2023 年 71 巻 4 号 p. 262-268
2023 年 71 巻 4 号 p. 262-268
Apple is an important dietary agent for human and apple polyphenols (AP) are the main secondary metabolites of apples. In this study, the protective effects of AP on hydrogen peroxide (H2O2)-induced oxidative stress damage in human colon adenocarcinoma Caco-2 cells were investigated by cell viability, oxidative stress change as well as cell apoptosis. Pre-adding AP could significantly increase the survival rate of H2O2-treated Caco-2 cells. Besides, the activities of antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and catalase (CAT) were elevated. While the malondialdehyde (MDA) content which is the major oxidant products of polyunsaturated fatty acids (PUFA) reduced after AP treatment. In addition, AP also suppressed the emergence of DNA fragment and decreased the expression of apoptosis-related protein Caspase-3. These results demonstrated that AP could ameliorate H2O2-induced oxidative stress damage in Caco-2 cells, which could serve as a reference for further studies of apple natural active products and deep study of the anti-oxidative stress mechanism.
Ulcerative Colitis (UC) is a type of Inflammatory Bowel Disease (IBD), which is characterized by the colon and rectal mucosa inflammation, with bloody diarrhea, abdominal cramps, and blood in the stool as the main clinical manifestations.1) The highest prevalence of UC has been reported to be in Europe, reaching 5‰. As the incidence and prevalence of UC in Asia have increased year by year, UC has now become a global disease.2,3) Besides, there are data showing that people with UC are at a high risk of developing into colorectal cancer.4) The pathogenesis of UC is multifactorial and has not been fully elucidated. Numerous studies have shown that oxidative stress due to excessive release of reactive oxygen species (ROS) is one of the important pathogenic factors in colon tissue. It is recognized that overproduction of ROS in intestinal mucosal cells could induce subcellular damage, destruct intercellular tight junctions, and increase mucosal permeability, thereby impairing the integrity of the intestinal mucosal barrier, and causing the occurrence of intestinal inflammation.5–8) Therefore, oxidative stress plays a key role in the occurrence and development of UC, and inhibiting the excessive release of ROS is of great significance to protect intestinal mucosal cells and control the development of UC.
At present, UC cannot be cured yet, due to the wide range of lesions and easy recurrence. In clinic, 5-aminosalicylic acid, steroid hormones and immunosuppressive drugs are the main medicine for the treatment. However, 5-aminosalicylic acid is a non-specific anti-inflammatory drug, which can only temporarily control and relieve the symptoms for UC. Side effects such as headaches and nephrotoxicity would occur after the administration, while it is easy to repeat after stopping the drug.9,10) Besides, treatment with steroids and immunosuppressive agents could also lead to secondary health problems, such as hormone dependence and drug resistance.11,12) Therefore, developing new drugs for treating UC remains a challenge.
Apple is an important dietary agent for human, and epidemiological studies have found that apple can reduce the risk of cancers, cardiovascular disease, asthma, and diabetes.13) Apple polyphenols (AP) are the secondary metabolites of apples, and are the general term for polyphenols contained in apples.14) It has been reported in the literature that AP exhibit various biological efficacy such as anti-allergy, regulating glucose/lipid metabolism, promoting growth, preventing atherosclerosis, anti-aging, as well as antioxidant and oxygen free radical scavenging effects.15) However, as far as we know, there are not many related reports on AP treatment for UC.16–18) Caco-2 cells, which could mimic human intestinal epithelial cells in the body when cultured in monolayer, have been widely used as a typical in vitro UC model for antioxidant study.19–22) Hydrogen peroxide (H2O2) which is an important ROS, could induce the oxidative stress damage and apoptosis of many cells.23–26) Therefore, this study evaluated the protective effect of AP on the oxidative stress damage of Caco-2 cells induced by H2O2, in which cell viability, apoptosis, as well as oxidative stress parameters of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT) and malondialdehyde (MDA) were explored.
AP (polyphenol, 81.8%; Batch: 1310019-14) were kindly provided by Tianjin Jianfeng natural product R&D Co., Ltd., China. Dulbecco’s modified Eagle’s medium (DMEM)/12F medium and fetal bovine serum (FBS) were purchased from Hyclone, U.S.A. 3-(4,5)-Dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT), bicinchoninic acid (BCA) protein assay kit and secondary HRP conjugated sheep anti-rabbit antibody were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd., China. DNA Ladder apoptosis detection kit was purchased from Shanghai Biyuntian Institute of Biotechnology. MDA assay kit and SOD assay kit were purchased from Nanjing Jiancheng Bioengineering Institute, China. GSH-PX kit and CAT kit, dimethyl sulfoxide (DMSO), sodium dodecyl sulphate (SDS), polyvinylidene difluoride filters (PVDF, Bio-Rad, U.S.A.) were purchased from Beijing Solarbio Science & Technology Co., Ltd., China. Caspase-3 primary antibody was purchased from Cell Signaling Technology, U.S.A. β-Tubulin antibody was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd., China. Thirty percent H2O2 solution was purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd., China. Human colon adenocarcinoma Caco-2 cells were provided by the Cell Resource Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences.
Chemical Characterization of APChemical constituents of AP were analyzed with an HPLC system (Shimadzu Scientific Instruments, Tokyo, Japan) equipped with a diode array detector (DAD). The detection wavelength was 280 nm. The sample was filtered through a 0.45 µm Millipore membrane filter before injection. The flow rate was 1.0 mL/min and a 10 µL aliquot was separated by a Phenomenex luna C18 column (250 × 4.6 mm, 5 µm) at 30 °C with the mobile phase consisted of solvent A (2% acetic acid in water, v/v) and solvent B (80% acetonitrile with 20% A). The gradient program was as follows: 0% B to 4% B in 6 min, to 10% B in 9 min, to 15% B in 15 min, to 23% B in 20 min, to 25% B in 10 min, to 30% B in 6 min, to 50% B in 14 min, to 80% B in 3 min, to 0% B in 5 min, and to stop after 20 min.
Cell CultureCaco-2 cells were cultured in 75 mL flasks in the presence of DMEM/12F medium with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
Grouping and DosingCaco-2 cells were divided into 6 groups, namely blank control group, negative control group, and administration groups (with four concentration gradients of 50, 10, 2, and 0.4 µg/mL). The cells in the logarithmic growth phase were seeded in the plates. After overnight incubation, culture medium containing different concentrations of AP were added, while for the blank control group and negative control group, equal volume of culture medium was added. After 24 h, except the blank control group, H2O2 was added to all the other groups to make the final concentration of H2O2 in each well was 100 µM. And the blank control group was added with an equal volume of culture medium. All the cells were incubated for another 24 h before examination.
Cell Viability AssayCaco-2 cells were seeded at 1.8 × 104 cells/well in a 96-wells plate with 100 µL per well. The cells were treated with the above-mentioned method and were observed for cell morphology and photographed under an inverted microscope. Then, 20 µL of MTT solution (concentration of 5 g/mL) was added to each well, and cells were incubated for another 4 h. After aspiration the supernatant, 150 µL of DMSO were added to each well and the plates were shaken for 10 min. Then the samples were tested by a microplate reader (Tecan Trading AG, Switzerland) with the absorbance at 490 nm.
Determination of MDA, SOD, GSH-PX and CATTake logarithmic growth phase cells and inoculate at 1.5 × 104 cells/well in a 24-wells plate. After treatment with the above-mentioned method, the cell culture medium was aspirated and centrifuged for 5 min. Two hundred microliters of the supernatant was taken and the contents of MDA, SOD, GSH-PX, and CAT in the cell supernatant were measured by each kit, respectively.
DNA Ladder DetectionCaco-2 cells in logarithmic growth phase were inoculated into 6-wells plates at 4 × 105 cells/well. After treatment with the above-mentioned method, DNA Ladder extraction was performed. Gel electrophoresis at 90V for 1 h was performed and observed under a UV gel imager.
Western Blot AssayAnother logarithmic growth phase Caco-2 cells were seeded in 6-wells plate, and the density was controlled to be 4 × 105 cells/well. After treatment with the above-mentioned dosing method, cells were lysed and the concentration of the total protein was measured by the BCA protein assay kit. Then equal amounts of protein (100 µg) were separated by 10% SDS polyacrylamide gel electrophoresis and electrotransferred on to PVDF. After blocking with 5% non-fat milk, the proteins were probed with the following specific primary rabbit antimouse antibodies: cleaved Caspase-3 (each at 1 : 1000 dilution) and β-tubulin (1 : 10000 dilution). The immunoreactive bands were then visualized with secondary HRP conjugated sheep anti-rabbit antibody, using an enhanced chemiluminescence (ECL) detection system.
Statistical AnalysesExperimental results are presented as means ± standard deviation (S.D.), and all measurements and analyses were repeated at least three times. SPSS V.20.0 statistical software was used for the statistical and graphical evaluations in this study. Statistical analyses were performed by one-way ANOVA with Tukey’s multiple comparisons and the Student’s t-test. All p-values <0.05 were considered statistically significant.
The chemical constituents of AP were isolated by HPLC (Fig. 1) and the peaks were identified according to the retention times of standards. AP contained chlorogenic acid (17.9%) followed by procyanidin B1 (3.21%), procyanidin B3 (2.93%), procyanidin B2 (6.48%), epicatechin (5.89%), phloridzin (25.19%), phloretin (2.23%), and other unidentified phenolics (36.17%).

Peaks: 1, procyanidin B1; 2, procyanidin B3; 3, chlorogenic acid; 4, procyanidin B2; 5, epicatechin; 14, phloridzin; 15, phloretin; 6, 7, 8, 9, 10, 11, 12, 13, unknown.
The morphological changes of Caco-2 cells after incubation with AP and H2O2 were observed under the light microscope (Fig. 2). Compared with the normal cells in the blank control group, in the negative control group (only H2O2 was added), the cells shrank and most of the cells were exfoliated. However, in the administration groups in which cells were treated with AP in advance, the cell morphology tended to be normal, and the number of cells were relatively increased. The results of the MTT colorimetric assay also verified it. Compared with the normal cells in the blank control group, the Caco-2 cell viability in the negative control group was significantly decreased (p < 0.01), while the cell viability in the AP administration groups were significantly higher than that in the negative control group (p < 0.05) with dose-dependent (Fig. 3).

(A) Pretreatment of cells with 50 µg/mL AP before exposed to H2O2. (B) Pretreatment of cells with 10 µg/mL AP before exposed to H2O2. (C) Pretreatment of cells with 2 µg/mL AP before exposed to H2O2. (D) Pretreatment of cells with 0.4 µg/mL AP before exposed to H2O2. (E) Negative control group: 100 µM H2O2 treatment for 24 h. (F) Blank group: cells without any treatment.

Caco-2 cells were treated with 100 µM H2O2 for 24 h in the presence of AP (50, 10, 2, 0.4 µg/mL). Results are presented as mean ± S.D. (n ≥ 6); ## p < 0.01, # p < 0.05 vs. blank control, ** p < 0.01, * p < 0.05 vs. negative control.
MDA, SOD, GSH-PX, CAT are the important indicators for evaluating oxidative damage. The experimental results showed that the content of MDA in the supernatant of cells after 100 µM H2O2 treatment increased significantly (15.4%), while the content of SOD, GSH-PX and CAT obviously decreased in the negative control groups (17.7, 11.6 and 23.9%, respectively), which showed that H2O2 could induce the oxidative stress in Caco-2 cells. However, pre-adding AP 24 h before damaging by H2O2 could reduce the content of MDA in the cell supernatant (p < 0.01) and increase the content of SOD, GSH-PX and CAT (p < 0.05) in varying degrees, as comparing with the negative control group (Fig. 4). In the administration groups with 50 to 0.4 µg/mL AP, the MDA content decreased by 45.0%, and SOD, GSH-PX and CAT content increased by 81.2, 33.3 and 40.7%, respectively. The results indicated that AP could effectively reduce the level of oxidative damage and enhance the antioxidant capacity of cells.

Results are presented as mean ± S.D. (n ≥ 4); ## p < 0.01, # p < 0.05 vs. blank control, ** p < 0.01, * p < 0.05 vs. negative control.
DNA Ladder, also known as DNA fragmentation, is an important indicator of apoptosis. In the process of apoptosis, DNA between nucleosomes is broken, resulting in oligonucleotide fragments, and at the same time there are more than 50kbp of genomic DNA, so a ladder electrophoresis pattern could be observed on agarose gel electrophoresis. In this study, after Caco-2 cells were treated with 100 µM H2O2, obvious DNA ladders could be observed on the electrophoresis bands, while the AP treatment groups could significantly inhibit the appearance of DNA ladders, and showed a dose-dependent relationship (Fig. 5).

(B) Pretreatment of cells with 10 µg/mL AP before exposed to H2O2. (C) Pretreatment of cells with 2 µg/mL AP before exposed to H2O2. (D) Pretreatment of cells with 0.4 µg/mL AP before exposed to H2O2. (E) Negative control group: 100 µM H2O2 treatment for 24 h. (F) Blank group: cells without any treatment. (M) DNA maker.
Caspase-3 is the most important terminal cleavage enzyme in the process of apoptosis. In this study, the expression of Caspase-3 in each group were determined and analyzed by Western blot method. The results showed that H2O2 up-regulated the expression of Caspase-3 in the negative control group, while in the groups treated with AP, the expression of Caspase-3 was down-regulated (p < 0.05). Specifically, varying the AP concentration from 50 to 2 µg/mL decreased the expression of Caspase-3 by 60.3, 49.1 and 17.6%, respectively. This indicated that AP could suppress the expression of apoptotic protein Caspase-3, thereby inhibiting H2O2-induced oxidative stress damage in Caco-2 cells (Fig. 6).

The statistical results were the mean ± S.D. (n = 3), ## p < 0.01, # p < 0.05 vs. blank control, ** p < 0.01, * p < 0.05 vs. negative control.
UC is a chronically idiopathic IBD that causes persistent mucosal inflammation extending from the rectum to the proximal colon. The pathogenesis of UC is complex and multifactorial, including genetics, environment, autoimmunity, gut microbiota and so on.27) Under adverse conditions, these factors induce the release of pro-inflammatory mediators, such as ROS. ROS have cytotoxic effects through lipid peroxidation and protein denaturation, resulting in a chain reaction that expands and increases oxidative stress and inflammatory damage, so ROS are considered as markers in the pathogenesis of UC.28,29)
Caco-2 cells, a colon adenocarcinoma cell line isolated from human intestinal tissue, have the characteristics of epithelial cells and are internationally recognized as a typical model for in vitro oral drug absorption experiments.30) H2O2 is an important ROS, which is prone to homolysis to form highly active ROS-hydroxyl radicals.31) Evidences suggested that excess H2O2 in vivo readily diffused through colonic epithelial cell membranes into the extracellular microenvironment, where its unique combination of longevity, strong oxidative capacity, and ability to attract leukocytes (neutrophil chemotaxis) could promote oxidative damage to the tightly joined colonic epithelium.32) In this study, we established an in vitro model of intestinal epithelial cell oxidative damage by using H2O2 treated Caco-2 cells. It was found that the number of viable cells was significantly reduced compared with untreated Caco-2 cells (p < 0.01). This indicated that H2O2 is suitable for constructing the oxidative damage model of intestinal epithelial Caco-2 cells in vitro.
AP are a class of important biologically active substances naturally presented in apples. In recent years, a large number of studies have shown that AP have a variety of activities, among which anti-oxidation is one of the most important pharmacological effects.33–35) From the HPLC results, we could see that AP is a mixture containing chlorogenic acid, procyanidin, epicatechin, phloridzin, etc. It is generally recognized that the structure of polyphenolic hydroxyl groups could endow the components with antioxidant activities. Specifically, chlorogenic acid can obviously alleviate the reduction of chlorophyll concentration and maximum potential photosystem II efficiency in plants. It can also reduce membrane damage and lipid oxidation, stimulate the activity of antioxidant enzymes, and change the transcription levels of antioxidant enzymes and phenolic metabolism-related genes, exhibiting effective antioxidant activities.36) Besides, chlorogenic acid chelated with Zn(II) exhibited even better antioxidant activities than the commercially used antioxidants such as L-ascorbic acid, butylated hydroxyanisol (BHA) and butylated hydroxytoluene (BHT).37) Procyanidin as a powerful antioxidant flavonoid, exhibited significant ROS scavenging ability on stimulated THP-1 macrophages, and effectively suppressed the inflammatory signaling pathways, down-regulated of matrix metalloproteinase 9 (MMP9) expression, inhibited nuclear factor-kappaB (NF-κB) signaling, and interrupted the formation of the NLRP3 inflammasome in a dose-dependent fashion.38) When complexed with chitosan, procyanidin-chitosan composite films showed higher 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ABTS+ scavenging rates, presenting outstanding antioxidant activity.39) Epicatechin was also verified to have potent and stable antioxidant activities for at least 14 d with low IC50 values.40) Single electron transfer-proton transfer was the most probable mechanism with the OH sites of the B ring in Epicatechin was the most preferential sites.41) Phloridzin alleviated and protected neuroinflammation in rats by enhancing antioxidants (SOD and GSH) in hippocampus and the cerebral cortex, and reducing the levels of inflammatory/oxidative markers (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and MDA).42) Moreover, myristic acid acylated derivative of phloridzin, which mainly occurred on the 6′-OH of phloridzin glycoside, was also demonstrated to have significantly higher total antioxidant activity than the parent phloridzin both in vitro and in vivo.43) Thus it is speculated that the chlorogenic acid, procyanidin, epicatechin and phloridzin in AP all contributed to its strong antioxidant activities. In our study, it could be found that adding different concentrations of AP 24 h before H2O2 treatment could effectively improve the activity of Caco-2 cells, maintain the cell morphology and significantly increase the number of viable cells (p < 0.05). At the same time, AP could inhibit the appearance of DNA Ladder with a concentration-dependent relationship. However, when AP co-incubated with H2O2, AP had no protective effect on H2O2-induced cellular oxidative damage (data not shown). This suggested that AP was not directly reacted with the added H2O2 in the medium, but AP activated certain protective signal pathway in the cells which suppressed the H2O2 cascade reaction. Importantly, pre-addition of AP is required to protect Caco-2 cells from oxidative stress damage (Fig. 7).
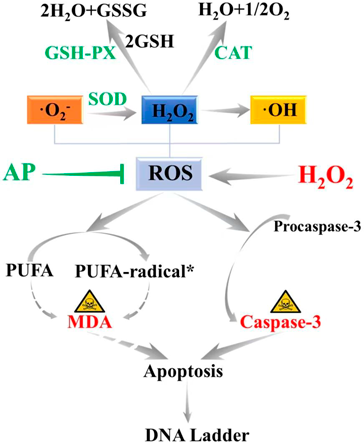
Oxidative stress caused by excessive release of ROS is one of the main mechanisms of UC development. In biological organisms, MDA is the major products of the oxidation of long-chain polyunsaturated fatty acids (PUFA),44) which could cause the cross-linking and polymerization of life macromolecules such as proteins and nucleic acids, thus exhibiting the cytotoxicity. MDA content manifests the degree of lipid peroxidation and indirectly reflects the degree of cellular oxidative stress damage.45) SOD plays a crucial role in the body’s balance between oxidation and anti-oxidation. SOD could scavenge superoxide anion free radicals in the body and protect cells from damage.46) That is to say, activity of SOD could verify the antioxidation capacity in vivo. GSH-PX and CAT are the important enzyme that catalyzes the decomposition of hydrogen peroxide widely in our body. When there are excess free radicals, superoxide radicals are disproportionated into H2O2 by SOD, and then H2O2 was converted into water under the catalysis of GSH-PX and CAT, thus protecting the body from peroxide poisoning.47,48) Therefore, to judge the degree of lipid oxidative damage in the body or cells, the level of lipid peroxidation product MDA should be detected to determine the severity of free radical attack on cells. And on the other hand, the activities of antioxidant enzymes such as SOD, GSH-PX and CAT should also be tested to determine the ability of drugs to scavenge oxygen free radicals.49,50) Simultaneous analyses of the levels of MDA, SOD, GSH-PX, and CAT could comprehensively evaluate the changes in oxidative stress and help verify the therapeutic effect of AP on oxidative damage. In this study, the contents of MDA, SOD, GSH-PX and CAT in the cell supernatant were measured. And the results showed that the contents of MDA in the negative control group were significantly increased, and the contents of SOD, GSH-PX and CAT were decreased (p < 0.01). The AP treatment groups could effectively reduce the content of MDA and increase the content of SOD, GSH-PX and CAT (p < 0.05), indicating that AP enhanced the antioxidant capacity of Caco-2 cells and reduced the degree of oxidative stress damage with a dose-dependent manner.
Caspase is a group of structurally similar cysteine-containing aspartate proteolytic enzymes presented in the cytosol and is a central component of apoptosis. In the Caspase family, the pro-apoptotic protein Caspase-3 plays an important role in inducing apoptotic events in intrinsic and extrinsic apoptotic pathways, and is also one of the major enzymes involved in the initiation of apoptosis.51,52) The results of this study showed that under the action of H2O2, the expression of Caspase-3 in the negative control group was up-regulated (p < 0.01). However, the expression of Caspase-3 in AP-treated groups were significantly down-regulated (p < 0.05) showing a certain concentration-dependent manner. This indicated that AP could inhibit the expression of apoptosis protein Caspase-3, thereby inhibiting the apoptosis of Caco-2 cells induced by H2O2-induced oxidative stress damage.
In conclusion, AP, the main secondary metabolites extracted from apples, could effectively protect Caco-2 cells from H2O2-induced oxidative stress damage. Pre-adding AP to Caco-2 cells significantly enhanced cell viability and improved cell morphology. At the same time, AP could inhibit the oxidative stress damage induced by H2O2 and enhance the antioxidant level of Caco-2 cells by increasing the activities of antioxidant enzymes of SOD, GSH-PX and CAT, as well as reducing the major oxidant products of PUFA, the MDA. Moreover, the obviously weakened DNA fragmentation and the positively down-regulation of cell apoptosis protein of Caspase-3 demonstrated that AP could ameliorate H2O2-induced oxidative stress damage in Caco-2 cells via Caspase-3 signaling. All in all, AP possessed good protective effect on H2O2-induced oxidative stress damage in Caco-2 cells, which provided a new way for the development of apple natural active products and the screening of anti-UC drugs. At present, it is generally believed that the significant features of natural products are multiple activities, multiple targets work together and less adverse effects. In this study, we fundamentally investigated its anti-oxidative stress injury and anti-apoptosis activities via Caspase-3 signaling in UC from the cellular level. AP have also been reported to alleviate UC by restoring bile acid metabolism disorder, reducing mitochondrial dysfunction as well as repairing gut microbiota dysbiosis.16–18) Therefore, AP possesses a good prospect for the development of anti-UC health food and drugs. It is believed that with the continuous development of research, AP would achieve promising results in clinical treatment.
This work was supported by National Natural Science Foundation of China (No. 22108225), China Postdoctoral Science Foundation (2018M643720), and Natural Science Foundation of Shaanxi Province, China (No. 2021JQ-434).
The authors declare no conflict of interest.