Article ID: CJ-22-0607
Article ID: CJ-22-0607
Fabry’s disease is caused by a GLA abnormality, in which globotriaosylceramide (Gb3) accumulates in the body. We previously revealed that 4 types of Gb3, with different fatty acid components, accumulate in the heart.1 The Gb3 profile is reported to differ according to the species and organ.1,2 In this study, we compared the profiles and amounts of Gb3 in the heart in various cases of Fabry’s disease (Supplementary Table).
We found the same mass spectrometry (MS) pattern despite differences in the type, pedigree, or mutation, which implied that these 4 Gb3 molecules play an essential role in the human heart. Interestingly, the accumulated Gb3 was largest in a classical-type patient, even though he was the youngest (Figure B). Figure C,D show the results for a 41-year-old patient and his 71-year-old mother. There was less accumulated Gb3 in the mother with heterozygous mutation than in her son with hemizygous mutation. Figure E1 shows the MS pattern of a 55-year-old patient. Although the initial amount of Gb3 was minimal, it increased despite 4 years of enzyme replacement therapy (ERT) (Figure E2), indicating the possibility of ERT efficiency variation by cell type in the heart.
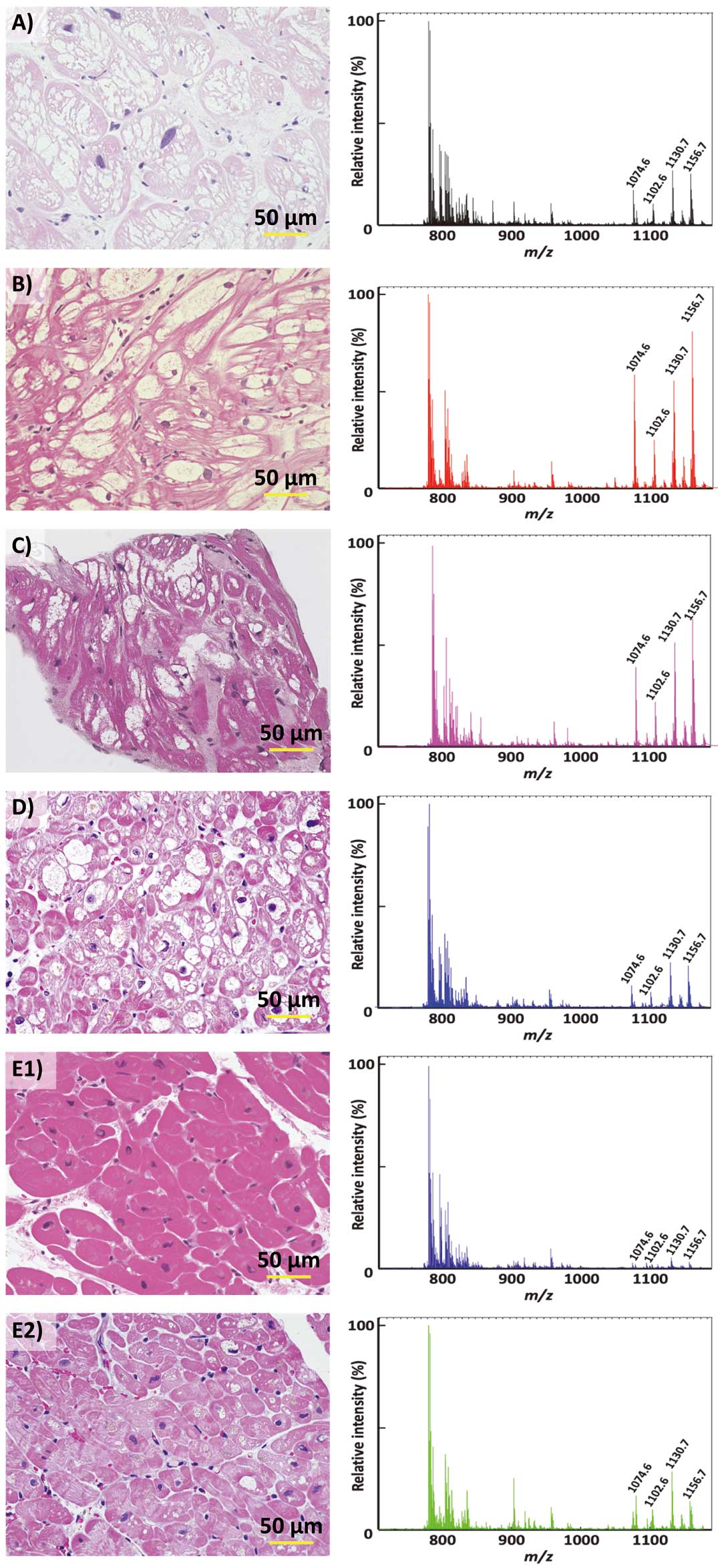
(A–E) Histology and mass spectra of the endomyocardial biopsy tissue. The relative intensity is displayed with the peak at a mass charge ratio of 782.5 set to 100%.
In addition to analyzing the characteristics of Gb3, MS can estimate the accumulated amount of Gb3.
The procedures followed were in accordance with the “Declaration of Helsinki” and the ethical standards of the Nara Medical University ethics committee on human experimentation (G107 and 1176).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-22-0607