initial pre-dilatation procedure because of the impression of strong recoil based on IVUS findings. IVUS was performed again after pre-dilatation, and the need for additional dilatation or stent placement was determined. If the minimum lumen area (MLA) according to IVUS was >8 mm2, then the procedure using BA was terminated; otherwise, a BMS was implanted. The final balloon size in the BA group was 8 mm, and the inflation pressure was 8 atm (standard deviation [SD], ±2 atm). In the BMS group, a 8×17 mm stent was placed with the inflation pressure of 12 atm. Two patients underwent additional post-dilatation with a 10-mm balloon. For patients treated with a DCB, 4-mm and 2-mm DCBs were used from the main trunk to the 2 branches because of the difficulty of stent placement. Seven patients who underwent PV angioplasty were evaluated using CT because of the recurrence of clinical symptoms and restenosis of the treated vessel. The mean follow-up period of the 7 patients was 27 months (SD, ±20 months). Three of seven patients had restenosis (BA group, n=2; DCB group, n=1). Two patients in the BA group required repeat treatment with stenting because of their symptoms; 1 of them had a chronic total occlusion on PV angiography (Figure 3F–L). In the remaining 1 patient with a DCB, repeat interventional treatment was not performed because of the absence of symptoms. Only 1 patient subsequently developed repeat restenosis after stenting (Figure 4). Although the stent structure was preserved, stent restenosis occurred. Regarding in-stent restenosis (ISR) lesions, we attempted to resect the intima using a directional coronary atherectomy device to evaluate the histological characteristics of the lesion; however, this was unsuccessful. We selected the BA technique (cutting balloon plus high-pressure dilatation balloon) as the repeat treatment technique (Figure 5). The quantitative coronary analysis results and IVUS measurements for each treatment are shown in Table 3. A total of 10 PV angioplasty procedures were performed with no major complications.

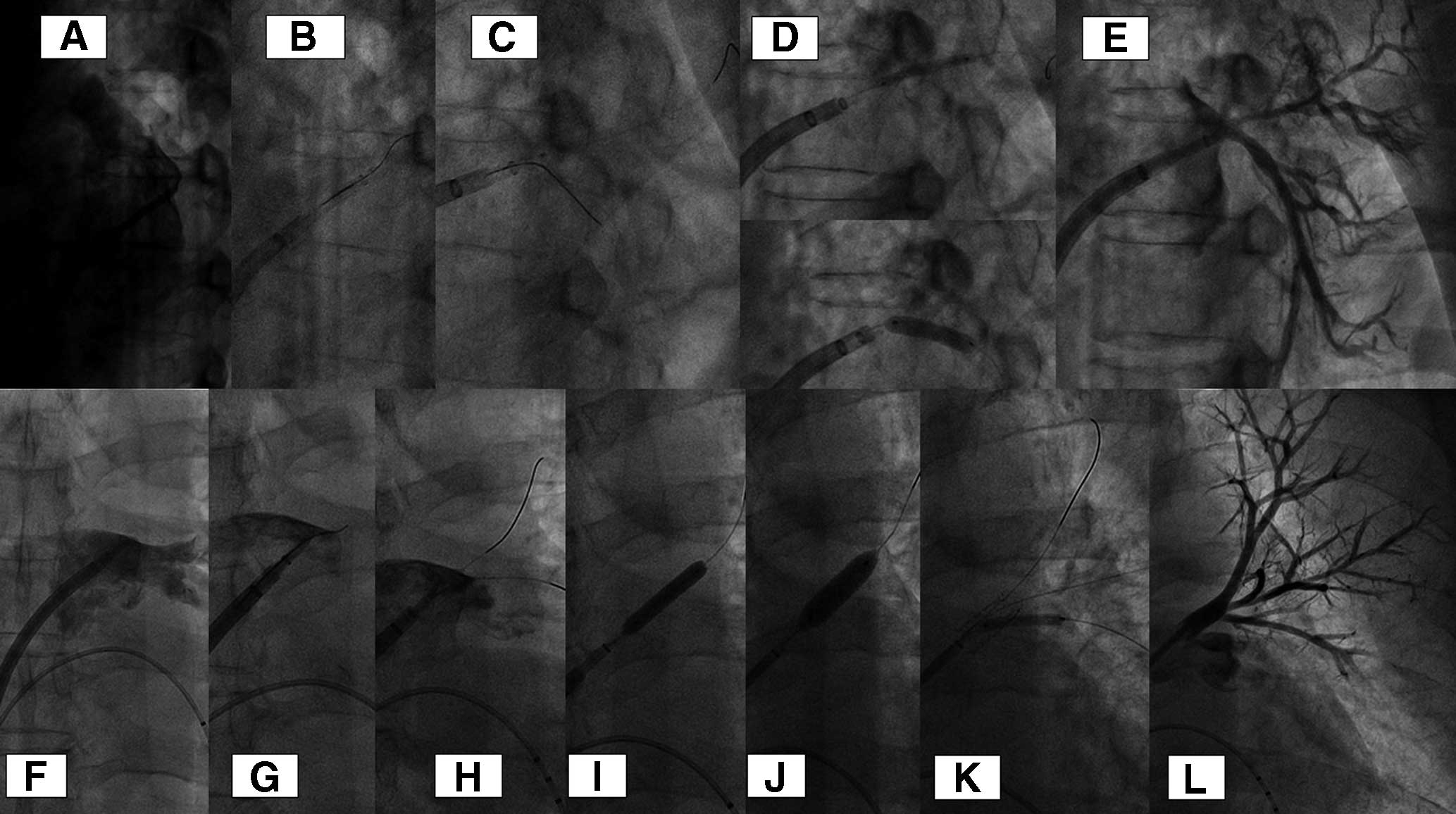


Table 3. Quantitative PV Angiography Analysis Results and IVUS Measurements Obtained for Each Treatment
| Case |
Final
procedure |
Pre
Ref D
(mm) |
Pre
MLD
(mm) |
Pre
%DS |
Lesion
length
(mm) |
Post
Ref D
(mm) |
Post
MLD
(mm) |
Post
%DS |
Post IVUS
MLA
(mm2) |
Post IVUS
Max LD
(mm) |
Post IVUS
Min LD
(mm) |
Re-Tr |
Long-
term
outcome |
| 1 |
BA |
9.7 |
3.1 |
68.2 |
4.6 |
9.7 |
5.2 |
46.9 |
36.5 |
7.4 |
6 |
0 |
Event free |
| 2 |
BA |
5 |
1.3 |
73.6 |
4 |
5.26 |
4 |
24 |
17.6 |
5.5 |
4 |
1 |
Restenosis |
| |
BMS |
5.5 |
1.7 |
69.9 |
3.4 |
6.1 |
5.5 |
10.4 |
37.1 |
7.2 |
6.6 |
0 |
Event free |
| 3 |
BA |
3.8 |
0.6 |
84.7 |
1.8 |
5 |
4.7 |
6 |
19.1 |
5.6 |
4.2 |
1 |
Restenosis |
| |
BMS |
5.7 |
0 |
100 |
2.2 |
5.7 |
5.5 |
2.9 |
32.7 |
6.8 |
6.2 |
1 |
ISR |
| |
BA |
5.7 |
2.6 |
54.3 |
9.4 |
6.5 |
6.3 |
3.5 |
49.1 |
8.4 |
7.4 |
0 |
Event free |
| 4 |
BMS |
4.5 |
0.7 |
83.5 |
4.8 |
7.4 |
7.1 |
3.9 |
53 |
7.6 |
7.9 |
0 |
Event free |
| 5 |
BMS |
6.5 |
1.7 |
74.4 |
3 |
7.4 |
6.8 |
7.9 |
54 |
9.4 |
7.5 |
0 |
Event free |
| 6 |
BMS |
6.7 |
2.2 |
67.1 |
2.6 |
7.1 |
6.6 |
8.3 |
56.7 |
9 |
7.9 |
0 |
Event free |
| 7 |
DCB |
4.1 |
0 |
100 |
9.4 |
4.1 |
2.8 |
32.5 |
8.3 |
3.8 |
2.7 |
0 |
Event free |
IVUS, intravascular ultrasound; Max LD, maximum lumen diameter; MLA, minimum lumen area; MLD, minimum lumen diameter; Ref D, reference diameter; Re-Tr, repeat treatment; %DS, percent diameter stenosis. Other abbreviations as in Table 2.
Postoperative antithrombotic therapy included triple antithrombotic therapy (direct oral anticoagulants [DOACs] plus dual antiplatelet therapy [DAPT]) for 3 patients, dual antithrombotic therapy (DOACs plus single antiplatelet therapy) for 2 patients, DOAC for 1 patient, and DAPT for 1 patient (Table 2).
Discussion
The frequency of PV stenosis has been estimated to range from 0.7% to 3.4%,6,7 and as high as 42.4%; however, it is decreasing because of advances in treatment. Previous studies have shown that between 0.29% and 0.74% of patients require treatment.11,12 We previously reported that the incidence of severe PV stenosis (>75% stenosis) after cryoballoon ablation was 1.3% (9 of 170 patients had severe PV stenosis),9 with 1 patient requiring PV angioplasty10 (this patient was included in this study). Additionally, the predictors of PV narrowing (>25% stenosis) and severe PV stenosis (>75%) have been reported to be a larger PV ostium, lower minimum freezing temperature, and smaller PV angle and large balloon contact ratio.9,13 These results indicate that excessive cryothermal application at the ostium of the PV may be associated with the incidence of PV stenosis.
Symptoms of severe PV stenosis have been reported, including dyspnea, cough, hemoptysis, and chest pain, as well as flu-like symptoms, such as fatigue and myalgia. The onset of symptoms is often gradual, occurring between 3 and 5 months after PV isolation. During our study, although some patients were asymptomatic, blood sputum, chest pain, and dyspnea were observed, and CT indicated stenosis from 3 months after ablation treatment. A CT scan of the lung at 3 months after PV angioplasty is useful; therefore, it is currently routinely performed for the early detection of PV stenosis. At this point, particularly if severe stenosis (>75%) is observed, careful follow up is needed. For patients who underwent pulmonary perfusion scintigraphy, a significant decrease in blood flow was observed. In addition to symptoms, a CT scan of the lungs and pulmonary blood flow scintigraphy are useful for diagnosis and treatment. In particular, it is important to evaluate symptoms and physiological examination findings rather than anatomical findings alone.
Treatment techniques have been extensively investigated, with many studies suggesting that the restenosis rate is high with percutaneous balloon angioplasty, and that stenting provides better outcomes.14–17 Our study findings are in accordance with the findings of those studies. During the early stage of the treatment, we used only balloon therapy because there is no stent with a suitable size for PV stenosis treatment because of the anatomical characteristics. The stents are too long for the lesion length or the PV diameters are too small or too large. However, 2 of 3 patients in the balloon group underwent repeat treatment, and 0 of the 3 patients in the BMS group underwent repeat treatment. The presence of restenosis in the BA group led us to believe that stenting is a better option if a sufficient lumen cannot be obtained. We also found that the larger the stent size and the greater the acute volume, the better the outcome. It has been reported that the restenosis rate is significantly lower with larger stents (>9 mm) than with smaller stents (<9 mm), and that severe PV stenosis stenting with a stent size ≥10 mm is appropriate for the treatment of severe PV stenosis.18,19 One study investigated histopathological changes of the PV after ablation. Ablation causes injury to the inner elastic membrane, progressive fibrosis, and narrowing of the lumen. In the mid-term to long-term, the lumen becomes even narrower because of intimal proliferation and subsequent negative remodeling.20 Therefore, we considered recoil to be a major factor in PV restenosis as well as in restenosis after plain old balloon angioplasty (POBA) for percutaneous coronary interventions. We also considered that a stent that prevents recoil and has a large acute volume increase might provide good long-term results. After treatment at our hospital, the minimum lumen diameter determined using quantitative coronary analysis, and the MLA determined using IVUS, tended to be greater in the BMS group than in the BA group. This trend was also observed in the BA group; furthermore, this trend was larger in the group without repeat treatment than in the group with repeat treatment. If the mechanism of restenosis in PV angioplasty is reactionary, then, based on previous results, the use of the BMS is optimal, and the “bigger is better” concept for percutaneous coronary intervention in relation to BMS use remains relevant. The present study is the first to investigate the detailed technique used for PV angioplasty and the clinical outcomes of an Asian population. Our data and results were similar to those of previous studies involving a Western population, although the body sizes of these populations were different.14–17 The factors associated with PV restenosis after PV angioplasty may be the PV dilatation size after BA and stenting rather than body size and race. In the clinical setting, the optimal strategy for PV angioplasty (POBA, stent, DCB) must be selected based on the baseline PV size, length, and distal vessel dimension because the balloon and stent length are large (>20 mm).
We recommend POBA alone if IVUS findings show an MLA >50 mm2
and a minimum lumen diameter >7.0 mm; otherwise, we recommend a BMS with an implantation size of ≥8 mm if possible, depending on the reference vessel diameter.
Regarding restenosis, we consider BMS implantation to be the primary procedure for restenosis after BA and for ISR. The mechanism of restenosis usually involves recoil immediately after BA, which may contribute to early restenosis; therefore, stenting may be necessary in such instances. At our hospital, 2 patients in the BA group required repeat treatment; at that point, stenting was performed. Subsequently, 1 of those patients underwent repeat treatment and 1 underwent restenosis without stenting and repeat BA. An autopsy evaluation of 5 patients with PV stenosis who underwent surgical resection showed the effects of neointimal hyperplasia and fibrosis.21 If neointimal hyperplasia is a possible cause of late restenosis observed in the retreated stent group, then a drug-eluting stent (DES) and drug-eluting balloon (DEB) may be effective for patients with stent restenosis.
The results of DES use and DCB use have been reported, but there have been no reports of better results obtained with DES use compared with BMS use. Although there are case reports of better results achieved with DCB use, the reported patient numbers are low (similar to studies of DES use); therefore, more studies are needed.22 The DCB may have potential for patients with an anatomically inadequate MLA and in those unsuitable for stenting. At our institution, we have no experience with the DES; however, we have used the DCB for one such case. Although our experience is limited, the anatomy of the LSPV has a relatively large vessel diameter, and balloons and stents as large as 8 mm can be implanted. However, regarding the 2 cases involving the LIPV, the quantitative coronary analysis and IVUS measurements showed that the vessel diameter was small, the distance to the bifurcation was short, and the vessel diameter after the bifurcation was small; therefore, we had to choose a rather small device size, and the surgical technique was challenging. We deemed it difficult to use the optimal balloon size, secure a sufficient MLA, and implant a stent; therefore, a DCB was used for 1 patient; however, this was not covered by insurance. Although a DCB was chosen because of its anatomical characteristics, because of acute exacerbation and restenosis, we consider DES implantation in similar patients using low-pressure dilatation to be a safer option; however, further studies are warranted to validate this.
Currently, there is no dedicated device for PV plasty. This may be because of the lower number of cases reported. Therefore, we use devices to treat peripheral arterial disease that are not suitable for the treatment of PV stenotic lesions. They are not suitable because the vessel diameter and lesion length of these lesions are shorter than those of peripheral arterial disease devices. Additionally, regarding stent selection, no appropriate length has been determined. If such treatment becomes necessary in future, then it is hoped that an anatomically appropriate device will be developed based on early results, including those reported by this study, and that research concerning the longevity and safety of these devices will be conducted. Although no major complications have been observed at our institution, embolisms, GW perforation, and cardiac tamponade associated with PV dilatation and PV rupture have been reported in POBA groups, and stent thrombosis and stent dislodgement have been reported in stent groups. In particular, PV rupture is a serious complication that requires emergency surgery; therefore, it must be avoided.16
Regarding the antithrombotic strategy after PV angioplasty, there are no available data from prospective studies comparing different medical treatment strategies. A recent expert consensus regarding antithrombotic therapy after venous stenting (not including PV angioplasty) recommended the use of anticoagulative therapy after venous stenting; however, a consensus regarding the recommendation for the use of antiplatelet therapy was not reached.23 We used these treatments for coronary intervention therapy and anticoagulation drugs for patients with AF recurrence. Although we know acute and chronic thrombosis occurred during follow up, further studies are needed to define the role of antithrombotic drugs.
Study Limitations
This was a single-center study of a low number of cases. The number of cases was low because of the rarity of the disease; however, this is the first report of consecutive cases in Japan, and the treatment techniques are described in detail.
Conclusions
Regarding PV angioplasty after ablation therapy for AF, the use of stenting resulted in superior long-term PV patency compared with BA alone; therefore, we believe that stenting should be used as a standard first-line approach.
Acknowledgments
We thank all the physicians and nurses from the participating hospitals for their important contributions to this study.
Sources of Funding
No funding was received for this study through any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors declare no conflicts of interest. M. Yoshimura is a member of Circulation Journal’s Editorial Team.
IRB Information
This study was approved by the ethics committee of the Jikei University School of Medicine (approval number: 33-034(10644)).
Data Availability
The deidentified participant data will not be shared.
References
- 1.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659–666.
- 2.
Chinitz LA, Melby DP, Marchlinski FE, Delaughter C, Fishel RS, Monir G, et al. Safety and efficiency of porous-tip contact-force catheter for drug-refractory symptomatic paroxysmal atrial fibrillation ablation: Results from the SMART SF trial. Europace 2018; 20: f392–f400.
- 3.
Saad EB, Marrouche NF, Saad CP, Ha E, Bash D, White RD, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: Emergence of a new clinical syndrome. Ann Intern Med 2003; 138: 634–638.
- 4.
Packer DL, Keelan P, Munger TM, Breen JF, Asirvatham S, Peterson LA, et al. Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation. Circulation 2005; 111: 546–554.
- 5.
Yu WC, Hsu TL, Tai CT, Tsai CF, Hsieh MH, Lin WS, et al. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2001; 12: 887–892.
- 6.
Saad EB, Rossillo A, Saad CP, Martin DO, Bhargava M, Erciyes D, et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: Functional characterization, evolution, and influence of the ablation strategy. Circulation 2003; 108: 3102–3107.
- 7.
Teunissen C, Velthuis BK, Hassink RJ, van der Heijden JF, Vonken EPA, Clappers N, et al. Incidence of pulmonary vein stenosis after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2017; 3: 589–598.
- 8.
Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245.
- 9.
Tokutake K, Tokuda M, Yamashita S, Sato H, Ikewaki H, Okajima E, et al. Anatomical and procedural factors of severe pulmonary vein stenosis after cryoballoon pulmonary vein ablation. JACC Clin Electrophysiol 2019; 5: 1303–1315.
- 10.
Tokutake K, Tokuda M, Ogawa T, Matsuo S, Yoshimura M, Yamane T. Pulmonary vein stenosis after second-generation cryoballoon ablation for atrial fibrillation. HeartRhythm Case Rep 2017; 3: 36–39.
- 11.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111: 1100–1105.
- 12.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3: 32–38.
- 13.
Narui R, Tokuda M, Matsushima M, Isogai R, Tokutake K, Yokoyama K, et al. Incidence and factors associated with the occurrence of pulmonary vein narrowing after cryoballoon ablation. Circ Arrhythm Electrophysiol 2017; 10: e004588.
- 14.
Fender EA, Widmer RJ, Hodge DO, Cooper GM, Monahan KH, Peterson LA, et al. Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: Presentation, management, and clinical outcomes. Circulation 2016; 134: 1812–1821.
- 15.
Fender EA, Widmer RJ, Hodge DO, Packer DL, Holmes DR Jr. Assessment and management of pulmonary vein occlusion after atrial fibrillation ablation. JACC Cardiovasc Interv 2018; 11: 1633–1639.
- 16.
Schoene K, Arya A, Jahnke C, Paetsch I, Nedios S, Hilbert S, et al. Acquired pulmonary vein stenosis after radiofrequency ablation for atrial fibrillation: Single-center experience in catheter interventional treatment. JACC Cardiovasc Interv 2018; 11: 1626–1632.
- 17.
Fink T, Tilz RR, Heeger CH, Schlüter M, Feickert S, Rottner L, et al. Management of arrhythmia recurrence in patients with pulmonary vein stenosis following atrial fibrillation ablation. Europace 2019; 21: 1494–1501.
- 18.
Neumann T, Kuniss M, Conradi G, Sperzel J, Berkowitsch A, Zaltsberg S, et al. Pulmonary vein stenting for the treatment of acquired severe pulmonary vein stenosis after pulmonary vein isolation: Clinical implications after long-term follow-up of 4 years. J Cardiovasc Electrophysiol 2009; 20: 251–257.
- 19.
Baranowski B, Saliba W. Our approach to management of patients with pulmonary vein stenosis following AF ablation. J Cardiovasc Electrophysiol 2011; 22: 364–367.
- 20.
Taylor GW, Kay GN, Zheng X, Bishop S, Ideker RE. Pathological effects of extensive radiofrequency energy applications in the pulmonary veins in dogs. Circulation 2000; 101: 1736–1742.
- 21.
Lu HW, Wei P, Jiang S, Gu SY, Fan LC, Liang S, et al. Pulmonary vein stenosis complicating radiofrequency catheter ablation: Five case reports and literature review. Medicine (Baltimore) 2015; 94: e1346.
- 22.
Rosenberg J, Fisher WG, Guerrero M, Smart S, Levisay J, Feldman T, et al. Drug-coated balloon venoplasty for in-stent restenosis in a patient with recurrent pulmonary vein stenosis post ablation for atrial fibrillation: Initial experience with a new treatment technique. J Invasive Cardiol 2016; 28: E44–E48.
- 23.
Milinis K, Thapar A, Shalhoub J, Davies AH. Antithrombotic therapy following venous stenting: International Delphi Consensus. Eur J Vasc Endovasc Surg 2018; 55: 537–544.






