2023 年 71 巻 4 号 p. 289-298
2023 年 71 巻 4 号 p. 289-298
Oral disulfiram (DSF) has been used clinically for alcohol dependence and recently has been found to have antitumor activity. A transdermal delivery system would be useful for maintaining drug concentration and reducing the frequency of administration of DSF for cancer treatment. Penetrating the stratum corneum (SC) barrier is a challenge to the transdermal delivery of DSF. Therefore, we investigated the promoting effects and mechanism of action of the combination of oleic acid (OA) and Tween 80 on the skin permeation of DSF. Hairless mouse skin was exposed to OA and Tween 80, combined in various ratios (1 : 0, 2 : 1, 1 : 1, 1 : 2, and 0 : 1). A permeation experiment was performed, and total internal reflection IR spectroscopic measurements, differential scanning calorimetry, and synchrotron radiation X-ray diffraction measurements were taken of the SC with each applied formulation. The combination of OA and Tween 80 further enhanced the absorption-promoting effect of DSF, compared with individual application. The peak of the CH2 inverse symmetric stretching vibration near the skin surface temperature was shifted by a high frequency due to the application of OA, and DSF solubility increased in response to Tween 80. We believe that the increased fluidity of the intercellular lipids due to OA and the increased solubility of DSF due to Tween 80 promoted the absorption of DSF. Our study clarifies the detailed mechanism of action of the skin permeation and promoting effect of DSF through the combined use of OA and Tween 80, contributing to the development of a transdermal preparation of DSF.
Drugs are frequently administered using the oral route of administration, but their bioavailability is reduced due to the first-pass effect of the liver; furthermore, gastrointestinal side effects may occur and they are challenging to administer to patients who have difficulty swallowing. Therefore, an alternative route of administration is a transdermal delivery system that delivers the drug into the body through the skin. The transdermal delivery system avoids the first-pass effect of the liver and can be given to patients who have difficulty swallowing. In addition, since the drug is continuously absorbed into the body, the blood concentration of the drug remains stable, so it is possible to reduce the occurrence of side effects caused by a rapid increase in blood concentration and reduce the frequency of administration. In addition, it enables easy discontinuation and visual confirmation of the medication.1) These advantages of transdermal preparation could improve the QOL of the patient and further contribute to a reduction of the burden on medical staff who administer treatments and long-term care.
The skin consists of the epidermis, dermis, and subcutaneous tissue,2) and the epidermis is further divided into four layers, from the outside: the stratum corneum (SC), stratum granulosum, stratum spinosum, and stratum basal. The SC is composed of SC cells containing approximately 90% keratin as the main component and intercellular lipids consisting of approximately 10% ceramide, cholesterol, and free fatty acids. These SC cells and intercellular lipids are stacked in a brick-and-mortar structure, and form a strong barrier. Therefore, the SC is considered to be the key to the skin’s barrier function.3–5)
Transdermal absorption of a drug through the skin is considered to occur through the transepidermal route. The transepidermal pathway is further divided into an intercellular pathway through the intercellular lipids of the SC and an intracellular pathway through the SC cells; it is believed that drugs are absorbed by the intercellular pathway rather than the intracellular pathway.6) Therefore, the barrier function of intercellular lipids in the SC must be overcome to administer drugs through the skin.
The intercellular lipids in the SC create a barrier by forming a regular arrangement called a lamellar structure and a packing structure.7) The lamellar structure is made of a long-period structure with a period of approximately 13 nm and a short-period structure with a period of approximately 6 nm; this has been confirmed in the small-angle region with small-angle and wide-angle X-ray diffraction.8–10) In the wide-angle region, hexagonal crystals composed of 0.42 nm lattices and orthorhombic crystals composed of 0.42 and 0.37 nm lattices have been confirmed.8–11) The peculiar structure of this intercellular lipid changes with a phase transition, identified by temperature scanning,12) and its relationship with the barrier function of the SC has been clarified.13–18)
Due to the barrier function of the SC resulting from these regular arrangements, it is difficult to deliver enough drug into the body for treatment.19) Therefore, it is difficult to develop transdermal formulations of a wide variety of drugs, and the drugs developed thus far meet the following conditions. Their “molecular weight must be less than 500 to increase drug diffusion in the skin”; they must have a “logarithmic oil–water partition coefficient of 1 to 4 for drug distribution from the base to the highly liposoluble SC and from the SC to the less liposoluble lower layer”; and “the melting point must be lower than 200 °C.”20) In addition, there are various methods to improve the skin permeability of drugs, such as physical permeation promotion methods using potential difference and ultrasonic waves21,22) and chemical facilitation methods using absorption promoters. Many compounds have been investigated for promoting absorption, and many compounds such as terpenes, fatty acids, esters, and surfactants have been identified.23,24) Among them, oleic acid (OA), which is an unsaturated fatty acid, and Tween 80, which is a surfactant, are used in some commercial preparations, and their promoting effects on various drugs have been reported.23,25) In addition, two major mechanisms for promoting transdermal absorption have been proposed.26,27) One mechanism increases the distribution of a drug by penetrating the SC and changing the solubility of the substance. Another mechanism penetrates the intercellular lipids of the SC, disturbs the lipid arrangement, and increases the diffusion of substances into the SC. It has been reported that the combined use of compounds having these different promoting effects and whose safety as an additive for pharmaceuticals has been established may show a strong promoting effect.28–30) For example, Tween 80, which is a hydrophilic surfactant, has been reported to extract intercellular lipids in the SC, increase the diffusion of drugs into the skin, and promote transdermal absorption of drugs.25) On the other hand, OA, which is an unsaturated fatty acid, is distributed to the skin and absorbed by increasing the fluidity of intercellular lipids due to its bulky and refracted structure. OA disturbs the intercellular lipid layer and causes phase separation in the SC. Previous studies have shown this increased fluidity promotes the transdermal absorption of drugs.3,23,31) These reports demonstrate that OA and Tween 80 have different promoting mechanisms, and the combined use of the two compounds may greatly promote the skin penetration of a drug. However, there are no reports of the effects of OA and Tween 80 on skin permeation when used in combination.
Research on the treatment of cancer is continually advancing, but there is still opportunity for further new discoveries. Cancer is one of the leading causes of death in developing countries and is thought to be due to aging and lifestyle habits such as lack of exercise, smoking, and eating a Western diet. Treatment of cancer, which has a high mortality rate and is an important issue, is roughly divided into three modalities: surgical excision, chemotherapy, radiation therapy, genetic therapies, and immunotherapies. In Japan, surgery has been the mainstay of cancer treatment, but through recent advances, chemotherapy and radiation therapy are expected to be as effective as surgery depending on the type and stage of cancer. Chemotherapy mainly uses anticancer drugs to kill or suppress the growth of cancer cells, but some tumors are resistant to existing anticancer drugs. Therefore, additional drugs to treat cancer are required, and in recent years, disulfiram (DSF), which has a long history of use as an anti-alcoholic drug, has been attracting attention.32–34)
DSF is a drug discovered in the 1940 s and has been used as an oral therapy for alcohol dependence for more than 60 years. In the body, it is metabolized to diethylthiocarbamic acid and exerts its effects by inhibiting alcohol dehydrogenase in the liver. The anti-cancer activity of DSF reported in recent years is wide-ranging, with potential applications to colorectal, prostate, and breast cancer,35,36) and clinical research is underway. The details of the mechanism of the antitumor activity of DSF are not clear, but it may exert its action by inhibiting proteasome activity and forming a complex with copper, which is a divalent metal that enhances antitumor activity.34)
In anticancer drug treatment, which is expected to be administered for a long period of time, a transdermal preparation that can consistently deliver a drug to the blood and can reduce the frequency of administration is considered very useful. To that end, DSF is a lipophilic drug with a molecular weight of approximately 296.54, an oil–water partition coefficient of 3.9, and a melting point of 70 to 73 °C, so it meets the conditions of established transdermal preparations. Despite the good potential permeability of DSF, studies confirming its skin permeability are limited.37) Since there have been no reports on the skin permeation-promoting effect of OA and Tween 80 in combination, this study examined the skin permeation-promoting effect of DSF combined with OA and Tween 80, and performed an in-depth study of its mechanism of action, focusing on its effect on intercellular lipids in the SC.
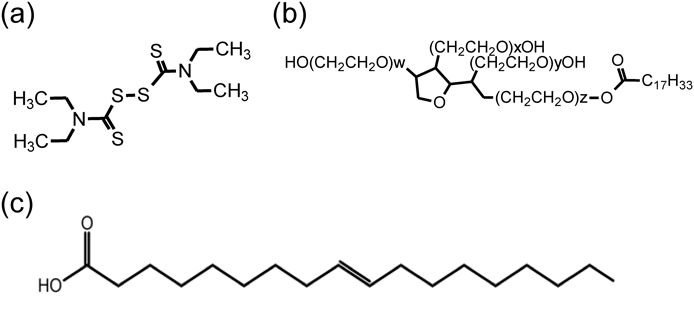
DSF, trypsin (from bovine pancreas), dimethyl sulfoxide (DMSO), ethanol, isopropanol (IPA), oleic acid, Tween 80, hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), disodium hydrogenphosphate, potassium dihydrogenphosphate, sodium chloride, and potassium chloride from FUJIFILM Wako Junyaku Co., Ltd. (Osaka, Japan) were purchased and used in this experiment. Other reagents were reagent grade. Figure 1 shows the structural formula of DSF, Tween 80, and OA.
Purified water was added to HPC and HEC, and the mixture was allowed to stand overnight to swell the base. Separately, a mixture of all of the components of each formulation, shown in Table 1 and excluding HPC, HEC, and purified water, was added to the swollen base with stirring to form a homogeneous hydrogel for the experiment.
| Formulation components | Control (%) | OA (%) | OA:Tween 80 (2 : 1) (%) | OA:Tween 80 (1 : 1) (%) | OA:Tween 80 (1 : 2) (%) | Tween 80 (%) |
|---|---|---|---|---|---|---|
| DSF | 3 | 3 | 3 | 3 | 3 | 3 |
| OA | — | 5 | 3.3 | 2.5 | 1.7 | — |
| Tween 80 | — | — | 1.7 | 2.5 | 3.3 | 5 |
| IPA | 20 | 20 | 20 | 20 | 20 | 20 |
| HPC | 1 | 1 | 1 | 1 | 1 | 1 |
| HEC | 1 | 1 | 1 | 1 | 1 | 1 |
| Water | 75 | 70 | 70 | 70 | 70 | 70 |
Labskin® (Hos/HR-1 male hairless mice, 7 weeks old, Sankyo Lab Service Co., Ltd., Tokyo, Japan) were mounted in a Franz-type diffusion cell (effective area; 2.01 cm2) with the SC side as the donor side and the skin basement membrane side as the receiver side. To maintain sink conditions for DSF, 16 mL of receiver solution (DMSO:EtOH = 10 : 90 (w/w)) was added to the receiver side, and the cell was heated in a 32 °C warm bath. The experiment began once one gram of the prepared hydrogel was applied to the donor side and the top was covered with parafilm. At predetermined time intervals (2, 4, 6, 8, 10, 12, 24 h), 8 mL of receiver solution was collected, and immediately after collection, 8 mL of warmed receiver solution was added. The concentration of DSF in the receiver solution was determined by HPLC.
Determination of DSFThe DSF concentration was determined using an absolute calibration curve method. The mobile phase was prepared by mixing purified water : methanol = 200 : 800 (v/v). The sample was filtered (Ekicrodisc®3 0.45 µm Versapore) prior to measurement and used as the sample for quantification under the following conditions. Detector: UV-Visible spectroscopic detector; detection wavelength: 254 nm; column: YMC-Pack ODS-A (150 × 4.6 mm I.D.); flow rate: 1.0 mL/min; injection volume: 20 µL.
Measurement of DSF Concentration in SkinAfter the permeation experiment was completed, the skin surface was wiped three times with a Kim wipe containing phosphate-buffered saline (PBS), and the hydrogel application site was tape stripped. The first tape of the stripping was discarded, and a total of 15 tapes were obtained. The resulting tapes were sonicated in 2 mL of methanol to extract the drug. Live epidermis after removal of SC by tape stripping was shredded, homogenized in 2 mL of methanol, and quantified by HPLC.
Solubility MeasurementThe solution of the formula of hydrogel in “Preparation of Hydrogel” was brought to 32 °C by a thermostatic bath, DSF was dissolved, and then stirred to saturate the solution. The saturated solution was filtered, diluted, and the DSF concentration was determined by HPLC.
Exfoliation and Preparation of the Stratum CorneumLabskin® (7-week-old male hairless mice of Hos/HR-1 strain, Sankyo Lab Service, Inc.) preparations were immersed in 0.1% trypsin solution and kept refrigerated for 24 h. The mice were then placed in a thermostatic chamber heated to 37 °C for 4 h, and the exfoliated SC was washed and dried. Each formula was applied to the exfoliated SC for 1 h at 32 °C and allowed to dry naturally until it reached 125% of its pre-application mass. All procedures involving animals and their care complied with the regulations of the Committee on Ethics in the Care and Use of Laboratory Animals of Hoshi University.
Fourier Transform IR Spectroscopy (FTIR) MeasurementsSC samples prepared in “Exfoliation and Preparation of the Stratum Corneum” were measured using ATR-FTIR (ATR-PRO attached to FTIR-4200, Japan Spectroscopy Co., Japan) as a preliminary data. Measurements were performed under the following conditions: measurement range, 500–4000 cm−1; temperature scanning range, 20–100 °C; temperature increase rate, 1 °C/min; measuring interval, 3 °C; integration, 32 times; and resolution, 2 cm−1. Synchrotron FTIR measurement was performed to obtain IR spectrum of SC at the BL43IR in SPring-8 (Hyogo, Japan). Measurements were performed under the following conditions; measurement range, 550–7500 cm−1, temperature scanning range, 20–80 °C; temperature increase rate, 1 °C/min; measuring interval, 3 °C; integration, 32 times; and resolution, 2 cm−1.
Differential Scanning Calorimetry (DSC)Five milligrams of the SC sample prepared in “Exfoliation and Preparation of the Stratum Corneum” was filled into an aluminum pan for DSC and measured using DSC (DSC-60 plus, Shimadzu Corporation, Kyoto, Japan). The temperature scanning range was 20–140 °C, the temperature increase rate was 10 °C/min, and alumina was used as the reference material. The phase transition temperature at which each lipid structure changed was determined from the obtained endothermic curve.
Synchrotron X-Ray DiffractionThe SC prepared in “Exfoliation and Preparation of the Stratum Corneum” was placed into a glass capillary (1 mm in diameter). The sample was irradiated with synchrotron radiation X-rays using BL6A (X-ray wavelength: 0.154 nm) or BL10C (X-ray wavelength: 0.1 nm) at the Photon Factory (PF) of the High Energy Accelerator Research Organization (KEK). In the wide-angle region, the distance from the sample to the detector was approximately 55 mm and the detector was a pixel array detector (PILATUS2 100 K-A: DECTRIS). In the PF (BL10C), the distance from the sample to the detector was approximately 500 mm and the detector was a pixel array detector (PILATUS3 2M : DECTRIS). A DSC (HCS302-LN190®, Instec, Inc., U.S.A.) was used for temperature scanning. The lamellar structure within the structure was analyzed from the results obtained in the small-angle region, and the packing structure was analyzed from the wide-angle region.
Statistical AnalysisStatistical analysis of the data obtained from this experiment was performed using one-way ANOVA or multiple comparisons using the Tukey–Kramer method, with a statistical significance of p < 0.05.
We investigated the improvement of skin permeability of DSF by a chemical promotion method using OA and Tween 80. The dosage form used in the experiment was a hydrogel that was easy to handle and also used in commercial formulations, and IPA, which is used as an additive in commercial formulations, was added to dissolve hydrophobic compounds in aqueous hydrogels. First, to investigate the skin permeation enhancement effect of the combination of OA and Tween 80 in DSF and the effect of the combination ratio, in vitro skin permeation experiments were conducted by adding OA, Tween 80, OA:Tween 80 = 2 : 1, OA:Tween 80 = 1 : 1, and OA:Tween 80 = 1 : 2 to hydrogels. The control was a hydrogel containing only 20% IPA, and the cumulative permeation time curves are shown in Fig. 2 (a), when the percentage of each compound added as a skin permeation enhancer was 5%. The concentration of the penetration enhancer was selected to be 5%, which has been previously reported to be effective in promoting penetration.25) Since steady-state flux and lag time are frequently used to evaluate skin permeability, the flux calculated from Fig. 2 (a) according to Fick’s first law in Eq. (1) is shown in Fig. 2 (b) and the lag time in Fig. 2 (c).
 | (1) |

(a) Cumulative amount of DSF permeated through hairless mouse skin from control (○), OA (△), OA:Tween 80 (2 : 1) (●), OA:Tween 80 (1 : 1) (×), OA:Tween 80 (1 : 2) (■), and Tween 80 (□) as donors. (b) Steady-state flux of DSF through the hairless mouse skin. (c) Lag time of DSF through the hairless mouse skin. (d) DSF uptake into the hairless mouse SC and skin 24 h after application of gels. (e) Permeability coefficient (P) of DSF through the hairless mouse skin. Each point or column represents the mean ± standard deviation (S.D.) of three experiments. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. the control. † p < 0.1 and †† p < 0.01 vs. each formulation containing different proportions of OA and Tween 80.
Here, J is the flux, D is the diffusion coefficient in the skin, and dC/dx is the concentration gradient in the skin.
The amount of DSF permeated into the skin was increased by the hydrogel containing either OA or Tween 80 alone, but the combination containing both OA and Tween 80 showed a remarkable increase in the amount of permeated DSF. The flux also showed the same tendency as the cumulative permeation amount. On the other hand, regarding lag time, only Tween 80 showed almost no decreasing tendency. These results suggest that the combined application of OA and Tween 80 significantly improves the skin penetration of DSF compared to the application of either individually.
The average skin concentration (C_ss) ̅ is expressed by Eq. (2), and the skin concentration does not depend on the skin diffusion coefficient, but on the skin/base partition coefficient and the base drug concentration.
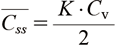 | (2) |
Here, (C_ss) ̅ is the average skin concentration, K is the partition coefficient between skin/substrate, and Cv is the drug concentration in the substrate.
Therefore, to examine distribution to the skin and the effect of the concentration in the base, skin tape stripping was performed after the in vitro skin permeation experiment was completed, and the DSF concentration in the skin was measured. Figure 2d shows the concentration of DSF in the skin. OA and Tween 80 significantly increased the DSF concentration in the skin in both the SC and the live epidermis, and the combined application further significantly increased the DSF concentration in the SC and the live epidermis. This corresponded to the results of the in vitro skin permeation experiments. The reason for the higher drug concentration in deep live epidermis than in SC may be due to the higher skin permeability of hairless mice compared to human skin.38) These results suggest that OA and Tween 80 may improve the skin permeability of DSF by improving the distribution of DSF to the skin.
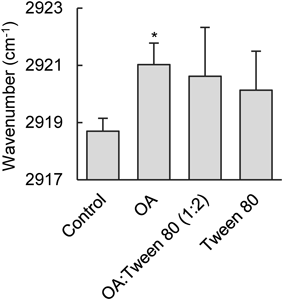
As described above, the concentration in the skin is largely related to the degree of distribution from the base to the skin and the DSF concentration in the base. Considering the DSF concentration in the base, the hydrogel used in the in vitro skin permeation experiment is suspended in all formulations and is considered to be an infinite dose system in which the drug concentration of the base is kept constant. Therefore, the drug concentration Cv in the base can be considered to be the solubility S, and so we investigated the solubility of DSF in the prescription. Figure 3 shows the DSF solubility of each formulation at 32 °C. OA did not significantly affect the solubility of DSF, but Tween 80 increased the solubility of DSF in response to the concentration of Tween 80. This suggests that the formulation containing Tween 80 increased the DSF concentration in the skin by increasing the DSF concentration in the base, and further, the increase in the DSF concentration in the base was a factor in the increase in flux.

**p < 0.01 and *** p < 0.001 vs. control.
On the other hand, Tween 80, which has the highest solubility, increases the skin concentration of DSF less than the combined application, so the combined application of OA and Tween 80 greatly improves the distribution of DSF from the base to the skin. Previous reports suggest that OA is oily in the intercellular lipids of the SC, increasing its apparent distribution from the skin.39) Based on this, the combined use of OA and Tween 80 may increase the distribution of DSF to the skin surface by increasing the amount of DSF dissolved in the part where oleic acid is an oil droplet.
To investigate the factors contributing to the promoting effect in more detail, we attempted to calculate the permeability coefficient from the cumulative permeation amount–time curve of the drug. As seen from Eq. (3), distribution from the base to the skin, the diffusivity in the skin, and the concentration of the drug in the base are important for the permeation of the drug into the skin.
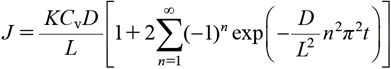 | (3) |
Here, L is the skin thickness.
From Eq. (4), the permeability coefficient can be regarded as a value that does not depend on the drug concentration in the base and changes due to the influence of the skin itself, and can be said to be very important in considering the skin permeability of a drug.
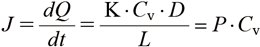 | (4) |
Here Q is the cumulative amount of transdermal absorption and P is the transmission coefficient.
Therefore, the permeability coefficient was calculated using the average values of flux and solubility obtained from the in vitro skin permeation experiment, and is shown in Fig. 2 (e). A single application of OA significantly increased the permeability coefficient, and a single application of Tween 80 was similar to that of the control, with only a slight increase. From this, the promoting effect of Tween 80 alone was considered to largely be due to the increase in DSF solubility compared to its direct effect on the skin. On the other hand, in the combined application of OA and Tween 80, the transmission coefficient was greatly increased compared to either single application; furthermore, a higher ratio of OA resulted in a higher transmission coefficient. From this, it was suggested that the combined application of OA and Tween 80 strengthened the promoting effect by directly acting on the skin. On the other hand, the flux and the permeability coefficient tended to be different in the combined application, and the flux increased as the proportion of Tween 80 increased, while the permeability coefficient increased as the proportion of OA increased. Since the flux is proportional to the permeability coefficient and the drug concentration in the base, the increase in flux when applied in combination is considered to be due to the DSF concentration in the base. In addition, the permeability coefficient increases in proportion to the partition coefficient and diffusion coefficient, but the combined application of OA and Tween 80 has a higher concentration in the skin than Tween 80, which has the highest solubility. OA and Tween 80 likely contributed to the distribution to the skin and increased the permeability coefficient. On the other hand, the diffusion coefficient is considered to increase as the movement of the drug increases in the intercellular lipids of the SC, which is the center of the barrier. Therefore, in the next section, considering the possibility that the combined application of OA and Tween 80 affected the intercellular lipids in the SC and increased the diffusivity in the skin, the skin permeability of DSF was improved. The effect of OA:Tween 80 (1 : 2) on the intercellular lipids in the SC was investigated.
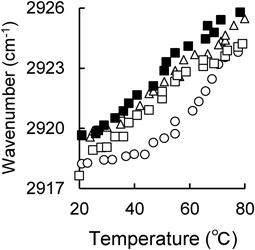
Effects of Formulation Components on Stratum Corneum MicrostructureEffect of Pharmaceutical Ingredients on IR Absorption Characteristics of Intercellular Lipids in the Stratum CorneumTo investigate the fluidity of intercellular lipids in the SC, FTIR measurement of the SC of hairless mice was performed. The FTIR spectrum of the hairless mouse SC is shown in Supplementary Fig. S1. The peaks around 2850 and 2920 cm−1 correspond to CH2 symmetric and CH2 antisymmetric stretching vibrations originating from lipids, while the two large absorption peaks at 1500–1800 cm−1 are reported to correspond to amide I and amide II from SC cells.40) Furthermore, the absorption peak of CH2 symmetric/inverse symmetric expansion and contraction vibration shifts to the high wave number side as the temperature rises, and the shift of the peak to the high wave number side is due to the increase in lipid fluidity.41) Therefore, the peak position derived from the CH2 inverse symmetric stretching vibration was calculated by quadratic differentiation of the FTIR spectrum, and the position change of the absorption peak derived from the CH2 inverse symmetric stretching vibration by temperature scanning is shown in Fig. 4. By applying OA and Tween 80 alone, the absorption peak derived from the CH2 inverse symmetric stretching vibration is shifted to the high frequency side compared to the control, and OA and Tween 80 may increase the fluidity of intercellular lipids. In addition, formulations containing OA showed a high wave number shift of the absorption peak due to CH2 inverse symmetric expansion and contraction vibration from low temperature, suggesting that lipid fluidization may have already occurred at a temperature lower than 20 °C. Furthermore, Fig. 5 shows the peak position of the CH2 inverse symmetric stretching vibration at 32 °C, which is near the skin surface temperature. OA alone tended to shift significantly to higher waves, followed by OA:Tween 80 (1 : 2) and Tween 80 alone. This suggests that a formulation containing OA may increase the fluidity of intercellular lipids in the SC and improve DSF skin permeability. In addition, Tween 80 did not show a significant difference, but showed an increasing tendency, suggesting that Tween 80 may contribute slightly to the increase in the fluidity of intercellular lipids.
Each column represents the mean ± S.D. of three experiments. * p < 0.05 vs. the control.
To investigate the effects of OA and Tween 80 on the ultrastructure of intercellular lipids, DSC and synchrotron radiation X-ray diffraction measurements were performed using the hairless mouse SC. As described above, the intercellular lipids in the SC have a packing and lamellar structure as shown in Supplementary Fig. S2. As can be seen in Supplementary Fig. S2, orthorhombic crystals have a denser structure than hexagonal crystals, so they have a structure that exerts a greater skin barrier function. In addition, it has been reported that these packing structures change due to temperature scanning and that orthorhombic crystals disappear at approximately 40 °C, high-temperature hexagonal crystals are formed at around 60 °C, and liquid crystals appear at approximately 80 °C.34) On the other hand, the lamellar structure includes a long-period lamellar structure with a period of 13 nm and a short-period lamellar structure with a period of 6 nm. These lamellar structures undergo structural changes similar to the packing structure upon temperature scanning. Previous studies report that the lamellar structure melts at approximately 80 °C.42)
DSC was performed to estimate the phase transition temperature due to the application of each compound, and the obtained endothermic peaks were separated into four peaks as phase transitions of the rectangular, hexagonal, long-period lamellar, and short-period lamellar structures. Table 2 shows the phase transition temperatures T1–T4 obtained by separating the DSC endothermic peaks.
| Formula code | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) |
|---|---|---|---|---|
| Control | 38.0 ± 0.66 | 49.2 ± 1.68 | 61.7 ± 4.15 | 76.7 ± 1.51 |
| OA | 36.5 ± 3.33 | 41.6 ± 1.50 | 47.7 ± 1.80 | 56.8 ± 0.94 |
| OA:Tween 80 (1 : 2) | 32.7 ± 2.99 | 41.7 ± 1.10 | 49.1 ± 4.39 | 59.4 ± 5.25 |
| Tween 80 | 35.6 ± 1.50 | 42.0 ± 1.96 | 48.0 ± 2.33 | 59.2 ± 1.25 |
Each value represents the mean ± S.D. of three experiments.
In the synchrotron radiation X-ray diffraction in the wide-angle region, strong diffraction peaks were obtained at 2.4 and 2.7 nm−1 by making the image obtained by the pixel array detector one-dimensional. It has been reported that 2.4 nm−1 is derived from orthorhombic and hexagonal arrangements, and 2.7 nm−1 is derived from an orthorhombic arrangement.43) Therefore, the phase transition temperature obtained by DSC is combined with the result of synchrotron radiation X-ray diffraction measurements; T1 was estimated as an orthorhombic phase transition and T3 as a hexagonal phase transition. Figure 6 shows the synchrotron radiation X-ray diffraction profile and the separated DSC endothermic peaks in the wide-angle region. The peak derived from the orthorhombic crystal of OA:Tween 80 (1 : 2) is obscured at low temperature, and the orthorhombic crystal, which is important for skin barrier function, undergoes a phase transition at 32.7 °C, which is close to the skin surface temperature. In addition, OA and Tween 80 significantly lowered the phase transition temperature of the hexagonal crystal when applied alone, suggesting that it has a large effect on the hexagonal crystal.
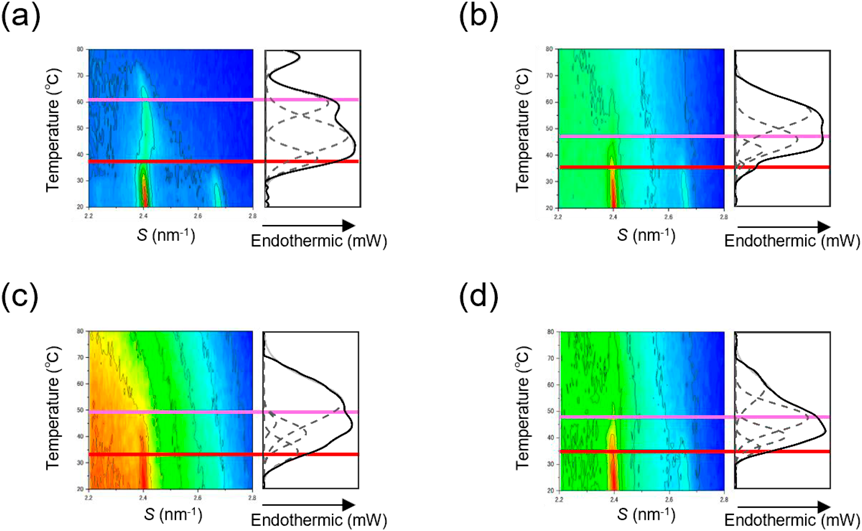
High absorbance is indicated by red, and low absorbance is indicated by blue. The red and light red lines are based on the phase transition temperatures of the orthorhombic and hexagonal packing structure origins, respectively, in the DSC measurements.
In order to investigate the thermal behavior in more detail, Fig. 7 shows the rate of change in intensity of the diffraction peak near 2.4 nm−1 obtained by synchrotron radiation X-ray diffraction with temperature scanning. The peak intensity of the SC to which the 20% IPA solution was applied as a control changed significantly between 20–80 °C and around 40, 60, and 80 °C, as previously reported.40) On the other hand, in the SC to which OA and Tween 80 were applied alone and in combination as enhancers, the peak intensity decreased to approximately 50 °C, but the peak enhancement near 60 °C, which was observed in the control, was not observed. This result indicates that the high-temperature hexagonal crystals that should have been formed by the increase in temperature are difficult to form due to increased fluidity. The peak intensity in the SC achieved when OA and Tween 80 were applied alone after the phase transition from approximately 50 °C was weaker when OA was applied alone than when Tween 80 was applied alone. This suggests that the application of OA contributed more to the increase in fluidity compared to the application of Tween 80. In the formulation using OA and Tween 80 in combination, no diffraction peak was observed from around 50 °C, suggesting that hexagonal liquefaction occurs at low temperatures. The combined application of OA and Tween 80 may have a stronger effect on the packing structure and make the skin barrier function more fragile than the single application of OA and Tween 80.
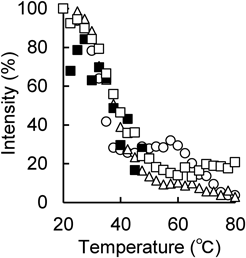
On the other hand, the lamellar structure in the intercellular lipids of the SC can be investigated by analyzing the peak in the small angle region. As mentioned above, the lamellar structure has a long period with a period of approximately 13 nm and a short-period lamellar structure of approximately 6 nm.
In the SC to which a 20% IPA solution was applied as a control, peaks were observed near 0.07, 0.15, 0.16, 0.21, 0.3, and 0.35 nm−1 when the peaks were separated. A long-period lamellar structure has been reported to be located near 0.07, 0.15, 0.21, and 0.3 nm−1 and a short-period lamellar structure is located near 0.16 and 0.35 nm−1.41) Prescriptions to which OA and Tween 80 were applied had broader peaks than the control, and it was difficult to analyze peaks below 0.3 nm−1, so peaks near 0.07, 0.15, and 0.16 nm−1 were the main focus. We proceeded with the examination. Figure 8 shows the synchrotron radiation X-ray diffraction profile and the separated DSC endothermic peak in the small-angle region. Since the vicinity of 0.07 and 0.15 nm−1 is derived from the long-period lamellar structure and the vicinity of 0.16 nm−1 is derived from the short-period lamellar structure, T2 was estimated as the phase transition temperature of the long-period lamellar structure and T4 as the phase transition temperature of the short-period lamellar structure. Application of each formulation lowered the phase transition temperature of each lamellar structure relative to the control, suggesting that there was no significant difference between OA and Tween 80 applied alone and in combination.

High absorbance is indicated by red, and low absorbance is indicated by blue. The green and light green lines are based on the phase transition temperatures of the short-period lamellar structure and the long-period lamellar structure origins, respectively, in the DSC measurements.
To investigate the thermal behavior of the lamellar structure in detail, we analyzed the peaks around 0.07 nm−1 as peaks derived from the long-period lamellar structure and 0.16 nm−1 as peaks derived from the short-period lamellar structure. The rate of change of peak intensity with temperature scanning is shown in Fig. 9. At the peak near 0.07 nm−1, the peak of the control diminished with temperature scanning, and the peak disappeared at around 50 °C. The formulation with OA applied alone showed a marked attenuation of the peak up to around 40 °C compared to the formulation with Tween 80 applied alone, suggesting that OA strongly affects the long-period lamellar structure and improves the diffusion ability of DSF in the SC. In the formulations applied in combination, the rate of temperature change was smaller than in other prescriptions, and no clear phase transition peak intensity change was observed. This suggests that the formation of the long-period lamellar structure at low temperatures may be minimal.
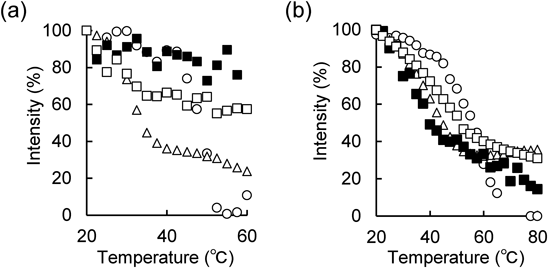
On the other hand, at the peak near 0.16 nm−1, the peak of the control diminished with temperature scanning as in the case at around 0.07 nm−1, and the peak disappeared at around 70 °C. Formulations of OA and Tween 80 applied alone or in combination showed marked attenuation of the peak up to around 60 °C, with no significant differences observed.
From the results of synchrotron radiation X-ray diffraction measurement and DSC, OA acts more strongly on the hexagonal and long-period lamellar structures than Tween 80, thereby enhancing the DSF diffusion in the skin. Furthermore, the combined application of OA and Tween 80 has a significant effect on the packing structure and lamellar structure, and in particular, it greatly affected the orthorhombic crystal and long-period lamellar structure, which are important for the skin barrier function, and improves the diffusivity of DSF in the skin.
The combined application of OA and Tween 80 significantly increased the amount of DSF which permeated into the skin. OA may improve the fluidity of intercellular lipids in the SC and Tween 80 may improve the skin permeability of DSF by increasing the solubility of DSF. The combined use of OA and Tween 80 was found to have a more pronounced effect on orthorhombic and long-period lamellar structures, improving the skin permeability of DSF. Based on these findings, the combined application of OA and Tween 80 made it possible to apply the skin permeability of DSF as a pharmaceutical product, and it was possible to clarify its detailed mechanism of action. From this, an excellent DSF transdermal preparation could be developed by using OA and Tween 80 together.
The synchrotron X-ray diffraction experiments were performed using a BL6A at the Photon Factory under approval of the Photon Factory Advisory Committee (2017G129, 2019G134, 2021G094) and a BL40B2 at the SPring-8 with the approval of the SPring-8 Proposal Review Committee (2018A1078, 2018B1273). The synchrotron FTIR experiments were performed using a BL43IR at SPring-8 with the approval of the SPring-8 Proposal Review Committee (2017B1449, 2018A1092, 2018B1287, 2019A1283, 2020A1411, 2021A1205). This work was supported by JSPS KAKENHI Grant Number 20K08699.
The authors declare no conflict of interest.
This article contains supplementary materials.