| To whom correspondence should be addressed: Hisato Shida, Laboratory of Molecular Cell Biology, Faculty of Medicine, Yamanashi University, 1110 Tamaho, Yamanashi 409-3898, Japan. Tel & Fax: +81–55–273–9375 Abbreviations: BSA, bovine serum albumin; Dsg, desmoglein; Dsc, desmocollin; HRP, horse radish peroxidase; PBS, phosphate buffered saline. |
Desmosomes are punctuate adhesive cell-to-cell junctions which are observed mainly in various epithelial tissues but secondarily in non-epithelial tissues, e.g. cardiac muscle. Although the molecular constituents of various desmosomes have been studied extensively (Garrod et al., 2002; Green and Jones, 1996; Green and Gaudry, 2000; Steinberg et al., 1987), relatively little is known about their essential molecules for adhesive function in desmosomal diversity (Amagai et al., 1991; Collins et al., 1991). Desmosomes have been classified into a single system with three constituents: (1) transmembrane desmosomal adhesion molecules (DAM, e.g. desmosomal cadherins classified as desmogleins (Buxton et al., 1993; Koch et al., 1990) and desmocollins (Buxton et al., 1993; King et al., 1995; Koch et al., 1991; Koch et al., 1992; Parker et al., 1991); (2) anchoring intermediate filaments (AIF, e.g. keratin filaments); and (3) desmosomal mediatory molecules (DMM, e.g. desmoplakins (Schmid et al., 1994) and plakoglobins (Karnovsky and Klymkowsky, 1995; Peifer et al., 1992; Witcher et al., 1996). We still need to obtain key data for the characterization of various desmosomal systems in order to determine whether they are basically uniform systems or not. For this purpose, the viewpoints from ontogeny and/or phylogeny would be useful.
Furthermore, hardly anything is known about the biological functions of desmosomes in such dynamic systems as association, dissociation, migration, arrangement, and sorting-out of cells in development. Thus for better understanding the biological role of desmosomes in the dynamic conditions that occur in morphogenesis and development, we have to develop a more suitable experimental system. Since the classical investigations on the early development events, Xenopus has been extremely an useful tool. Among many types of vertebrates, Xenopus, the South African clawed toad, is accepted as an important experimental animal especially in the field of developmental biology on account of its easy manipulation of oocyte and embryo at different developmental stages. Though such research saw moderate progress until 1990s, a kind of explosion occurred the 2000s with more than 2,000 Xenopus-related works being published within 2 years time during the so-called “post-seqence era”. To clarify the real finding, that link biological systems with genetic information, again we need to stress the importance of developing more dynamic experimental systems in the field of desmosome research.
In the first stage of the investigations of desmosomes, the major molecular constituents of desmosomes have been studied mainly by using isolated bovine snout desmosomes (Gorbsky and Steinberg, 1981). The information on the constituents molecules then expanded rapidly to include the sequential data such as the homologous proteins originating from the different mammalian tissues (Cowin and Garrod, 1983; Cowin et al., 1984).
Strange to say, despite all of these studies we still have no report on the isolation of Xenopus desmosomes except for one preliminary report on the isolation and molecular components of desmosomes from another frog, Rana pipiens (Suhrbier and Garrod, 1986). This previous work used the standard isolation method of bovine desmosomes which was developed by Gorbsky and Steinberg (1981), and non-monospecific antibodies against Dsgs or Dscs for the immunochemical identification of the constituent molecules from the frog (Rana pipiens) epidermal desmosomes.
In the present study, we successfully improved on an isolation method of desmosomes from the Xenopus skin. Although studies on the molecular constituents of Xenopus desmosomes have been concentrated on the fields of cytoplasmic factors (Fouquet et al., 1992), we still lack complete basic information on the adhesion molecules in Xenopus desmosomes, although important secondary data were obtained from primary data using immunoelectron microscopy and related techniques (data not shown). All of these results taken together revealed the presence of Xenopus desmogleins (XDsg) which might have unique intercellular epitopes. Surprisingly, none of the data positively supported the presence of Dscs at Xenopus epidermis. If our results are correct, we may have to reconsider the concept that both Dsgs and Dscs are essential to build up stratified epithelium.
Young adult male frogs, purchased from the local animal supply company, were fed and housed in the aquariums of the animal center of Yamanashi Medical University at room temperature until experiment.
The basic procedures of the isolation was a modified method which was originally developed for the isolation of bovine snout desmosomes (Gorbsky and Steinberg, 1981). The frog skin was removed from the whole body with scissors under general paralyzed condition using ice-water. Ten animals were used for each experiment.At the first step, all animals were thrown into the ice-water at once and then were lain on their backs for the purpose of immediate induction of paralysis by low temperature. After complete paralysis, each animal was laid on aluminum foil chilled with ice-flakes. After surgically removing the sheets of skin, the sheets were soaked in cold 0.1 M citric acid sodium citrate buffer, pH 2.6 containing 5 μg/ml pepstatin (Sigma Chemical Co., St. Louis, MO) as an inhibitor of acid protease (CASC+) and minced into small pieces with razor blades. After a wash with the same cold buffer solution, the incubation solution was changed from the CASC+ solution to the 05NP-CASC+ solution (CASC+ containing 0.05% NP-40) approximately 400–500 ml total volume in a ratio of 40–50 ml/animal. Following vigorous stirring for 2 hr at 4°C, tissue debris was removed by filtration with double layers of gauze. The remaining filtrate, mainly composed by epidermal-cell sheets which were released from the basement membrane, was subjected to subsequent stirring for next 2 hr at 4°C. After the second filtration with Kimwipe s-200 (Kimberly-Clark Co., Tokyo, Japan), the suspension was centrifuged at 20,000×g for 15 min at 4°C in a RPR20-2 angle rotor of a Hitachi 20PR-52 centrifuge (Hitachi Co. Ltd., Ibaraki, Japan). The precipitate (ppt) was re-suspended in CASC+ containing 0.1% NP-40 (10NP-CASC+) and stirred for 1 hr at 4°C. For the purpose of removing debris and non-junctional membrane, the suspension was overlaid on a pair of discontinuous sucrose gradients (50%, 30%; all of the sucrose solutions were prepared with 01NPCASC+ containing 0.01% NP-40) in two glass centrifuge tubes and centrifuged in a RPR20-2 rotor at 20,000×g for 20 min at 4°C. The crude desmosomal layer was concentrated at the 50%/30% interface separating from the ppt debris and the nonjunctional membrane fraction at the interface between 30%/0%. This desmosome-rich and was collected with a Pasteur pipette and equal volume of 01NPCASC+ was added. After re-suspension by pipetting and brief sonication in a sonic washing bath, the pooled fraction was overlaid on a pair of discontinuous sucrose gradients (50%, 40%, 30%; all of the sucrose solutions were prepared with 01NP-CASC+ containing 0.01% NP-40) in two polyallomer centrifuge tubes (13×51 mm; Beckman Instruments Inc., PaloAlto, CA) and centrifuged in a SW-50 rotor at 30,000×g for 60 min at 4°C. Purified desmosome-rich fractions were collected at the interfaces between 30%/40% and 40%/50%, and washed twice by centrifugation at 20,000×g in 01NP-CASC+. The pellets were suspended in small volumes of 01NPCASC+. A part of these pellets was processed for elecron microscopy.
 View Details | Fig. 1. Diagram of the procedure for isolating Xenopus epidermal desmosomes. |
Next 3 monospecific antibodies against desmosomal cadherins were used. Rabbit polyclonal antibody against bovine Dsgs (which presumably contains all of the 3 isoforms); RPAffi Anti-BEd Dsgs (BEd DsgsAS/BEd DsgsIA). This antibody was purified from the corresponding rabbit antisera using blot-affinity procedure with specific antigens, described in the following procedure. The designation of three antibodies are summarized in Table I. These three antibodies, RPAffi Anti-BEd Dsgs (BEd DsgsAS/BEd DsgsIA), GPAffi Anti-XEdXDB3 (XEd XDB3AS/BEd DsgsIA) and GPAffi Anti-XEdXDB3 (XEd XDB3AS/XEd XDB3IA), were also purified by blot-affinity procedure with specific or selective antigens.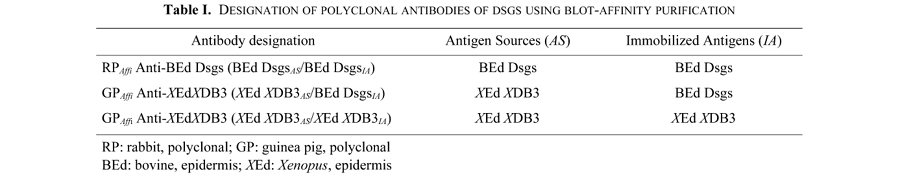
Antisera directed against bovine Dsgs and bovine Dscs for the sources of monospecific antibodies were rabbit ones both originally prepared at Dr. M.S. Steinberg’s laboratory, Princeton University, Princeton, NJ; and a guinea pig serum against Xenopus XDB3 was prepared in our laboratory, using a method previously described (Giudice et al., 1984). Briefly, Xenopus desmosomal fraction concentrated at the interface between 30%/40% sucrose was dissolved in Laemmli’s solution (Laemmli, 1970) and applied on a 7.5% polyacrylamide gel for SDS-PAGE. XDB3 band stained with Coomassie Brilliant Blue (CBB) was cut out carefully with razor blades. The gel slices were neutralized in PBS and ground in a glass homogenizer. A volume of acrylamide homogenate containing approximately 200 μg of protein was mixed with an equal volume of complete Freund adjuvant and injected subcutaneously into male (Hartley) guinea pigs. One of the resulting antiserum against Xenopus XDB3 protein reacted mainly with its original band but subsequently with other minor bands. To obtain monospecific antibodies, all of the sera were processed by the following blot-affinity procedure.
The blot-affinity procedure originally developed by Olmsted (1981) is a useful method not only for eliminating contaminated antibodies, but also for positively selecting the antibodies which share common epitopes with the blotted antigens in sera or antibody pool. The present blot-affinity procedure was performed as in our previous work (Shida and Ohga, 1990). Both purification and positive selection procedure are basically identical. In brief whole desmosomal fraction isolated from the bovine muzzles or Xenopus epidermis was applied for electrophoresis on one-dimensional 7.5% SDS-PAGE. After electrophoresis, the fractionated proteins were electroblotted onto polyvinylidene difluoride paper (PVDF Immobilon; Millipore, Bedford, MA) with a transfer buffer (25 mM Tris base, 192 mM glycine) in a semi-dry blotting apparatus (Horizblot; Atto Co., Tokyo, Japan) at a constant current of 200 mA for 90 min to gels (the size of which was approximately 10×11×0.1 cm). After a brief wash with PBS, blotted sheets of paper were immersed in 10% BSA (Sigma, St. Louis, MO) in PBS for at least 12 hr at 4°C. For the purpose of preparing the isolated antigens immobilized on the paper, a band which should correspond to Dsgs or XDB3 was detected by putting the post-transferred gels stained with CBB on the blots via transparent sheets of Saran Wrap, and the strips of this specific antigen were cut off with a pair of scissors. To collect the monospecific antibody, at least four pieces of the strips were incubated in each corresponding antiserum solution diluted to 1:20 with 1% BSA in PBS for 30 min at room temperature (rt). Next, they were washed three times with 5 ml of ice-cold 0.1 M glycine buffer (pH 2.3) containing 1% BSA for 10 min at rt. This 5 ml of acidic solution of antigens was neutralized with 225 μl of 1 M Tris base. The monospecificity of antibodies was checked by immunoblotting (Fig. 6).
For transmission electron microscopy of isolated desmosomes, collected samples from the discontinuous bands with disposable glass Pasteur pipettes (53/4, Corning-Iwaki Co., Tokyo, Japan) were pelleted at 40,000 g for 30 min in 01NP-CASC+ solution. These pellets were processed for fixation and embedding in Spurr’s resin (Spurr, 1969) according to standard procedures. Ultrathin sections were stained with uranyl acetate and lead citrate, and observed on a Hitachi H-600 transmission electron microscope.
Glycoprotein detection was performed by an original method (Shiozawa et al., 1987). Briefly, after electroblotting, the paper was incubated with 50 mM Tris-HC1, 0.1 M NaCl, 1 mM MnCl2, and 1 mM CaCl2, PH 7.0 (ConA buffer), containing 1% BSA for 1 h at rt. ConA (1 mg/ml in ConA buffer) was added directly to the BSA-containing buffer to a final concentration of 50 μg/ml. The paper was incubated for 30 min or longer. It was washed twice for 10 min each with 0.1% BSA in ConA buffer and then incubated for 30 min or longer at room temperature in ConA buffer containing 0.l% BSA and 13 μg/ml HRP. HRP activity was detected by 4-chloronaphthol followed by two washes with 0. l% BSA in ConA buffer for 10 min each and then once with ConA buffer for 5 min.
By a step sucrose gradient; 30%, 40%, and 50% in 01NPCASC+, three layers were observed at the interface between 0% and 30% (XDL0/30), 30% and 40% (XDL30/40), 40% and 50% (XDL40/50) sucrose solutions, respectively, with SW-50 rotor at 18,000 rpm for 1 h at 4°C. The uppermost layer (XDL0/30) mainly consisted of fragmented membranes. Electron microscopic observation of the second (XDL30/40) (Fig. 2A, B) and the third layer (XDL40/50) (Fig. 3A, B) revealed numerous desmosomes as the major constituent condensed in both layers. However, electron microscopic properties of the fractions included in each layer had different characteristics. In XDL30/40, almost all of the isolated desmosomes were observed as an isolated form without any type of chain-like shapes, losing their association with 10 nm filaments and non-junctional membranes. In contrast with XDL30/40, the isolated whole desmosomes which were main constituents of XDL40/50, presented as an associated form including junction-free plasma membranes. The ultrastructural details of desmosomes isolated in XDL30/40 showed distinct organizations of desmosomes, e.g., central dense stratum, ODP and inner dense plaque (IDP). Though both layers, XDL30/40 and XDL40/50, were available as desmosme fractions, it was reasonable to assume that we might eliminate minor contaminations of non-junctional membranes by using the XDL30/40 one.
 View Details | Fig. 2. Electron microscopic images of Xenopus desmosomes-enriched fraction obtained from the interface between 30% and 40% sucrose solution. A relatively homogeneous preparation of whole desmosomes having basically no interdesmosomal membrane was observed at lower magnification (A). Inset (B): A higher magnification image of isolated desmosomes. They preserved typical desmosomal features; a pair of adjacent plasma membranes, a central midline, and cytoplasmic dense plaques. |
 View Details | Fig. 3. Electron microscopic images of Xenopus desmosomes-enriched fraction obtained from the interface between 40% and 50% sucrose solution. Though whole desmosomes were greatly condensed, these were still connected through interdesmosomal membranes as shown in figure 3A at lower magnification. Inset (B): A higher magnification image of isolated desmosomes. |
In the region over 75 kD of the gel obtained by SDS-PAGE of the Xenopus desmosomal fraction (XDL30/40), at least 12 major bands (from XDB1 to XDB12) were identified by CBB staining (Fig. 4). Though XDB1 could contain more than one band, we tentatively summarized it as into a broad single band as samewhat under an unstable property depending on the preparation. This band would sometimes contain at least 3 sub-bands that could be possible be named in alphabetical order, 1a, 1b and 1c (data not shown). In the present experiment, we focused our concern on the adhesion molecules in Xenopus desmosomes. For the purpose of this, the glycosylated bands should be detected first. Within the 12 bands, glycosylation was recognized at XDB3, XDB7, and XDB8 by ConA binding assay (Fig. 5). Estimated MW of these bands is around 175 kD, 124 kD, and 112 kD, respectively. This MW of XDB3 estimated from the Rf value of SDS-PAGE profile was slightly higher than the average value of bovine snout Dsgs. On the other hand, both MWs of XDB7 and XDB8 estimated from the Rf values of SDS-PAGE profile, were lower than those of Dscs a/b.
 View Details | Fig. 4. SDS-PAGE analysis (7.5% gel) of desmosomal bands isolated from bovine snout (L1) and Xenopus epidermis (L2, 3). Filled circles: molecular weight markers of 67 kD, 100 kD, 140 kD, and 180 kD in this order. DP: desmoplakin; Dsg: desmoglein; Dsc: desmocollin; PG: plakoglobin; and PP: plakophilin. Note Rf values of XDB 7 and XDB 8 which were larger than Dsc a and Dsc b respectively. |
 View Details | Fig. 5. GIycoprotein detection after SDS-PAGE using ConA binding. BED: bovine epidermal desmosomes; XED: Xenopus epidermal desmosomes. Proteins were stained with Coomassie Brilliant Blue (CBB) or, to detect carbohydrates, with ConA. Glycosylated bands were detected at XDB3, XDB7, and XDB8 of XED. |
A rabbit monospecific polyclonal antibody; RPAffi Anti-BEd Dsgs (BEd DsgsAS/BEd DsgsIA) which reacts only with bovine Dsgs, exclusively bound with Xenopus XDB3. On the contrary, a guinea pig monospecific antibody, GPAffi Anti-XEdXDB3 (XEd XDB3AS/XEd XDB3IA) which reacts only with Xenopus XDB3, exclusively bound with bovine Dsgs. Surprisingly, a rabbit monospecific polyclonal antibody, RPAffi Anti-BEd Dscs (BEd DscsAS/BEd DscsIA) which reacts specifically only with bovine Dscs a/b, never bound with any bands observed in the SDS-PAGE of isolated desmosomes from the Xenopus epidermis (data not shown).
 View Details | Fig. 6. Western blotting analysis of antibodies used. Designation of each antibody was summarized in Table I. XDB3 which was recognized by antibody against XDB3 was located at exactly the same position as Dsg of bovine epidermal desmosomes. XED: Xenopus epidermal desmosomes, and BED: bovine epidermal desmosomes. |
In the present modification of original technique developed for the isolation of desmosomes from bovine snout epidermis using NP-40 (Gorbsky and Steinberg, 1981), the most effective factors were the concentration of NP-40 and incubation time. The first incubation with 05NP-CASC+ for 2 hr at 4°C was an essential step to remove the epithelial layer from the underlying connective tissue, the dermis. We might minimize contamination and protease degradation by separating the epithelial layer from the dermis by this first incubation step. The next successive incubation with the same solution, 05NP-CASC+, was a step for cutting out desmosomes from the nonjunctional membranes and from the cytoplasmic integration. However, even in the incubation with 05NP-CASC+ for 4 hrs in total, a lot of nonjunctional membrane regions were still associated with desmosomes (Fig. 3A). To reach complete degradation, the third step, incubation with 10NP-CASC+ for 1 hr at 4°C, was successfully adopted. In another previous work on the isolation of frog desmosomes (Suhrbier and Garrod, 1986), Suhrbier and Garrod did not use higher concentration of NP-40, and instead homogenized the pellet by Dounce type homogenizer before final differential centrifugation and metrizamide or sucrose gradient centrifugation. However, the fraction obtained in their experiment apparently contained associated desmosomes with nonjunctional membranes in zig-zag chains. SDS-PAGE pattern showed a complicated profile on account of numerous numbers of CBB positive bands. By contrast in the present isolation, the final fraction contained highly enriched Xenopus whole desmosomes without any nonjunctional membranes.
By a ConA-binding assay applied to the blotted SDS-PAGE of 30%–40% XD, three glycosylated bands; XDB3, XDB7, and XDB8, were detected (Fig. 5). All of major desmosomal cell adhesion molecules are classified as two desmosomal cadherins sub-families of the cadherin superfamily desmogleins (Dsgs) and desmocollins (Dscs) (Buxton et al., 1992; Magee and Buxton, 1991). Although average MW of XDB3 (175 kD) was slightly higher than that of bovine Dsgs, XDB3 could be classified as a member of desmogleins, at least from the present immunochemical results. Monospecific antibody against bovine Dsgs designated as RPAffi Anti-BEd Dsgs (BEd DsgsAS/BEd DsgsIA) cross-reacted specifically with XDB3 of Xenopus desmosomes. In a similar manner as above, monospecific antibodies against Xenopus XDB3 designated as GPAffi Anti-XEdXDB3 (XEd XDB3AS/BEd DsgsIA) and GPAffi Anti-XEdXDB3 (XEd XDB3AS/XEd XDB3IA) reacted not only with the Xenopus XDB3, but also with bovine Dsgs. These results clearly show tnat Xenopus XDB3 glycoprotein(s) is a homologous antigen of Dsgs.
Other major glycoproteins of Xenopus, XDB7 and XDB8, whose average MW were estimated as 124 kD and 112 kD, respectively, did not cross-react with the monospecific antibody against bovine Dscs. These MWs of bovine Dscs a/b (128 kD and 118 kD) were slightly larger than those of XDB7 and XDB8. The antibody against bovine Dscs was rabbit polyclonal antibody prepared from Dscs bands of isolated bovine desmosomes. It is rather hard to accept that the polyclonal antibody could not recognize any Xenopus Dscs isoforms, even if these are rarely present.
Both subclasses of Dsgs and Dscs are members of the cadherin superfamily which is classified into two major groups named classical cadherins and nonclassical cadherins (Kemler, 1992). The “homophilic” interaction between the same subtype cadherins which should give the most distinctive feature to cadherin-mediated cell-cell adhesion, was mainly obtained from the analyses of the Ca2+- dependent cell-cell adhesion in L cells first expressing these classical cadherins (Nagafuchi et al., 1987; Nose et al., 1987; Hatta et al., 1988). This prescribed form of homophilic interaction showing cadherin tropism with subtype-specific binding (Takeichi, 1988, 1990) has been widely accepted and now we can find such a conclusive remark as “E-cadherin binds cells together through the interaction of two E-cadherin molecules on different cells” in a standard textbook, Molecular Biology of the Cell (2002). To our surprise, a strong and clear experimental objection was published quite recently against the subtype specific interaction by Duguay, Foty, and Steinberg (2003). They used a similar experimental system to the original one which appeared frequently in the first stage of investigations, but under precisely controlled cadherin expression levels and shear forces. One of the most amazing results was an intermixed pattern of coaggregation using two different types of L cells; one was transfected with E-cadherin and the other was transfected with P-cadherin under the same expression levels. It might be hard to avoid the theoretical consequence that there would be no difference between the adhesion forces of homophilic interaction (E-E or P-P) and heterophilic interaction (E-P).
On the other hand, in the case of desmosomal cadherins which belong to nonclassical cadherins, neither mechanism whether heterophilic interaction or homophilic interaction, has been hypothetically excluded from the beginning (Steinberg et al., 1987), and various investigations using transfected cells expressing one of the Dscs or Dsgs subtypes, or both subtypes including one Dscs and one Dsgs have experimentally supported this molecular mechanism (Marcozzi et al., 1998; Amagai et al., 1994; Chidgey et al.,1996; Kowalczyk et al, 1996; Chitaev and Troyanovsky, 1997). These two interaction systems were also observed more directly by other analyses (e.g., analytical centrifugation, chemical cross-linking, far-UV CD spectrum, and BIAcore analysis), using Escherichia coli cells expressing the first two extracellular domains of Dsg2 and Dsc2 (Syed et al., 2002).
Thus, we should reconsider the molecular basis of cell-cell adhesion mechanism including homophilic interaction. Actually, cadherin-mediated segregation could be induced only by quantitative differences in expression level of the single cadherin subtype (Steinberg and Takeichi, 1994). We have no theory about the biological significance of a multimolecular system of cell-cell adhesion molecules in a single cell. Generally speaking, desmosomes are composed of more than two subtypes of cell-cell adhesion molecules but adherence junctions are not. In the present experiments, only X-Dsg molecules were detected in Xenopus desmosomes. We need to further investigate whether other desmosomal adhesion molecules or other subtypes of Dscs are present or not in Xenopus desmosomes. From an evolutional point of view, the prototypes of desmosomes should be pursued in various nonmammalian epithelial tissues.
We are grateful to Dr. M.S. Steinberg, Princeton University, for providing rabbit antiserum prepared against bovine Dsgs. We have also benefitted from the central equipment facilities for medical research at Yamanashi Medical University.
|