| To whom correspondence should be addressed: Akira Matsuno, Department of Biological Science, Faculty of Life and Environmental Science, Shimane University, Matsue 690-0823, Japan. Tel: +81–852–32–6430, Fax: +81–852–32–6449 Abbreviations: CPP, calcium pump protein; RyR, ryanodine receptor; SR, sarcoplasmic reticular system. |
It is well known that sarcoplasmic reticula (SRs) in cross striated muscle cells have close contact with the T-tubules which are infolded from the cell membrane into myofibrils. Collectively, these elements comprise the so-called “triad”. Excitation of cell membranes is transmitted to SRs through T-tubules, because the “triad” transmits signals easily. Ultrastructural studies have identified microstructures called foot structures in the interspaces between SRs and T-tubules of cross striated muscles (Franzini-Armstrong, 1980; Franzini-Armstrong and Nunzi, 1983; Costello et al., 1986; Timmerman and Ashley, 1988; Sharma et al., 1998), and those of smooth muscle (Sugi and Suzuki, 1978). These foot structures were first isolated from SR fractions, and were named foot protein. They are thought to be biochemically equivalent to the ryanodine receptor (RyR). RyRs have been characterized as an important candidate for the Ca-gate which initiates Ca-release from SRs (Campbell et al., 1980; Inui et al., 1987; Fill and Coronado, 1988; Lai et al., 1989). However, the relationship between foot structures and biochemical RyRs is not clear, and no reports to date have proved that foot structures observed in intact muscle cells are for certain RyRs.
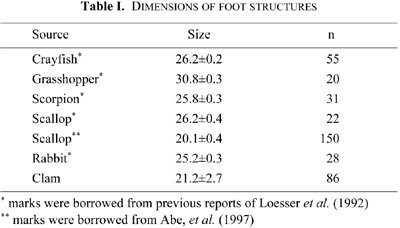
Ultrastructural studies of foot structures have revealed many details by thin sectioning or shadowing methods. Foot structures about 20×20×22 nm in size with cubic form and a central pore have been described in these reports (Saito et al., 1984; Lai et al., 1988; Block et al., 1988; Fill and Coronado, 1988; Wagenknecht et al., 1989; Radermacher et al., 1992). These foot structures were distributed in a regular lattice on the surface of the junctional face membrane of SR (Franzini-Armstrong and Jorgensen, 1994; Sun et al., 1995; Protasi et al., 1998). The pattern of the lattice varies among the “triad” of the muscle cells of various vertebrate and invertebrate animal species (Loesser et al., 1992).
Biochemical studies of foot structures (foot proteins) have also revealed much detail. The foot proteins are assumed to be composed of two main proteins and some unknown ones. The two main proteins are dihydropyridine receptor (DHPR) and RyR, respectively (Kim et al., 1990). These two proteins are usually closely bonded to each other, but the RyR in rabbit appears as an about 450 kD band on polyacrylamide gels analyzed by sodium dodecyl sulfate electrophoresis (SDS-PAGE). Among them, RyRs are believed to release Ca2+ from SR (Shoshan-Barmatz and Ashley, 1998; Leong and MacLennan, 1998).
In contrast, few reports of foot structures of invertebrates are available (Franzini-Armstrong and Nunzi, 1983; Franzini-Armstrong et al., 1986; Sanger and Sanger, 1985; Abe et al., 1997). In general, invertebrate cross striated muscle cells lack T-systems, and SR systems have been identified only in a few cases. Accordingly, it is not clear if foot structures/RyRs occur in the cross striated muscle cells of invertebrates. It is equally obscure if catch muscle cells contain foot structures, and how Ca-release is regulated. However, catch muscle contraction is almost certainly also regulated by Ca ions, and we must therefore assume that foot structures/RyRs occur somewhere within molluscan catch muscle cells.
According to these circumstances, we have attempted to locate the foot structures/RyRs of catch muscle cells in the adductor muscles of a clam species. The aims were to determine (a) whether foot structures were present in the catch muscle cells, and if so, where they occurred, and (b) if we could establish the ultrastructural and biochemical relationships between the foot structure and RyR in the catch muscle cells.
The animal used in this work was a clam specimen (Meretrix lusoria) measuring about 7–10 cm in diameter. The adductor of the shellfish is anatomically divided into a larger translucent muscle part and a smaller opaque part. The smaller opaque part is constructed of special smooth muscle cells called catch muscle cells. The material used in this work was taken from the opaque part of the adductors.
A) Intact muscle cells
Catch muscles were prefixed at an elongated state with an aldehyde fixative solution for electron microscopy. The aldehyde fixative solution used contained 1.5% glutaraldehyde and 1.5% para-formaldehyde in 0.1 M cacodylate buffer pH 7.3 with 0.5% sucrose. Prefixed catch muscles were tied into small bundles, fixed again with a newly prepared aldehyde fixative for 90 min, and then post-fixed for 120 min with a 1% osmium tetroxide solution containing 0.1 M phosphate buffer pH 7.3 with 0.5% sucrose. The fixed specimens were dehydrated in a series of ethanol solutions and embedded in Epoxy resin after being passed through PQ-1 emerging agent. Specimens in Epoxy resin were cut into thin sections and examined using a JEM 1010 type electron microscope.
B) SR fraction
SR fractions were next examined under an electron microscope to identify the foot structures. SR fractions which were prepared as described below were prefixed in the same fixative solution as described above. After prefixation for 60 min, the SR fractions were collected by centrifuge, and then post-fixed in the 1% osmium tetroxide fixative described above. Epoxy-embedded specimens were thin-sectioned and observed by an electron microscope.
Preparation of SR fractions was carried out using a modified form of the protocol used previously for pecten SR (Ohkusa et al., 1991; Abe et al., 1997). Briefly, opaque parts of adductors were homogenized in cold water using a Waring blender, and centrifuged for 5 min at 7,000 g to remove myofilaments or connective tissues in the muscles. The supernatant was filtered through filter paper and re-centrifuged for 90 min at 16,700 g to isolate the SR fraction. The pellet obtained from this was resuspended in a solution containing 0.1 M KCl, 5 mM CaCl2, 5 mM MgCl2 and 5 mM EDTA. The fraction was stored in a freezer at –80°C. Foot proteins from these SR fractions were identified by general SDS-PAGE. SDS-PAGE was carried on polyacrylamide gels (9%) and stained with Coomassie Brilliant Blue. Samples for the SDS-PAGE were denatured for 3 min at 95°C in 0.1 M Tris-HCl, pH 6.8, containing 1.4% SDS, 3.3% 2-mercaptoethanol, 7% glycerol.
Foot proteins were also identified to be RyRs by the Western blotting method. Briefly, the foot proteins were transferred from the polyacrylamide gel (9%) to a piece of nitrocellulose membrane, which was then incubated in an anti-ryanodine-receptor Ca2+ channel. The antibody used of this study was made from triad preparation of rabbit skeletal muscle, and was able to recognize the approximately 450 kD skeletal muscle ryanodine receptor Ca2+ channel (Upstate Biotechnology Ltd). After washing out the secondary antibody, the membrane was then stained with DAB-Ni-Co.
A) Intact muscle cells
Examination of cross sections of catch muscle cells showed the nuclei were centrally situated, and myofilaments were homogeneously distributed around them. Other cell organelles, such as mitochondria and SRs were distributed at the cell peripheries. T-systems were not found anywhere (Fig. 1, A and B), but foot structures were observed. These were in close contact with the inner surface of a cell membrane, in about 12 nm interspace (Fig. 2, A–F). The SRs were oval in shape, measured about 0.4 μm to 0.9 μm in diameter, and were randomly distributed. Half to two-thirds of their surfaces were in close contact with the cell membrane. Electron-dense materials were recognized in the interspaces between the SR and cell membrane, binding the two membranes (Fig. 2, A–F). This distribution was observed equally in both longitudinal or cross sections of the cells (Fig. 2, A–C and D–F). Higher magnification observation of the most resolvable section showed the electron dense materials occurred as particles measuring about 21 nm in diameter, spaced at about 20 nm intervals (Fig. 2, A–F). The size of these electron-dense particles resembled that of foot structures on SRs at the “triad” of cross striated muscle cells in vertebrates and invertebrates (Table I).
 View Details | Fig. 1. Electron micrographs of intact muscle cells. T-systems were not observed. A: cross section of muscle cells. SRs directly face cell membranes, and electron-dense materials occur in the interspaces (arrowheads). B: longitudinal section of muscle cells. Similar electron-dense substrates occur in the interspaces (arrowheads). ×16,200 scale bar 1 μm |
 View Details | Fig. 2. Enlarged views of SRs. Many foot structures occur in the interspaces between the SRs and the cell membranes(arrowheads). The size and distribution of the foot structures are similar in cross section (A–C) and in longitudinal section (D–F). ×97,700 scale bar 100 nm |
B) SR fractions
SR fractions from the clam catch muscles appeared ultrastructurally as vesicles of varying size, ranging from about 1.5 μm to 7 μm in diameter. Among them, some vesicles were attached to each other, forming pairs (Fig. 3, A–D). Closer observation of the electron micrographs showed that paired vesicles were attached by regular interspaces at about 12 nm in intervals. The spacing between the paired vesicles was similar to that between SRs and cell membranes described from the intact muscle cells. Higher magnification electron micrographs of these spaces, showed electron-dense particles were present in the interspaces between paired vesicles. These particles measured about 21 nm, and were regularly arranged in the space at 20 nm intervals (Fig. 3, B–D). They thus resembled those observed in the intact muscle cells during section observation. Their size is also similar to those of foot structures reported from other species (Table I).
 View Details | Fig. 3. Electron micrographs of SR fractions. A: lower magnification. The SR fractions contain many types of vesicles. B–D: enlarged views of the interspaces. Foot structures (arrowheads) are observed as similar sizes to those of intact muscle cells as shown in Fig. 2. A: ×16,200 scale bar 1 μm, B: ×97,700 scale bar 100 nm |
SR fractions were then analyzed by SDS-PAGE to identify RyR. The PAGE results demonstrated that the major components of the SR fraction resembled those of the rat, as shown in Figure 4. According to the similarities between the band patterns, the 450 kD band on the clam lane represents the RyR band. For the same reason, we assumed that the 110 kD band represents Ca-pump protein. Thus, catch muscle cells of this clam contain proteins which have similar molecular weight RyRs to vertebrate cross striated muscle cells (Fig. 4).
 View Details | Fig. 4. SDS-PAGE of the SR fraction. The two lanes on the right show Western Blotting of the gel by anti-ryanodine-receptor. Lane 1; molecular weight marker; lane 2, clam SR; lane 3, rat SR; Lane 4, Western blotting of the clam 450 kD band; Lane 5, Western blotting of the rat 450 kD band. The SR fraction from the clam and rat both contain the 450 kD band (RyRs). The 450 kD bands clearly reacted to anti-ryanodine-receptor. RyR, ryanodine receptor; CPP, calcium pump protein. |
The Western blotting showed that the clam 450 kD protein reacts to anti-ryanodine-receptor in the same way as rat RyR (about 450 kD; Fig. 4). Therefore, the 450 kD protein in clam catch muscle cells is proved to be RyR.
Electron microscopic observations of foot structures have previously been reported from cross striated muscle cells of several vertebrates and invertebrates, including the rabbit, frog, snake, turtle and pecten. Among these reports, it has been demonstrated that the foot structures in rabbit skeletal muscles are arranged regularly on the surface of the terminal cisternae of SRs at “triad” (Inui and Fleischer, 1988; Franzini-Armstrong et al., 1986). Moreover, the patterns of these arrangements were specific to individual animal species (Loesser et al., 1992). In contrast, the foot structures of a pecten cross striated muscle cells are found in the interspaces between SRs and cell membranes in intact muscle cells, and are rather smaller in size (Abe et al., 1997).
Foot structures were also recognized in clam catch muscle cells in this study, occurring in the interspaces between SRs and cell membranes in intact muscle cells. The SR fraction from the clam catch muscle cells studied here were prepared using the same procedure as in previous studies examining cross striated muscle cells. The SR faction showed similar ultrastructures to those from rat and pecten in terms of vesicle size and distribution of foot structures. These observations suggest that we have successfully isolated SR fractions from clam catch muscle cells.
Foot structures in the clam catch muscle cells measured about 21 nm in width in both intact muscle cells and the SR fractions, and were slightly smaller than those reported for other species (Table I). The cause of the apparently smaller foot structures in clam is probably related to the fact that we measured foot size in thin sections of intact cells and SR fractions, whereas the measurements reported in other studies were made on samples which had been isolated biochemically. However, these small foot structures can be suspected to have somewhat different functions from that of the cross striated muscle cells.
Electron microscopic investigations of intact muscle cells and SR fractions from the clam revealed that its catch muscle cells bear foot structures in interspaces between SRs and cell membranes, and that these foot structures are of rather small size compared to those from vertebrates.
We observed foot structures in SR fractions, and these were identical to those found in intact cells. However, it is not yet clear whether the foot structures in intact catch muscle cells correspond completely to RyRs. To confirm the relationship between the foot structure and the RyR, we tried to detect the RyR biochemically from SR fractions in which foot structures had already been recognized. As described in the Results, the 450 kD band can be clearly identified by SDS-PAGE. This band must be the RyR of clam catch muscle cells, because it has almost identical molecular weight to rat RyR (Fig. 4), and is similar to bands reported for rabbit (Lai et al., 1989, Guo and Campbell, 1995), frog (Murayama and Ogawa, 1992) and pecten (Abe et al., 1997).
It is already known that bands of such RyRs can react with anti-RyRs in the Western blotting method (Campbell et al., 1980; Inui et al., 1987). We tried this blotting to confirm if the foot protein band of clam catch muscle cells reacted with anti-RyRs. The results clearly showed that our 450 kD clam band reacted with the anti-RyRs (Fig. 4).
The overall results indicate that clam catch muscle cells have foot structures in the interspaces between SR and cell membranes, and that the foot structures are smaller than those of cross striated muscle cells. Although, we could not clarify the details of the small foot structure, it is certain that the foot structure contains RyRs similar to those of cross striated muscle cells.
|