2021 年 69 巻 12 号 p. 1200-1205
2021 年 69 巻 12 号 p. 1200-1205
A protocol for the ruthenium-on-carbon (Ru/C)-catalyzed solvent-free oxidation of alcohols, which proceeds efficiently under solid–solid (liquid)–gas conditions, was developed. Various primary and secondary alcohols were transformed to corresponding aldehydes and ketones in moderate to excellent isolated yields by simply stirring in the presence of 10% Ru/C under air or oxygen conditions. The solvent-free oxidation reactions proceeded efficiently regardless of the solid or liquid state of the substrates and reagents and could be applied to gram-scale synthesis without loss of the reaction efficiency. Furthermore, the catalytic activity of Ru/C was maintained after five reuse cycles.
The oxidation of alcohols is one of the most fundamental molecular transformation reactions. In comparison to oxidation methods using stoichiometric amounts of oxidative reagents (such as pyridinium chlorochromate,1,2) Dess-Martin periodinane,3) manganese dioxide,4,5) and so on6–8)), catalytic aerobic9–17) and dehydrogenative alcohol oxidation reactions18–23) are environmentally-friendly organic syntheses in which less residual reactants (such as oxidants) are generated. In green chemistry, solvent-free reactions are advantageous because they decrease environmental waste, avoid the emission of organic solvents, and allow for down-scaling of the reaction vessels.24–28) However, under solvent-free conditions, the activity of homogeneous metal catalysts in the oxidation reactions may be reduced because coordinating the metal catalysts with ligands is challenging and can result in catalyst aggregation.29–34) On the other hand, in the case of heterogeneous platinum group metal catalysts, such as palladium and rhodium, immobilized on activated carbon or alumina, the metallic particles can be dispersed under solvent-free reaction conditions. Furthermore, these catalysts can be reused without significantly degrading their catalytic activity.35) It has been reported that oxidizing alcohols by using heterogeneous metal catalysts under solvent-free conditions can proceed without aggregation of the catalytic metals.36–44) However, special techniques and devices, which are complicated, are required to prepare such catalysts. Therefore, the development of novel and practical solvent-free alcohol oxidation methods using commercially available metal catalysts is required from the perspective of process chemistry.
Our research group has used commercially available activated carbon-supported platinum group metal catalysts, such as palladium-on-carbon (Pd/C), platinum-on-carbon (Pt/C), rhodium-on-carbon (Rh/C), or ruthenium-on-carbon (Ru/C), to develop aerobic45,46) and dehydrogenative47,48) reactions that oxidize alcohols. Furthermore, our studies revealed that hydrogenation reactions catalyzed by Pd/C proceeded efficiently under solvent-free conditions in the solid–solid or (liquid)–gas phase.49) From these results, we hypothesize that the solvent-free, aerobic and dehydrogenative oxidation of alcohols using heterogeneous platinum group metal catalysts should proceed efficiently in a solid–solid or (liquid)–gas phase system (Fig. 1). During aerobic oxidation process, the catalyst would come into direct contact with a high concentration of oxygen gas in a solvent-free environment. Thereafter, the hydrogen gas generated from the metal-catalyzed dehydrogenation of the alcohol would easily separate from the solid-phase reaction mixture. Herein, we report the heterogeneous platinum group metal-catalyzed oxidation of various alcohols under solvent-free reaction conditions.
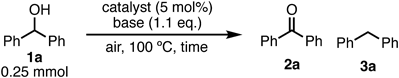
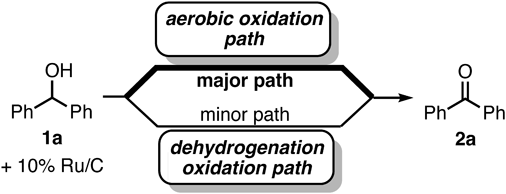
Benzhydrol (1a, 0.25 mmol) was first oxidized in the presence of a commercially available heterogeneous catalyst (5 mol%) and Na2CO3 (1.1 equivalent (equiv.)) at 100 °C under neat conditions (Table 1, entries 1–7). The dehydrogenative oxidation of 1a catalyzed by 10% Ru/C proceeded for 24 h under argon atmosphere to give 11% of the corresponding benzophenone (2a), with 89% of unreacted 1a (entry 1). Under aerobic conditions (entry 2), the oxidation of 1a was completed within 6 h to generate 2a. These results indicate that the oxidation proceeded mainly via the 10% Ru/C-catalyzed aerobic pathway (major path), together with the dehydrogenation pathway (minor path)50) (Fig. 2). The efficiency of the various heterogeneous platinum group metal catalysts (10% Ru/C, 10% Rh/C, 10% Pt/C, 10% Ir/C, and 10% Pd/C) under air atmosphere was then evaluated (entries 2–6). The 10% Ru/C catalyst had the highest activity when compared to the other platinum group catalysts (entry 2 vs. entries 3–6). In the case of 10% Pd/C or 10% Pt/C, a significant amount of diphenylmethane (3a) was generated as a byproduct via the hydrogenolysis of 1a by hydrogen which was provided from dehydrogenation of 1a (entries 5 and 6). Oxidation of 1a proceeded for 3 h with the use of 1.1 equiv. of potassium hydroxide (KOH) or tripotassium phosphate (K3PO4) instead of Na2CO3 (entries 10 and 11). However, some unreacted 1a remained in the case of potassium carbonate (K2CO3), sodium hydroxide (NaOH), or potassium hydrogen carbonate (KHCO3, entries 8, 9, and 12). The reaction could be completed with 5% ruthenium on alumina (5% Ru/Al2O3) in the presence of KOH, although ruthenium trichloride (RuCl3)-catalyzed conditions gave lower yields (entries 13 and 14).
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Base | Time (h) | 1H-NMR ratio of 1a/2a/3a |
| 1a) | 10% Ru/C | Na2CO3 | 24 | 89/11/0 |
| 2 | 10% Ru/C | Na2CO3 | 6 | 0/100/0 |
| 3 | 10% Rh/C | Na2CO3 | 6 | 77/23/0 |
| 4 | 10% Ir/C | Na2CO3 | 6 | 39/61/0 |
| 5 | 10% Pt/C | Na2CO3 | 6 | 49/44/7 |
| 6 | 10% Pd/C | Na2CO3 | 6 | 15/60/25 |
| 7 | 10% Ru/C | Na2CO3 | 3 | 15/85/0 |
| 8 | 10% Ru/C | K2CO3 | 3 | 8/92/0 |
| 9 | 10% Ru/C | NaOH | 3 | 12/88/0 |
| 10 | 10% Ru/C | Na3PO4 | 3 | 0/100/0 |
| 11 | 10% Ru/C | KOH | 3 | 0/100 (quant.)b)/0 |
| 12 | 10% Ru/C | KHCO3 | 3 | 13/87/0 |
| 13 | 5% Ru/Al2O3 | KOH | 3 | 0/100/0 |
| 14 | RuCl3 | KOH | 3 | 15/85/0 |
a) The reaction was carried out under argon atmosphere. b) isolated yield.
The 10% Ru/C-catalyzed oxidation of aromatic primary alcohols under solvent-free conditions was also investigated. Veratryl alcohol (4a) was used as the model substrate (Table 2). The ratio of veratryl aldehyde (5a) that reacted under aerobic conditions was improved by increasing the reaction temperature from 100 to 140 °C, despite partial over-oxidation of 5a, which gave the corresponding carboxylic acid (6a) (entries 1 and 2). Under oxygen atmosphere (entry 3), the oxidation efficiency decreased at 100 °C. Compound 4a was selectively transformed in the absence of KOH without over-oxidation (entry 4) to its corresponding aldehyde (5a), and the selective oxidation of 4a was completed within 6 h to yield 86% of 5a (entry 5).
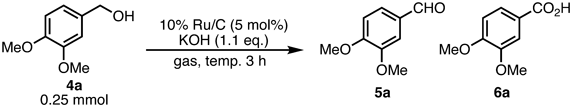 | |||
|---|---|---|---|
| Entry | Temp. (°C) | Gas | 1H-NMR ratio of 4a/5a/6a |
| 1 | 100 | Air | 52/29/19 |
| 2 | 140 | Air | 12/49/39 |
| 3 | 100 | O2 | 64/4/32 |
| 4a) | 100 | O2 | 14/86/0 |
| 5a,b) | 100 | O2 | 0/100 (86%)c)/0 |
a) Without KOH. b) 6 h. c) isolated yield.
The solvent-free oxidation was also applied to an aliphatic secondary alcohol. 4-Decanol (7a) was transformed to yield 25% of 4-decanone (8a) (Table 3, entry 1) under the same reaction conditions in Table 1, entry 11. The efficiency of oxidation of 7a was improved by increasing the reaction temperature, and 7a was obtained in 77% isolated yield at 160 °C after 24 h (entries 4 vs. 1–3).
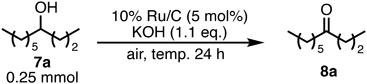 | ||
|---|---|---|
| Entry | Temp. (°C) | 1H-NMR ratio of 7a/8a |
| 1 | 100 | 75/25 |
| 2 | 120 | 63/37 |
| 3 | 140 | 10/90 |
| 4 | 160 | 0/100 (77%)a) |
a) Isolated yield.
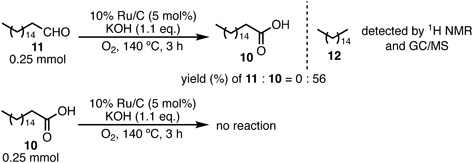
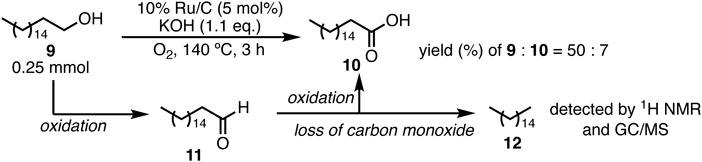
The 10% Ru/C-catalyzed solvent-free oxidation was also applied to 1-heptadecanol (9), an aliphatic primary alcohol. Similar reaction conditions to those used for the secondary aliphatic alcohols in Table 3, entry 3 (Chart 1) were used for the aliphatic primary alcohol. The desired heptadecanoic acid (10) was obtained in a yield of 7%; however, 50% of unreacted (9) remained. A trace amount of hexadecane (12) was detected in the reaction mixture using 1H-NMR and GC/MS. These results indicate that aldehyde elimination occurred during the reaction, which involved removing carbon monoxide from a heptadecanal intermediate (11).51)
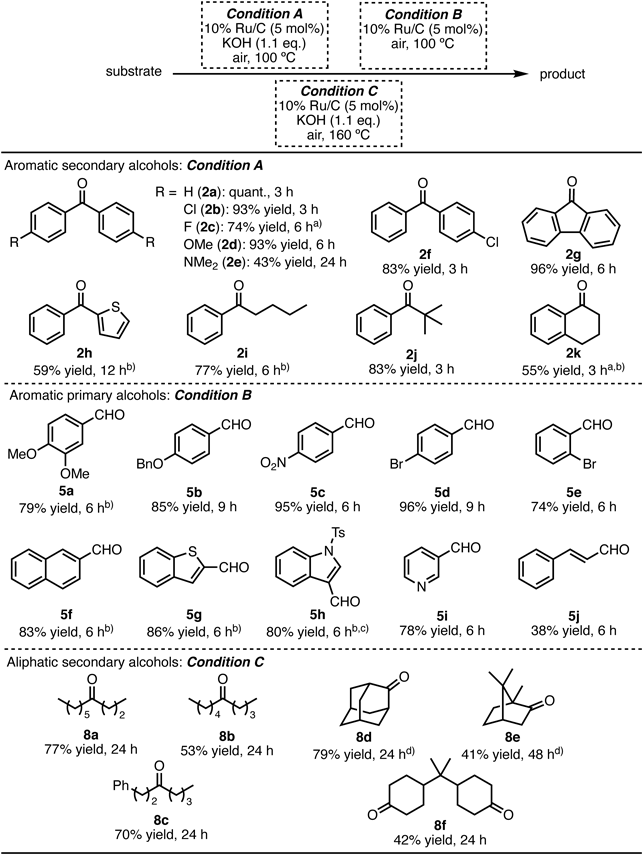
The substrate scope was then investigated under the reaction conditions optimized for aromatic secondary alcohols (Table 1, entry 11; Condition A), aromatic primary alcohols (Table 2, entry 5; Condition B), and aliphatic secondary alcohols (Table 3, entry 4; Condition C) (Table 4). Various benzhydrol derivatives (1a–1f), which had halogens or electron-donating groups on the aromatic rings, were efficiently oxidized under Condition A to their corresponding benzophenone derivatives (2a–2f) in 43% to quantitative yields. 9-Fluorenol (1g) was also efficiently transformed to 9-fluorenone (2g) in 96% yield. A secondary benzyl alcohol, which had the thienyl group at the benzylic position (1h), was converted to 2-thienyl phenyl ketone (2h) in 59% yield. The 10% Ru/C catalyzed oxidation of 1-phenyl alkyl alcohol derivatives (1i–1k) under solvent-free conditions produced the corresponding phenyl alkyl ketones (2i–2k) in good to high yields. 3,4-Di-MeO, 4-BnO, 4-NO2, 4-Br, and 2-Br-benzyl alcohol derivatives (4a–4e) were efficiently oxidized under Condition B to the corresponding aldehydes (5a–5e) in good to excellent yields, without over-oxidization. Aromatic primary alcohols, where the mother aromatic nucleus was a naphthalene (4f), benzothiophene (4g), or indole (4h) skeleton, were transformed to the corresponding aldehyde derivatives (5f–5h) in high yields. 3-Pyridinemethanol (4i) and cinnamyl alcohol (4j) were oxidized to obtain the corresponding 3-formyl pyridine (5i) and cinnamaldehyde (5j) in 78 and 38% yield under Condition B. Under Condition C, various aliphatic secondary alcohols (7a–7c) were transformed to the corresponding ketones (8a–8c) in moderate to good yields. The oxidization of 2-adamantanol (7d) and isoborneol (7e), which are alcohols that tend to be sterically rigid (relatively), proceeded smoothly to give 2-adamantanone (8d) and camphor (8e), respectively. Under Condition C, 2,2-bis(4-hydroxycyclohexyl)propane (7f) was converted to 2,2-bis(4-oxocyclohexyl) propane (8f) in 42% yield; this product is a useful intermediate for synthetic polymers, antioxidants, and heat stabilizers.52,53)
 |
a) Without KOH. b) The reaction was carried out under O2. c) 15 mol% of 10% Ru/C was used. d) 140 °C.
Combining the 10% Ru/C-catalyzed oxidation of alcohols under solvent-free conditions with aerobic and dehydrogenative oxidation resulted in high reaction efficiency (Table 5). The solvent-free oxidation of 1j, 1b, and 4c resulted in high yields within a short reaction time, when compared to the aerobic oxidation reactions catalyzed by the platinum-group metals (Condition D)45) and dehydrogenative oxidation (Condition E),47) which we previously reported.
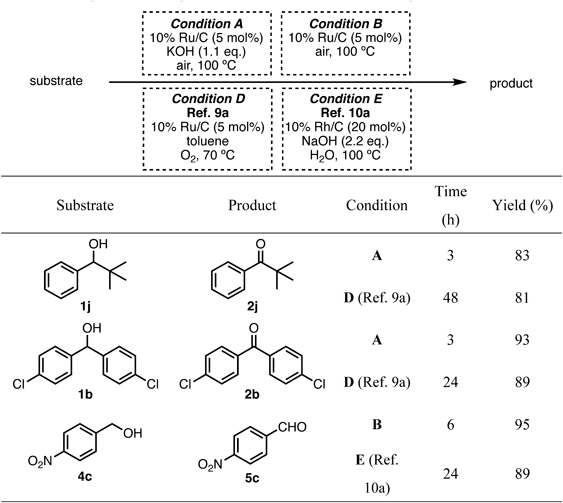 |
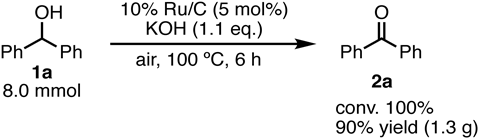
The solvent-free oxidation could be applied on the gram scale (Chart 2). Regardless of the stirring efficiency, 2a (7.2 mmol, 1.3 g) was obtained in a yield of 90% without significantly loss of reactivity.
Reusing heterogeneous catalysts is important for sustainable and green processes. Simple isolation and washing (only filtration, washing with H2O and CH2Cl2 then drying) allowed for 10% Ru/C to be reused at least five times without losing its catalytic activity (Table 6).
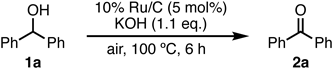 | ||
|---|---|---|
| Run | 1H-NMR ratio of 1a/2a | Yield of 2a (%) |
| 1st | 0/100 | 90 |
| 2nd | 0/100 | Quant. |
| 3rd | 0/100 | Quant. |
| 4th | 0/100 | Quant. |
| 5th | 0/100 | Quant. |
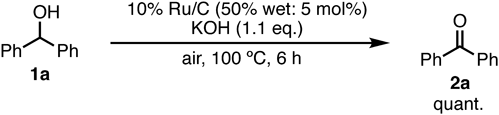
Using wet 10% Ru/C was also desirable for avoiding the potential risk of igniting the catalyst, which is important for safety and practicability. The 10% Ru/C catalyst containing water (50 w/w%) had almost the same catalytic reactivity as dry 10% Ru/C under the conditions of solvent-free oxidation (Chart 3).
A protocol for the solvent-free 10% Ru/C-catalyzed aerobic and dehydrogenative oxidation of alcohols to various carbonyl derivatives was developed. Furthermore, the selective oxidation of alcohols to aldehydes was accomplished without over-oxidation, and was applied on the gram scale. The catalyst could be reused at least 5 times without significantly degrading its activity. Notably, using wet 10% Ru/C instead of dry 10% Ru/C did not decrease the reaction efficiency. Therefore, the 10% Ru/C-catalyzed oxidation under solvent-free conditions is as a clean, safe, and practical methodology for industrial processes.
All reagents were obtained from commercial sources and used without further purification unless otherwise noted. 10% Ru/C, 10% Pd/C, 10% Rh/C, 10% Ir/C, 10% Pt/C, and 5% Ru/Al2O3 were obtained from N.E. Chemcat Corporation (Tokyo, Japan). Flash column chromatography was performed with Silica Gel 60 N (Kanto Chemical Co., Inc., Tokyo, Japan, 63–210 µm spherical, neutral). 1H- and 13C-NMR spectra were recorded on a JEOL ECZ 400 (1H: 400 MHz, 13C: 100 MHz) or ECA 500 spectrometer (1H: 500 MHz, 13C: 125 MHz) at room temperature in CDCl3 as a solvent and an internal standard (1H-NMR: δ = 0.00 for CDCl3; 13C-NMR: δ = 77.0 for CDCl3). GC-3200 [gas chromatograph equipped with thermal conductivity detector (GC-TCD; GL Science, Japan)] was used for gas analysis with Molecular Sieve 5 Å (60/80 mesh) packed column (3 × 2.2 mm i.d., 1/8 inch: GL Science).
General ProceduresCondition A: A mixture of the substrate (250 µmol), KOH (15.4 mg, 275 µmol) and 10% Ru/C (12.6 mg, 12.5 µmol) was stirred at 100 °C using a test tube equipped with air balloon. After the corresponding reaction time, the mixture was filtered through a membrane filter (pore size: 0.2 µm). The catalyst on the filter was washed with H2O and CH2Cl2 and extracted with CH2Cl2 (5 mL × 3). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was further purified by silica-gel column chromatography.
Condition B: A mixture of the substrate (250 µmol) and 10% Ru/C (12.6 mg, 12.5 µmol) was stirred at 100 °C using a test tube equipped with air balloon. After the corresponding reaction time, the mixture was filtered through a membrane filter (pore size: 0.2 µm). The catalyst on the filter was washed with H2O and CH2Cl2 and extracted with CH2Cl2 (5 mL × 3). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was further purified by silica-gel column chromatography.
Condition C: A mixture of the substrate (250 µmol), KOH (15.4 mg, 275 µmol) and 10% Ru/C (12.6 mg, 12.5 µmol) was stirred at 160 °C using a test tube equipped with air balloon. After the corresponding reaction time, the mixture was filtered through a membrane filter (pore size: 0.2 µm). The catalyst on the filter was washed with H2O and CH2Cl2 and extracted with CH2Cl2 (5 mL × 3). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was further purified by silica-gel column chromatography.
We thank N. E. Chemcat Corporation for the kind gift of various metal catalysts supported carbons.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.