| To whom correspondence should be addressed: Masa H. Sato, Graduate School of Human and Environmental Studies, Kyoto University, Yoshida-nihonmatsu, Sakyo-ku, Kyoto 606-8501, Japan. Tel & Fax: +81–75–753–6857 Abbreviations: BFA, brefeldin A; ER, endoplasmic reticulum; EST, expression sequence tag; GFP, green fluorescent protein; PVC, prevacuolar compartment; SNARE, soluble N-ethyl-maleimide sensitive factor attachment protein receptor; TGN, trans-Golgi network; VAMP, vesicle-associated membrane protein; YFP, yellow fluorescent protein. |
The trafficking of proteins by vesicular transport is essential for all eukaryotic cells. Membrane bound transport vesicles carry cargo proteins from one compartment to another, and discharge the cargo into a specific compartment by fusing with the target membrane. The specificity of membrane fusion is mainly mediated by membrane-associated proteins called SNAREs (soluble N-ethyl-maleimide sensitive factor attachment protein receptors) (Rothman, 1994; Sollner et al., 1993a; Sollner et al., 1993b). The SNARE molecules have a highly conserved coiled-coil domain, called the SNARE motif, and are anchored to the membrane by a hydrophobic transmembrane domain (TMD) at the C-terminus or post-translational lipid attachment. Before the membrane fusion, a trans-SNARE complex consisting of four-helical bundles of the SNARE motif from three target-SNAREs (t-SNAREs) and one vesicle-associated SNARE (v-SNARE) is formed (Antonin et al., 2002; Bonifacino and Glick, 2004; Chen and Scheller, 2001; Jahn and Sudhof, 1999; Sutton et al., 1998).
In the case of homotypic membrane fusion between identical compartments, however, one cannot define which is the vesicle and which is the target. To avoid confusion, the SNARE proteins have recently been reclassified as “Q-SNAREs” and “R-SNAREs” according to the conserved layer of interacting amino acids (glutamine or arginine) in the center of the helix bundle, the “zero-layer” (Fasshauer et al., 1998). Furthermore, they are classified into five classes based on their similarities to the synaptic SNARE molecules: Qa-SNAREs (Syntaxin), Qb-SNAREs (SNAP Ns), Qc-SNAREs (SNAP Cs), R-SNAREs (VAMPs) and SNAP (SNAP Ns and Cs) (Bock et al., 2001). A typical SNARE complex contains one copy of the Qa-, Qb-, Qc-, and R-SNARE motifs. Since a specific SNARE complex is involved in membrane fusion in each vesicular transport pathway, specific organelles are marked by the presence of specific resident SNARE proteins (Jahn et al., 2003).
Higher plants have more complicated membrane traffic than other eukaryotes. For instance, plant cells have multiple vacuoles with different functions, and the existence of several distinct pathways from the Golgi apparatus and even directly from the endoplasmic reticulum (ER) have been demonstrated by biochemical and electron microscopic studies (Hillmer et al., 2001; Hara-Nishimura et al., 1998; Hohl et al., 1996; Matsuoka et al., 1995). Recently, membrane fusion events have proved important for various aspects of plant development and signal transduction (Pratelli et al., 2004; Surpin and Raikhel, 2004; Ueda and Nakano, 2002). In the Arabidopsis genome, 53 genes have been annotated to encode SNARE molecules (Sanderfoot et al., 2000). Some SNARE proteins in plants have already been reported to be involved in physiological processes such as cytokinesis (Lauber et al., 1997; Lukowitz et al., 1996), abscisic acid-related signaling (Geelen et al., 2002; Leyman et al., 1999), shoot gravitropism (Kato et al., 2002; Morita et al., 2002) and osmotic stress tolerance (Zhu et al., 2002). However, due to lack of the information on subcellular localization of most SNARE proteins, the complexity of membrane traffic of higher plant cells remains to be explained at the molecular biological level.
In the present study, we conducted an extensive analysis on the subcelluler localizations of all Arabidopsis SNAREs. Green fluorescent protein (GFP)- and/or yellow fluorescent protein (YFP or its brighter version, Venus)-tagged SNARE molecules were examined under a confocal microscope using a transient expression system in the protoplasts of Arabidopsis suspension cultured cells. We localized 38 SNAREs to single compartments, 7 to dual compartments, and 3 to triple compartments. The subcellular distribution of Arabidopsis SNARE molecules showed that higher plant cells have a unique and complex post-Golgi membrane traffic system.
A total of 54 SNARE protein sequences of A. thaliana SNAREs were detected in a screening of the protein database in the National Center for Biotechnology Information (NCBI: http://www.ncbi.nlm.nih.gov/) using the BLAST program. An amino acid sequence alignment of 54 A. thaliana SNAREs was performed with the Clustal_X program (Thompson et al., 1997). Based on conserved regions (67 residues), a phylogenetic analysis was performed with the PROTDIST and NEIGHBOR programs of the PHYLIP 3.2 package (Felsenstein, 1989). A phylogenetic tree was then inferred by the neighbor-joining method (Saitou and Nei, 1987) tested with 1000 replications in the bootstrap analysis carried out with the SEQBOOT and CONSENSE programs in the same package, and visualized using the TREEVIEW program (Page, 1996).
Total RNA was extracted from root, stem, leaves, flowers and suspension cultured cells of Arabidopsis using an RNeasy Mini Kit (QIAGEN), and each cDNA was generated using TrueScript Reverse Transcriptase (SAWADY Technology Inc., Tokyo) according to the manufacturer’s protocol. A specific primer for each gene was used for PCR amplification. cDNAs for 53 SNARE genes were obtained by RT-PCR. Open reading frames (ORFs) of these cDNAs were fused to the ORFs of sGFP of CaMV35S-sGFP(S65T)-NOS3’ vector (provided by Yasuo Niwa) or a modified CaMV35S-sGFP(S65T)-NOS3’ vector in which the sGFP coding region was replaced with the coding region for Venus (Nagai et al., 2002).
The transient expression of GFP-fused proteins in Arabidopsis suspension cultured cells was performed as described previously (Ueda et al., 2001). Briefly, 2 g of cultured cells was incubated in 25 ml of enzyme solution (0.4 M mannitol, 5 mM EGTA, 1% cellulase R-10 and 0.05% pectolyase Y-23) for 1–2 hr at 30°C and filtered with a nylon mesh (125 μm pore). Protoplasts were washed twice with 25 ml of solution A (0.4 M mannitol, 70 mM CaCl2, and 5 mM MES-KOH, pH 5.7) and resuspended in 1 ml of MaMg solution (0.4 M mannitol, 15 mM MgCl2, and 5 mM MES-KOH, pH 5.7). After the addition of 20 μg of plasmid and 50 μg of carrier DNA to 100 μl of protoplast solution, 400 μl of DNA uptake solution (0.4 M mannitol, 40% polyethylene glycol (PEG) 6000 and 0.1 M Ca(NO3)2) was added. The protoplasts were incubated on ice for 20 min and then diluted with 10 ml of dilution solution (0.4 M mannitol, 125 mM CaCl2, 5 mM KCl, 5 mM glucose and 1.5 mM MES-KOH, pH 5.7). Collected protoplasts were resuspended in 4 ml of MS medium containing 0.4 M mannitol and incubated with gentle agitation at 23°C for 16 hr in the dark.
The constructs expressing the GFP-fused proteins were introduced into the protoplasts of Arabidopsis suspension cultured cells as described above. The cells expressing GFP-fused protein were incubated in MS medium containing 0.4 M mannitol and 50 μM FM4-64 for 1 hr. After the incubation, cells were washed twice with the medium and resuspended in the same medium without FM4-64.
Observations were made using confocal laser scanning microscopes, Olympus BX60 fluorescence microscope equipped with a Model CSU10 confocal scanner (Yokogawa Electric) (Nakano, 2002), and Zeiss LSM510 META or LSM5 PASCAL equipped with green HeNe and argon lasers.
GFP-fused proteins were introduced into the protoplasts of Arabidopsis suspension cultured cells as described above. The cells expressing GFP-fused protein were incubated in the medium containing 0.4 M mannitol and 200 μM BFA for 2 hr. After the incubation, cells were observed under a confocal microscope.
A total of 53 SNARE genes have already been identified in the Arabidopsis genome (Sanderfoot et al., 2000). We found one additional SNARE gene, the product which shares significant similarity to Arabidopsis VTI1 proteins. We named this gene (At5g39630) AtVTI14. Phylogenic analysis suggests that the 54 SNARE proteins can be classified into 5 distinct SNARE subfamilies (Qa, Qb, Qc, R, and SNAP25) according to the amino acid sequences of their SNARE domains (Fig. 1).
 View Details | Fig. 1. Anunrooted phylogenic tree of the SNARE family in Arabidopsis. The phylogenetic tree was constructed based on the SNARE motifs including 67 amino acid residues. The scale bar shows the Dayhoff distance among the SNARE molecules. The numbers on the tree are bootstrap values with 1000 replications of SNARE motifs. The SNARE proteins were categorized into four distinct groups (green, Qa-SNARE/Syntaxin; purple, Qb-SNARE; blue, Qc-SNARE; red, R-SNARE) according to Jason et al. (2001) depending on the similarity of their SNARE domains. |
Using the RT-PCR method, all genes but AtYKT62 and AtVTI14 were found to be expressed in almost all tissues at various levels (Fig. 2). AtVTI14 was detected only in suspension-cultured cells. The expression of YKT62 was not detected in any tissues under our experimental conditions (Table I). Although no EST (expression sequence tag) clone for AtYKT62 is found in any database, there is a putative TATA box sequence in its promoter region. AtYKT62 might be expressed in specific cells or under certain conditions.
 View Details | Fig. 2. Expression patterns of VTI (Qb) and YKT6 (R) groups. Total RNA was extracted from suspension cultured cells, roots, stems, leaves and flowers. First strand cDNA was generated by reverse transcription from each total RNA. PCR amplification was performed by specific primers for each transcript. |

To determine the subcellular distribution, GFP-tagged SNARE proteins were transiently expressed under the control of the CaMV 35S promoter in protoplasts prepared from Arabidopsis suspension cultured cells, and the fluorescence of GFP-fused proteins was examined by confocal laser scanning microscopy. To determine the localization of each SNARE molecule, we selected protoplasts having weak GFP-fluorescence to avoid mislocalization by the effect of overexpression, and observed approximately 100 independent protoplasts per construct.
We identified 6 ER-localized SNARE molecules, SYP81/AtUfe1 (Qa), SYP71, SYP72, and SYP73 (Qc), AtSec22 and AtVAMP723 (R). Among the 54 SNAREs, no Qb SNARE was identified in the ER. The fluorescence patterns of GFP-tagged proteins showed a tubular/reticular shape (mesh-like ER patterns) (Fig. 3), as a typical structure of the plant ER (Takeuchi et al., 2000).
 View Details | Fig. 3. The ER-localized SNARE molecules. GFP-tagged SNARE molecules (SYP81, SYP71, SYP72, SYP73, AtVAMP723 and AtSec22) were transiently expressed in the protoplasts of Arabidopsis suspension cultured cells. Confocal and Nomarski images were captured from the same cells expressing each GFP-tagged SNARE. Scale bars=10 μm |
The fluorescence of GFP-tagged SYP31 (Qa) protein was distributed in a dot-like pattern throughout the cell (Fig. 4A). Treatment with BFA, an inhibitor of Arf GTPase guanine nucleotide exchange factors known to induce morphological changes in the Golgi apparatus (Boevink et al., 1998; Lippincott-Schwartz et al., 1990; Nebenfuhr et al., 2002), drastically changed the fluorescence profile to the typical ER pattern (Fig. 4B). This led us to conclude that SYP31 is localized to the Golgi apparatus. We localized 8 more SNAREs (SYP32 (Qa), AtMEMB11, AtMEMB12, AtGOS11 and AtGOS12 (Qb), BS14a and BS14b (Qc), and AtVAMP714 (R)) proteins to the Golgi apparatus (Fig. 4), by BFA treatment and colocalization with Venus (a bright version of YFP (Nagai et al., 2002))-SYP31 (Fig. 4, C, F, I, L, O, R, U and X).
Although AtMEMB11, AtMEMB12, AtGOS11, AtGOS12, AtBS14a and AtBS14b are all located in the Golgi apparatus, the patterns of their fluorescence differed after the BFA treatment. Namely, AtMEMB11, ATMEMB12 and AtBS14b showed a mesh-like ER-pattern (Fig. 4, H, K and W) whereas AtGOS11, ATGOS12 and BS14a exhibited a so-called “BFA-compartment” pattern (Fig. 4, N, Q and T) (Satiat-Jeunemaitre et al., 1996). Since the cis, medial and trans-Golgi cisternae differ in their sensitivity to BFA treatment (Ritzenthaler et al., 2002), these SNARE proteins may be located in different Golgi cisternae: AtMEMB11, AtMEMB12 and AtBS14b in cis and AtGOS11, AtGOS12 and BS14a in trans-Golgi cisternae.
We concluded that a total of 15 SNARE molecules (6 in the ER and 9 in the Golgi apparatus) are involved in the vesicular transport in and between the ER and the Golgi apparatus.
 View Details | Fig. 4. The Golgi apparatus-localized SNARE molecules. The GFP- or Venus-tagged SNARE molecules were transiently expressed in the protoplasts of Arabidopsis suspension cultured cells. The localization in the Golgi apparatus was confirmed with a Golgi-marker, Venus-SYP31, which was coexpressed with each SNARE molecule. Fluorescence and Nomarski images are shown for GFP-SYP31/SED5 (A), GFP-VAMP714 (D), GFP-MEMB11 (G), GFP-MEMB12 (J), GFP-AtGOS11 (M), GFP-AtGOS12 (P), GFP-AtBS14a (S), and GFP-AtBS14b (V). Fluorescence images are also shown for BFA-treated cells expressing GFP-SYP31/SED5 (B), GFP-VAMP714 (E), GFP-MEMB11 (H), GFP-MEMB12 (K), GFP-AtGOS11 (N), GFP-AtGOS12 (Q), GFP-AtBS14a (T), and GFP-AtBS14b (W). The Golgi apparatus marker Venus-SYP31 was coexpressed with GFP-SYP32 (C), GFP-VAMP714 (F), GFP-MEMB11 (I), GFP-MEMB12 (L), GFP-AtGOS11 (O), GFP-AtGOS12 (R), GFP-AtBS14a (U), and GFP-AtBS14b (X). Arrowheads show the GFP fluorescence merged with Venus-SYP31. Scale bars=10 μm |
We identified 18 plasma membrane-localized SNARE molecules: SYP111/KNOLLE, SYP112, SYP121/AtSYR1, SYP122, SYP123, SYP124, SYP125, SYP131 and SYP132 (Qa), AtVTI12, NPSN11, NPSN12 and NPSN13 (Qb), and AtVAMP721, AtVAMP722, AtVAMP724, AtVAMP725 and AtVAMP726 (R). The fluorescence from the GFP-tagged form of these proteins was mainly observed on the plasma membrane and occasionally detected as cytoplasmic dots (Fig. 5A). To characterize these dot structures, we labeled cells expressing GFP-SYP111 or GFP-AtVAMP721 with a lipophilic styryl dye, FM4-64, which is known to follow an endocytic pathway from the plasma membrane via endosomes to the vacuole in various eukaryotes including plants (Ueda et al., 2001; Vida and Emr, 1995). The labeling demonstrated colocalization of the dye with GFP-SYP111 and GFP-AtVAMP721 (Fig. 5, B and C). AtVTI12 was distributed in the trans-Golgi network (TGN) and the plasma membrane (see below). These plasma membrane-localized SNARE molecules are likely to be brought to an endocytic compartment, most probably early endosomes, via transport from the plasma membrane.
In our experimental system, fluorescence from the GFP-tagged SNAP25 subfamily (AtSNAP29, AtSNAP30 and AtSNAP33) was dispersed in the cytosol (Fig. 5D). Since this subfamily lacks a TMD and is considered to be anchored to the membrane via a post-transcriptional modification (Gonzalo et al., 1999; Steegmaier et al., 1998), the conjugation of GFP might interfere with their post-translational modification and thus organelle localization.
Only fluorescence of GFP-AtVAMP727 (R), among 14 R-SNAREs, was detected in endosomes, but not on the plasma membrane under any conditions (Fig. 5E). Colocalization of AtVAMP727 with early endosomal Rab GTPases, Ara7 and Rha1, has also been observed (T. Ueda, T. Uemura, M. H. Sato, and A. Nakano, unpublished data). The fluorescence of GFP-AtVTI14 (Qb) exhibited aggregated ring-like structures, which were identified as endosomes by FM4-64 staining (Fig. 5F). Since these aberrant endosomal structures were not observed in cells expressing other GFP-SNARE proteins, we concluded that the abnormal morphology of endosomes was caused by the effect of overexpression of GFP-AtVTI14.
 View Details | Fig. 5. SNARE molecules involved in transport to the plasma membrane and endosomes. Fluorescence images of cells expressing the GFP-tagged SNARE molecules, SYP111, SYP112, SYP121, SYP122, SYP123, SYP124, SYP125, SYP131, SYP132, AtVTI12, NPSN11, NPSN12, NPSN13, AtVAMP721, AtVAMP722, AtVAMP724, AtVAMP725 and AtVAMP726, show plasma membrane localization (A). Protoplasts of Arabidopsis cultured cells expressing GFP-SYP111/KNOLLE (B) or GFP-AtVAMP721 (C) were labeled with FM4-64. Fluorescence images of GFP-tagged SNAP-25 subfamily (AtSNAP29, AtSNAP30 and AtSANP33) are shown in (D). Protoplasts of Arabidopsis cultured cells expressing GFP-AtVAMP727 and those expressing GFP-AtVAMP727 labeled with FM4-64 are shown in left and right panels of (E), respectively. Protoplasts of Arabidopsis cultured cells expressing GFP-AtVTI14 and those expressing GFP-AtVTI14 labeled with FM4-64 are in left and right panels of (F), respectively. Arrowheads show the GFP fluorescence merged with FM4-64. Scale bars=10 μm |
SYP21/AtPep12 and SYP22/AtVam3 (Qa), AtVTI11 and AtVTI13 (Qb), SYP51 and SYP52 (Qc), and AtVAMP711, AtVAMP712 and AtVAMP713(R) are present on the vacuolar membrane (Fig. 6). The fluorescence of GFP-SYP21 predominantly exhibited a dot-like pattern, although weak vacuolar membrane staining of GFP-SYP21 was occasionally detected (Fig. 6A). This vacuolar localization of GFP-SYP21 may perhaps be due to the effect of overproduction of the protein. In contrast, GFP-SYP22 was mainly localized to the vacuolar membrane as well as the dot-like structures (Fig. 6C). These structures were not completely stained with FM4-64 (Fig. 6, B and D). To determine whether the structures with fluorescence from GFP-SYP21 and GFP-SYP22 are the same organelle or not, these fusion proteins were transiently coexpressed. The SYP21-residing and the SYP22-residing dot structures completely merged (Fig. 6, E and F), indicating that SYP21 and SYP22 are in the same organelle. SYP21 is known to localize in the prevacuolar compartment (PVC) (da Silva Conceicao et al., 1997) which is considered to be synonymous with the multivesicular bodies/late endosomes in yeast and animals (Tse et al., 2004). Taken together, we conclude that SYP21 and SYP22 are localized to the vacuolar membrane and PVC with different densities. The fluorescence patterns of GFP-tagged AtVTI11, AtVTI13, SYP51, SYP52, AtVAMP711, AtVAMP712 and AtVAMP713 were the same as the pattern of GFP-SYP22 (Fig. 6 G–M). Therefore, we concluded that these SNARE molecules are involved in the transport to the vacuole from the PVC.
 View Details | Fig. 6. Vacuole- and PVC-localized SNARE molecules. The GFP- or Venus-tagged SNARE molecules were transiently expressed in the protoplasts of Arabidopsis suspension cultured cells. Fluorescence and Nomarski images are shown for GFP-SYP21/AtPep12 (A), GFP-SYP22 (C), GFP-AtVTI11 (G), GFP-AtVTI13 (H), GFP-SYP51 (I), GFP-SYP52 (J), GFP-ATVAMP711 (K), GFP-AtVAMP712 (L) and GFP-AtVAMP713 (M). The cells expressing GFP-SYP21/AtPep12 (B) and GFP-SYP22/AtVam3 (D) were labeled with FM4-64. GFP-SYP22/AtVam3 and Venus–SYP21/AtPep12 (E) or GFP-SYP21/AtPep12 and Venus–SYP22/AtVam3 (F) were transiently coexpressed in the same cells. Arrowheads show GFP fluorescence merged with FM4-64 (B and D) or the fluorescence of Venus-tagged proteins (E and F). Scale bar=10 μm |
It was shown that SYP4 (Qa) group is localized to the TGN in the forms of complex with AtVTI12, AtVPS45 and SYP61 (Bassham et al., 2000; Sanderfoot et al., 2001). We will follow this and use the term TGN in this paper.
The fluorescence of GFP-tagged SYP41 (Qa) protein exhibited a distinct dot-like pattern (Fig. 7A). The fluorescence from GFP-SYP42 and GFP-SYP43 (Qa) merged with that of YFP-SYP41, indicating that these proteins are located in the same organelle (Fig. 7, B and C). The fluorescence of GFP-SYP41 were usually separated clearly from, but occasionally existed in very close proximity to, the Golgi apparatus (Fig. 7E, arrowheads). Treatment of the cells expressing GFP-SYP41 with BFA did not cause any changes of the dot-like structures to the mesh-like pattern as seen in the case of SYP31, but did induce aggregation of the structures (Fig. 7D). Neither a Golgi marker (YFP-SYP31) nor a PVC marker (YFP-SYP21) colocalized with GFP-SYP41 (Fig. 7, E and F), indicating that the SYP4 (Qa) group is not localized to the Golgi apparatus, the PVC or endosomes. We again define this unique compartment as the TGN according to previous work. The unique feature of the plant TGN will be discussed later (see Discussion). Only fluorescence from YFP-SYP61, among 8 Qc-SNAREs, completely merged with that of SYP41 (Fig. 7H), but not the Golgi marker (Fig. 7 I), indicating that SYF61 is localized to the TGN.
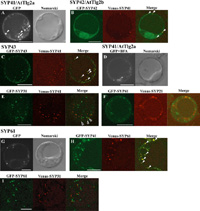 View Details | Fig. 7. Subcellular localization of the SYP4 (Qa) and SYP61 (Qc) group. GFP- or Venus-tagged SNARE molecules were transiently expressed in the protoplast of Arabisopsis suspension cultured cells. Fluorescence and Nomarski images are shown for GFP-SYP41/AtTlg2a (A) and GFP-SYP61 (G). Venus-SYP41 was coexpressed with GFP-SYP42/AtTlg2b (B) and GFP-SYP43 (C). Fluorescence and Nomarski images of BFA-treated cells expressing GFP-SYP41/AtTlg2a are in (D). SYP41 was coexpressed with the Golgi apparatus-marker GFP-SYP31 (E) or a PVC-marker, Venus-SYP21 (F). SYP61 was coexpressed with a TGN-marker, GFP-SYP41 (H), or the Golgi marker GFP-SYP31 (I). Closed arrowheads in (B), (C) and (H) show the GFP fluorescence merged with the TGN marker. Open arrowheads in (E) show a few TGN structures standing in close vicinity to the Golgi apparatus. Scale bar=10 μm |
AtVTI11 and AtVTI13 (Qb) were distributed in the TGN and the vacuolar membrane (Fig. 6, G and H, Fig. 8B), whereas AtVTI12 (Qb) was found in the TGN and the plasma membrane (Fig. 5A and Fig. 8D). The dot-like patterns of YFP-AtVTI11 and GFP-AtVTI12 fluorescence completely merged, consistent with the idea that both proteins reside in the TGN (Fig. 8A). Almost all fluorescence merged with that of GFP-SYP41 (TGN marker) (Fig. 8, B and D), but some of them merged with SYP22 (PVC marker) (Fig. 8, C and E). These results indicated that AtVTI11, AtVTI12 and AtVTI13 were mainly present in the TGN and partly in the PVC. This differential localization of the VTI1 (Qb) group proteins may indicate that AtVTI11 and AtVTI13 are involved in the vacuolar transport through the TGN and/or the PVC, and that AtVTI12 is involved in the transport to the plasma membrane via the TGN and/or the PVC.
 View Details | Fig. 8. Subcellular localization of the VTI1 (Qb) group. GFP- and Venus-tagged SNARE molecules were transiently coexpressed in the protoplasts of Arabidopsis suspension cultured cells. Fluorescence image are shown for cells coexpressing GFP-AtVTI12 and Venus-AtVTI11 (A), TGN-marker SYP41 and Venus-AtVTI11 (B), PVC-marker GFP-SYP22 and Venus-AtVTI11 (C), TGN-marker SYP41 and Venus-AtVTI12 (D), and PVC-marker GFP-SYP22 and Venus-AtVTI12 (E). Arrowheads show GFP fluorescence merged with the TGN-marker (B and D) and the PVC-marker (C and E). Scale bars=10 μm |
In our analysis, the subcellular distributions of SYP23/AtPLP (Qa) and AtYKT61 (R) could not be determined, because fluorescence from these GFP-tagged proteins was observed only in the cytosol (data not shown). SYP23 (Qa) may be unable to bind to membranes due to the lack of a C-terminal TMD caused by a frame-shift mutation (Zheng et al., 1999). AtYKT61 (R) is considered to be anchored to the membrane by a post-transcriptional modification similar to the YKT6 group in yeast and animals (Fukasawa et al., 2004; McNew et al., 1997), hence GFP conjugation might interfere with the post-translational modification site of this protein.
The length of the TMD is thought to be important for the retention of membrane proteins in yeast and animal cells (Kasai and Akagawa, 2001; Bulbarelli et al., 2002; Kim et al., 1999). In plants, the distribution of chimeric GFP-fusion proteins has been reported to vary depending on the lengths of their TMD, also suggesting that the length of the membrane anchor is important for localization (Brandizzi et al., 2002). We estimated the lengths of TMD of Arabidopsis SNARE proteins using the TMHMM program (Krogh et al., 2001) and found that, in most cases, they were conserved in phylogenetic groups but differed among the groups. In particular, the lengths of TMD for the VAMP-group SNAREs were almost identical. However, the VAMP proteins showed various localizations among the organelles. This suggests that the determinant of subcellular localization does not lie in the TMD at least for the case of Arabidopsis SNAREs.
The amino acid sequence analysis of a variety of SNARE proteins from Arabidopsis, Drosophila, C. elegans, S. cerevisiae and mouse showed that the SNARE domain (four-helical bundle region in the SNARE fusion complex) was highly conserved in each SNARE group. We could not find any conserved motif required for the localization in a particular organelle. However, among the Arabidopsis VAMP7 proteins, we found that there is an interesting region within the SNARE motif that differs depending on the subcellular localization (Fig. 9). For example, SNARE proteins localized in PM/endosomes have the “RSQAQD” sequence in common between +4 and +6 layers of the SNARE domain. Likewise, SNAREs localized in vacuoles, endosomes and the Golgi apparatus have “QGNTFR”, “QFQADS” and “QDSSFH” motifs, respectively. While these motifs are not conserved in VAMP proteins of other eukaryotes,they might be determinants for the localizations of Arabidopsis VAMP proteins.
 View Details | Fig. 9. Sequence alignment of the Arabidopsis VAMP group. The full-length amino acid sequences were used in the analysis. Conserved residues of the Arabidopsis VAMP group are shaded in black. Conserved residues of each group of VAMPs are shaded in several colors. A bar under the alignment represents the regions where the possible localization signals of Arabidopsis VAMP proteins. |
In this study, by systematic analysis utilizing a protoplast expression system, we determined the subcellular distributions of the Arabidopsis SNARE proteins: 6 in the ER, 9 in the Golgi apparatus, 4 in the TGN, 2 in endosomes, 17 in PM, 7 in the PVC/vacuole, 2 in the TGN/PVC/vacuole, and 1 in the TGN/PVC/PM. The results, summarized in Fig. 10, suggest that SNARE complexes are involved in all steps of vesicular traffic in Arabidopsis. We were aware of the possibility that the fusion proteins may be mistargeted to improper locations due to the overproduction of the fusion proteins driven by the 35S promoter. In fact, we occasionally observed in the cells emitting strong fluorescence that overexpressed SNARE molecules tend to be mistargeted to the plasma membrane. We were therefore very careful to choose cells expressing moderate or low levels of tagged proteins before drawing conclusions. In the several cases in which the localization of SNARE molecules was examined by other methods (Bassham et al., 2000; Leyman et al., 1999; Sato et al., 1997; Sanderfoot et al., 2001; Uemura et al., 2002), our results were consistent with their findings, thus confirming the validity of this approach.
 View Details | Fig. 10. Schematic model of the Arabidopsis intracellular trafficking pathways. All determined locations of SNAREs are shown. Many molecules are localized to organelles involved in the post-Golgi network such as TGN-endosome-plasma membrane and TGN-PVC-vacuole pathways. TGN- and endosome-localized SNARE molecules may play key roles in the Arabidopsis post-Golgi trafficking. |
The distributions of SYP21 and SYP22 (Qa), AtVTI11, AtVTI12 and AtVTI13 (Qb), SYP51 and SYP52 (Qc) and AtVAMP711 AtVAMP712 and AtVAMP713 (R) were partly overlapped in the PVC, although these proteins are also found in another organelle, the TGN or the vacuolar membrane. The dual localization of these SNARE proteins indicates that they are cycled between the TGN and PVC or the PVC and the vacuole. AtVTI11 (Qb) was shown to form a SNARE complex with SYP2 (Qa) and SYP5 (Qc) in the PVC and vacuole, but has low affinity for SYP4 (Qa) and SYP6 (Qc) in the TGN, whereas AtVTI12 (Qb) forms a complex with SYP4 (Qa) and SYP6 (Qc) in the TGN (Sanderfoot et al., 2001; Yano et al., 2003). These results suggest that VTI11 is involved in the anterograde transport from the TGN to PVC/vacuole, while VTI12 functions in the retrograde transport from the PVC to TGN. This raises a question as to what types of R-SNAREs are involved in these complexes. Our newly characterized vacuolar R-SNAREs (AtVAMP711, AtVAMP712 and AtVAMP713) may form a ternary SNARE complex in the vacuole and PVC membranes.
SYP21 (Qa) is predominantly localized to the PVC membrane, while SYP22 (Qa) is located in both the PVC and vacuolar membrane (Fig. 6) (da Silva Conceicao et al., 1997; Sato et al., 1997; Uemura et al., 2002). The Arabidopsis sgr3 has been isolated as a mutant having a defect in the shoot gravitropic response. Positional cloning of sgr3 has shown that the SGR3 gene encodes SYP22 (Yano et al., 2003). These results suggest that SYP21 and SYP22 are not functionally redundant and have different roles in the vesicular transport to the PVC and vacuole. As the existence of two vesicular transport pathways to the vacuoles (wortmannin sensitive and insensitive) has been reported (Koide et al., 1999; Matsuoka et al., 1995), SYP21 and SYP22 might function independently in these two pathways.
Eighteen SNARE proteins are represented in the plasma membrane (Fig. 5), notably several Qa-SNAREs and R-SNAREs. These proteins are not considered to be tissue-specific isoforms because the expression of their genes was detected in all tissues examined in the RT-PCR analysis. In addition, these SNARE proteins do not seem to share a redundant function in vesicular transport to the plasma membrane, because mutations in some of these plasma membrane SNARE proteins cause particular defects in cytokinesis (SYP111) (Lauber et al., 1997; Volker et al., 2001) or pathogen resistance (SYP121) (Collins et al., 2003).
As to there are so many SNAREs on the plasma membrane, one possible explanation is that the plasma membrane-localized SNAREs may function in distinct transport pathways to the subdomains of the plasma membrane. In fact, SYP111/KNOLLE protein is specifically expressed during mitosis and recruited to the cell plate that is formed in the plane of cell division (Lauber et al., 1997; Lukowitz et al., 1996; Muller et al., 2003). Some integral membrane proteins are indeed localized to special subdomains of the plasma membrane. For instance, the auxin uptake carrier, AUX1, is localized to the apical domain of the plasma membrane, whereas the auxin efflux carrier, PIN1, shows basal localization (Galweiler et al., 1998; Geldner et al., 2003; Steinmann et al., 1999). COBRA, a GPI-anchored protein, and PIN3, another auxin efflux carrier, are localized to the lateral plasma membrane (Friml et al., 2002; Roudier et al., 2002; Schindelman et al., 2001). These proteins have to be delivered to distinct destinations on the cell surface via specific polarized vesicular transport pathways. Although Qa and R-SNAREs of the plasma membrane showed a ubiquitous distribution in the plasma membrane under our transient expression conditions, these SNAREs may be subjected to specific subdomain localizations in polarized cells in plants.
We demonstrated that the SYP41-residing organelle (TGN) does not always behave together with the Golgi apparatus. If the TGN could be defined as a separate organelle distinct from the Golgi apparatus in Arabidopsis cells, based on the distribution of SYP4 (Qa), VTI1 (Qb) and SYP6 (Qc), it is clearly not a continuation of the Golgi apparatus as has been constructed in mammals. In fact, Paris and coworkers have shown that the network structure of the TGN is not always seen at the trans-face of the Golgi apparatus, when optimal fixation procedures such as the high-pressure freezing are used (Saint-Jore-Dupas et al., 2004). According to the cisternal maturation model, each Golgi cisterna matures as it migrates outwards through a stack (Mironov et al., 1997; Pelham and Rothman, 2000). The TGN may detach itself from the Golgi apparatus after maturation. If such a separate nature of the plant TGN holds true, we may need to redefine this intracellular compartment as a novel organelle, not a special subregion of the Golgi complex, which might be the control center for the post-Golgi membrane traffic.
We thank T. Okamura for helpful discussion on the manuscript. We thank Y. Niwa for kindly providing the CaMV35S-sGFP(S65T)-NOS3’ GFP fusion plasmid. We also thank A. Miyawaki for the Venus plasmid. This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology: a Grant-in-Aid for Encouragement of Young Scientists, and Scientific Research on Priority Areas (B), the Toyota High-Tech Research Grant Program and Asahi Glass Foundation (M. H. S), and a Grant-in-Aid for JSPS Research Fellowships for Young Scientists (T. Uemura).
|