2016 Volume 64 Issue 8 Pages 1079-1083
2016 Volume 64 Issue 8 Pages 1079-1083
Natural products are well recognized as an important source of lead compounds in drug development. During the past >30 years, we have discovered >1000 novel bioactive natural products from Okinawan marine organisms (sponges, tunicates, cone shells, etc.) and microorganisms (fungi, bacteria, dinoflagellates, etc.). Some of them are used as bioprobes useful for basic studies of life sciences, while others are expected to be candidates of drug leads.
In recent years, as Japan’s population ages the numbers of patients with cancer, circulatory diseases, dementia, etc. are rapidly increasing; hence the development of new drugs is urgently required. Under these circumstances, new natural products have been recognized as an important source of lead compounds in drug development. During the past >30 years, we have discovered >1000 novel bioactive natural products from Okinawan marine organisms (sponges, tunicates, cone shells, etc.) and microorganisms (fungi, bacteria, dinoflagellates, etc.). Some of them are used as bioprobes useful for basic studies of life sciences, while others are expected to be candidates of drug leads. This review describes some representative examples of our work.
To date, >40 cytotoxic macrolides, amphidinolides, have been isolated from symbiotic dinoflagellates of the genus Amphidinium, which were isolated from the inner cells of acoel flatworms Amphiscolops sp.1) Amphidinolide H, a potent cytotoxicic macrolide, targets actin cytoskeleton and stabilizes F-actin2,3) (Fig. 1). Amphidinolide H covalently binds on actin. The binding site was determined as Tyr200 of actin subdomain 4.4) The incorporation patterns of 13C-labeled acetate for amphidinolides revealed that the main chain of these macrolides was generated from unusual units derived only from C-2 of acetates in addition to successive polyketide chains and all C1 branched carbons were derived from C-2 of acetates1) (Fig. 1).

Ircinal A, a putative biosynthetic precursor of a new antimararial drug candidate manzamine A, has been isolated from a marine sponge Ircinia sp.5) (Fig. 2). The article about the isolation of ircinal A was introduced in the journal Science as one of the most cited articles by Japanese scientists in 3 years (1992–1994). Nakadomarin A, a unique manzamine-related alkaloid isolated from a sponge Amphimedon sp., has been recognized as an attractive synthetic target and synthesized totally by several groups.6) We have isolated many manzamine-related alkaloids, such as zamamidine C and zamamiphidin A, from sponges of the genus Amphimedon7–9) (Fig. 3). Zamamidine C exhibited antimalarial and antitrypanosomal activities and the structure–activity relationship (SAR) has been studied.8) Zamamiphidin A showed antibacterial activity against Staphylococcus aureus.9)


Hymenin, a bromopyrrol alkaloid isolated from a marine sponge Hymeniacidon sp., showed α-blocker-like activity10,11) (Fig. 4). The development of a new antihypertensive agent from hymenin as a lead compound was carried out by a pharmaceutical company. Agelamadin A, a bromopyrrol alkaloid isolated from a marine sponge Agelas sp., exhibited antimicrobial activity against some bacteria and fungi12) (Fig. 4).

Nakijiquinone D, a sesquiterpenoid quinone isolated from a marine sponge family Spongiidae, inhibits tyrosine kinases recognized as molecular targets in cancer13) (Fig. 5). SAR studies have been conducted at Max Planck Institute using nakijiquinone D as a lead compound. Keramamide D, a unique cyclic peptide isolated from a marine sponge Theonella sp., inhibited superoxide generation in human neutrophils elicited with a chemotactic peptide, N-formyl-Met-Leu-Phe (fMLP), but did not inhibit that induced by phorbolmyristate acetate or immune complex14) (Fig. 5). Recently, the real producer of keramamide D was revealed to be an unculturable symbiotic bacterium of Theonella sp.15)

Manzamenone A, a dimeric fatty acid derivative isolated from a marine sponge Plakortis sp., exhibited inhibitory activity against DNA polymerases16,17) (Fig. 6). SAR studies were carried out. Manzamenone O, a trimeric fatty acid derivative isolated from a marine sponge Plakortis sp., exhibited antibacterial activity against Micrococcus luteus18) (Fig. 6).

Taurospongin A, an acetylenic fatty acid derivative isolated from a marine sponge Hippospongia sp., exhibited inhibitory activity against DNA polymerase β and human immunodeficiency virus (HIV) reverse transcriptase19) (Fig. 7). Synthetic studies of taurospongin A and related compounds were carried out.

Iejimalide A, a unique antitumor macrolide isolated from a marine compound tunicate Eudistoma cf. rigida, showed antitumor activity due to its potent V-ATPase inhibitory activity20–23) (Fig. 8). The V-ATPase inhibitory activity of iejimalide A was comparable to bafilomycin A1, a representative and commercially available inhibitor of V-ATPase.24,25) Iejimalide A is also expected to be a promising lead compound for a novel type of antiosteoporosis agent, since V-ATPase inhibitors trigger apoptotic cell death of osteoclasts.24,25)

Eudistomin C, a β-carboline alkaloid isolated from a Caribbean compound tunicate Eudistoma olivaseum, displayed extremely potent antiviral activity against DNA and RNA viruses26,27) (Fig. 9). Synthetic studies of eudistomin C were carried out by many synthetic chemists. N-Methyl bromoeudistomin D (MBED), a β-carboline alkaloid derived from eudistomin D, is a powerful Ca2+ releaser from sarcoplasmic reticulum (SR) (1000 times more potent than caffeine) and has been used as a powerful tool for studying molecular mechanisms of Ca2+ release processes in various mammalian cells28) (Fig. 9).
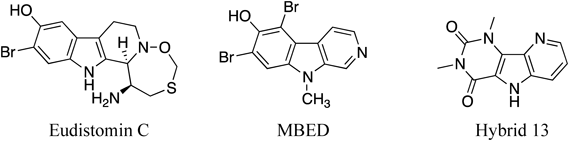
Caffeine exhibits a variety of physiological activities (or action) including regulation of blood pressure, respiratory functioning, gastric and colonic activity, urine volume, and exercise performance. The mechanism of action of caffeine is reported to be competitive antagonism to A1 and A2A adenosine receptors, induction of Ca2+-release from SR, inhibition of phosphodiesterase, and so on. The hybrid molecule of caffeine and eudistomin D, a β-carboline alkaloid from a marine tunicate, was synthesized and its affinity and selectivity for adenosine receptors A1, A2A, and A3 were examined. Among them, hybrid-13 showed the most potent affinity for adenosine receptor A3 subtype (Fig. 9). SAR study was carried out on a series of hybrid-13 analogs.29)
Alteramide A, a new tetracyclic alkaloid from a bacterium Alteromonas sp. associated with the marine sponge Halichondria okadai, exhibited cytotoxicity against murine leukemia P388, murine lymphoma L1210, and human epidermoid carcinoma KB cells in vitro30) (Fig. 10). Because of the unique structure, the biosynthesis of alteramide A has also attracted the attention of many scientists. Seragakinone A, a new anthracycline-type pentacyclic metabolite isolated from the mycelium of an unidentified marine-derived fungus, was found significantly to enhance the effect of antifungal agents31,32) (Fig. 10). Synthetic studies of seragakinone A and related compounds were carried out.

We also found many peptide toxins from cone shells. Geographutoxin I (μ-conotoxin GIIIA), a peptide-based potent selective sodium channel inhibitor, was isolated from the venom glands of cone shell Conus geographus33) (Fig. 11). The activity of this peptide was four times more potent than tetrodotoxin. Since geographutoxin I has no discernible effect on nerve sodium channels but potent effect on muscle sodium channel, this peptide is commercially available as a useful bioprobe for investigating sodium channels of tissues.

The strategy to search for bioprobes and drug leads from marine and terrestrial natural sources is shown in Fig. 12. I look forward to further development of natural products chemistry in the field of pharmaceutical sciences.

These studies have been carried out at Mitsubishi-Kasei Institute of Life Sciences and Graduate School of Pharmaceutical Sciences, Hokkaido University. I would like to thank collaborators at other universities and research institutes as well as the staff and students of my research group.
The author declares no conflict of interest.