| To whom correspondence should be addressed: Kohji Takei, Department of Neuroscience, Okayama University Graduate School of Medicine and Dentistry, 2-5-1 Shikata-cho, Okayama 700-8558, Japan. Tel: +81–86–235–7120, Fax: +81–86–235–7126 Abbreviations: mRNA, messenger RNA; Na, Sodium; KKRK, Lysine-Lysine-Arginine-Lysine; RT-PCR, reverse transcription polymerase chain reaction; EDTA, ethylenediaminetetraacetic acid; EGTA, ethyleneglycol bis(2-aminoethylether) tetraacetic acid. |
Klotho mutant mouse (kl-/-) has been established as a model mouse for aging. In the mutant mouse, the coding region of klotho gene is preserved but nonfunctional gene is introduced within its promoter region, which results in markedly decreased expression of Klotho protein (Kuro-o et al., 1997; Takahashi et al., 2000; Nabeshima, 2002). The mouse exhibits a phenotype resembling human aging including infertility, arteriosclerosis, neural degeneration, skin and gonadal atrophy, pulmonary emphysema, calcification of soft tissues, and dies within 10 weeks (Kuro-o et al., 1997). In addition, recent analysis revealed cognition impairment in kl-/- mice (Nagai et al., 2003).
Klotho gene encodes 130 kDa membrane protein comprised of an amino-terminal signal sequence, a putative extracellular domain, and transmembrane domain near its carboxyl terminus. The extracellular domain contains a short stretch of basic amino acids (KKRK), which is proposed to be a proteolytic cleavage site. In addition to the membrane protein, klotho gene also encodes a splicing variant lacking the transmembrane domain, which is termed secreted isoform (Matsumura et al., 1998; Shiraki-Iida et al., 1998). Because of the presence of potential cleavage site in the membrane Klotho and the presence of secreted isoform, Klotho protein has been suggested to be a humoral factor, and actually, Klotho protein was recently detected in serum and cerebrospinal fluid (CSF) (Imura et al., 2004). While the phenotypes of kl-/- mouse are observed in a wide range of organs, klotho mRNA is expressed only in limited organs, i.e., brain, kidney, reproductive organs, and pituitary gland (Kuro-o et al., 1997).
Kl-/- mice exhibit electrolyte abnormalities such as hyperphosphatemia and hypercalcemia (Yahata et al., 2003), and the most of kl-/- phenotypes are recovered by feeding the mice with low phosphorus diet (Morishita et al., 2001). These findings suggest a strong linkage between Klotho protein and electrolyte homeostasis. However, it is poorly understood how Klotho protein is implicated in the function, partly because localization of Klotho protein in various organs is not fully examined.
In this study, we examined cellular and subcellular localization of Klotho protein in brain, kidney, and reproductive organs, where klotho gene has been detected by RT-PCR (Kuro-o et al., 1997). Klotho proteins were localized at choroid plexus in brain, and distal renal tubules in kidney. In reproductive organs, Klotho protein was expressed only in mature germ cells. We also examined brain and kidney of kl-/- mice, and found that the number of synapses was reduced at hippocampus, and that type IIa Na/phosphate (Pi) cotransporters were intracellularly translocated in kidney. Relations between the localization of Klotho protein and the morphological changes in these organs in kl-/- mice were discussed.
Six-week-old kl-/- mice and the control wild-type (WT) mice established at the Nabeshima’s laboratory (Kyoto University, Japan, Kuro-o et al., 1997) were purchased from CLEA Japan Inc. (Osaka, Japan). Samples for Western blot analysis were prepared from C3H mice purchased from the Department of Animal Resources of Okayama University (Okayama, Japan).
Rat monoclonal antibody KM2076 against 55–261 amino acids of human Klotho protein (Kato et al., 2000) was a gift from Kyowa Hakko Kogyo Co. Ltd. (Tokyo, Japan). Mouse monoclonal antibody against synaptophysin was purchased from Synaptic Systems (Göttingen, Germany). Rabbit polyclonal antibody against type IIa Na/Pi cotransporter was a gift from Prof. K. Miyamoto (Tokushima University, Tokushima, Japan). Secondary antibodies used were Cy3 conjugated goat anti-rat IgG (KPL Co. Ltd., Gaithersburg, MD), Rhodamine Red-X conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR), and Alexa Fluor 488 conjugated goat anti-rabbit IgG (Molecular Probes), 5-nm colloidal gold-conjugated goat anti-rat IgG (British Biocell International Ltd., Cardiff, U.K.), peroxidase-conjugated anti-mouse IgG (Pierce, Rockford, IL), and goat peroxidase-conjugated anti-rat IgG (Amersham Pharmacia Biotech, Freiburg Germany).
Mice were anesthetized with ether, and they were subjected vascular perfusion through ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB). Fixed tissues were cut into slices, and further fixed by immersion in the same fixative at 4°C overnight. The fixed tissues were cryoprotected by sequentially immersing in 12%, 15%, and 18% sucrose in 0.1 M PB, embedded in O.C.T. compound (Sakura Fine Technical Co. Ltd., Tokyo, Japan), and then quick-frozen in liquid nitrogen. Ten-micrometer-thick frozen sections were cut with Leica CM 1850 cryostat, and mounted on Silane-coated glass slides. For immunostaining, the sections were incubated with 17% goat serum in a dilution buffer (450 mM NaCl and 0.3% Triton X-100 in 20 mM phosphate buffer, pH 7.4) for 30 min to block nonspecific labeling. Sections were then incubated with the primary antibodies in the dilution buffer for 2 h. After being washed with the dilution buffer, the sections were incubated with the secondary antibodies in the dilution buffer for 1 h. Sections from the testis and ovary were counterstained with 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) (KPL Inc. Gaithersburg, MD). The sections were mounted in PermaFluor (Thermo, Pittsburgh, PA), and examined with Zeiss LSM 510 laser scanning confocal microscope or with Olympus BX60 fluorescence microscope.
For immuno-electronmicroscopy, mice were anesthetized with ether, and fixed by perfusion of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M PB (pH 7.4). The choroid plexus from the third ventricle was cut into small pieces, and further fixed with the same fixative. The fixed tissues were immersed in 2.3 M sucrose, 50% polyvinylpyrrolidone in 0.1 M PB (pH 7.4), for several hours, and then rapidly frozen in Reichert KF-80 Universal Cryofixation System. Frozen ultrathin sections (60–70 nm of thickness) were cut using Reichert Ultracut S ultramicrotome, and picked up on formvar- and carbon-coated nickel grids. For immunogold staining, the grids were washed with 0.1 M PB containing 0.1 M glycine and 1% bovine serum albumin (BSA), blocked with 1% BSA in 0.1 M PB for 10 min, and then incubated with the antibody KM2076 for 90 min at room temperature. Specimens were rinsed in 0.1% BSA-PB for 15 min, incubated for 30 min with the gold-conjugated goat anti-rat IgG, washed in 0.1% BSA-PB and 0.1 M PB, and fixed with 2% glutaraldehyde in 0.1 M PB for 10 min. After washing with 0.1 M PB and distilled water, the sections were further fixed with 2% OsO4 for 20 min, washed in distilled water, stained in a solution of 2% uranium acetate for 20 min, and embedded with 2.2% polyvinyl alcohol. The sections were observed with Hitachi H-7100 transmission electron microscope.
For conventional EM, hippocampi were dissected into small pieces, and fixed in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M PB (pH 7.4) for 2 h at 4°C. After washing in 0.1 M PB and 0.1 M sodium cacodylate buffer (pH 7.4) three times, the specimens were postfixed in 2% OsO4 in the same buffer for 2 h, block-stained with 2% uranyl acetate in water for 2 h, dehydrated through a series of ascending concentrations of ethanol, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with Hitachi H-7100 electron microscope. For morphometry of synaptic density, stratum lucidum at CA3 region of the hippocampus was randomly photographed, and the number of synapses in total area of 0.12 mm2 was counted.
Choroid plexus, testis and ovary obtained from 10 wild-type mice at 8-week-old were homogenized in the homogenizing buffer (0.25 M sucrose, 1 mM EDTA, 10 mM EGTA, 10 mM 2-mercaptoethanol, 20 mM Tris-HCl, pH 7.5) with a Potter-type glass-Teflon homogenizer at 800 rpm, 7 strokes. The homogenate was centrifuged at 100,000×g for 10 min, and the supernatant was centrifuged at 100,000×g for 1 h. The pellet was re-suspended in the homogenizing buffer, and added 1% Triton X-100. For hippocampus, post-nuclear supernatant of 6-week-old hippocampus was sampled in sampling buffer. Samples were boiled for 5 min and subjected to SDS-PAGE in 7.5% polyacrylamide gel and transferred electrophoretically to a nitrocellulose membrane. The membrane was blocked with 140 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl buffer (pH 7.4), containing 0.1% Tween 20 and 5% skimmed milk overnight, and incubated with rat primary antibodies for 2 h followed by incubation with the goat peroxidase-conjugated anti-rat or mouse IgG for 1 h. Bands were visualized by using the ECL Western blotting detection system (Amersham Bioscience UK Ltd., UK).
Expression of Klotho protein has been tested so far only in kidney and inner ear tissue (Kato et al., 2000; Kamemori et al., 2002). Since klotho gene is highly expressed in brain, kidney, testis, and ovary (Kuro-o et al., 1997), we examined the expression of Klotho protein in these tissues by Western blot. As shown in Fig. 1, Klotho protein was detected in all of the tissues tested. Therefore, localization of Klotho protein in each tissue was examined next.
 View Details | Fig. 1. Expression of Klotho protein (130-kDa) demonstrated by Western blot analysis. Samples were prepared from the kidney (lane 1), choroid plexus (lane 2), testis (lane 3), and ovary (lane 4). |
Epifluorescent microscopy of brain sections revealed that Klotho protein was exclusively present at the choroid plexus of both the lateral ventricles and third ventricle. By confocal microscopy, Klotho protein predominantly localized at the apical plasma membrane of ependymal cells, which faces to the ventricles (Fig. 2a). In order to further elucidate the precise localization of Klotho protein at the plasma membrane, immunogold electron microscopy was performed. As shown in Fig. 2b–e, the apical surface of the ependymal cells formed microvilli, and the immunogold particles were present mostly on the outside of the microvilli, suggesting that Klotho protein is oriented so that the amino acid residues 55–261 corresponding to the epitope of the antibody is extracellularly located.
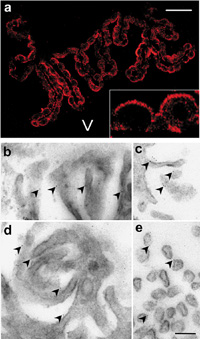 View Details | Fig. 2. Localization of Klotho protein in choroid plexus. (a) Confocal micrograph of choroid plexus section stained by immunofluorescence using KM2076 antibodies. Note strong immunoreactivity at the apical plasma membrane of the ependymal cells (inset). V: ventricle. Bar represents 50 μm, and 225 μm in the inset. (b–e) Immunogold electron micrograph of apical plasma membrane of ependymal cells. The immunogold particles are often present at extracellular side of the microvilli. Bar represents 200 nm. |
Choroid plexus produces CSF, which serves as extracellular fluid for neurons. Recently, secreted Klotho protein was detected in CSF (Imura et al., 2004), and in kl-/- mice, cognition is impaired (Nagai et al., 2003). These observations prompted us to investigate potential degenerative changes in kl-/- hippocampus. Expression of synaptophysin, a marker for synaptic vesicles, was reduced in kl-/- hippocampus (Fig. 3b). By immunofluorescent microscopy, immunoreactivity for synaptophysin was weaker in kl-/- hippocampus than in WT, and the reduction was especially evident at stratum lucidum, where mossy fiber nerve terminals are innervated (Fig. 3c). Morphometric analysis based on EM morphology revealed significant reduction of the nerve terminals in number at stratum lucidum of CA3 region (Fig. 3d). However, individual nerve terminals were normal in size, and contained a significant number of synaptic vesicles (Fig. 3e).
 View Details | Fig. 3. Reduction of the synapses in kl-/- hippocampus. (a) Drawing of coronal section of rodent brain demonstrating hippocampus in yellow. Arrow indicates CA3 region of hippocampus. (b) Western blot of WT and kl-/- hippocampus for synaptophysin. One hundred μg of proteins was loaded in each lane. (c) Confocal micrograph of WT and kl-/- CA3 region of hippocampus stained for synaptophysin, SL: stratum lucidum. Bar represents 20 μm. (d) Morphometric analysis of synaptic density at stratum lucidum at CA3 region. (e) Electron micrograph of synapse at stratum lucidum at CA3 region, showing little difference in numbers of synaptic vesicles. Bar represents 500 nm. |
In kidney, Klotho protein localized exclusively at the distal renal tubules (Fig. 4a). By confocal microscopy, the majority of immunoreactivitiy was recognized as fine puncta in the cytoplasm of the tubular epithelia, and accumulation of Klotho protein at the apical plasma membrane was not so evident as in ependymal cells in choroid plexus (Fig. 4b).
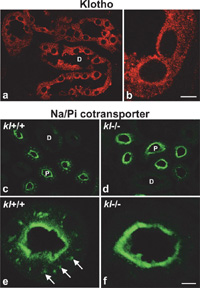 View Details | Fig. 4. (a, b) Confocal micrograph of distal renal tubule immunostained for Klotho protein. The immunoreactivity is observed as fine punctate present mainly in the cytoplasm. Bar represents 23 μm in (a) and 5 μm in (b). (c–f) Confocal micrograph of proximal renal tubule immunostained for type IIa Na/Pi cotransporter in WT and kl-/- mice. Note that the subpopulation of cotransporters present in the cytosol in WT (c, e, arrows in e), and absent in kl-/- (d, f). D: distal renal tubule; P: proximal renal tubule. Bar represents 25 μm in (c, d), 5 μm in (e, f). |
Kl-/- mice exhibit hyperphosphatemia (Yahata et al., 2003), and most of the phenotypes of kl-/- mice are recovered by putting on a low phosphorus diet (Morishita et al., 2001), suggesting that the mechanisms to maintain phosphate ion balance are distorted in kl-/- mice. As phosphate homeostasis is mainly regulated by reabsorption of phosphates by type IIa Na/Pi cotransporters in kidney (Biber et al., 1996; Murer et al., 2000), we examined the localization of type IIa Na/Pi cotransporters in WT and kl-/- mice. In the WT, the cotransporters were exclusively present at the proximal renal tubules, where the proteins were present not only at the apical plasma membrane (Fig. 4c, e), but also inside of the cells (Fig. 4e, arrows). Surprisingly, however, most of the type IIa Na/Pi cotransporters in the cytoplasmic pool were absent in kl-/- mice, suggesting translocation of the proteins to the apical surface (Fig. 4d, f).
Although klotho gene in mouse testis and ovary has been detected by RT-PCR (Kuro-o et al., 1997), the localization of Klotho protein has not been determined. Therefore, we examined the localization of Klotho protein in these organs. As shown in Fig. 5, Klotho proteins in testis were present only at the inner region of seminiferous tubules containing elongating spermatids (Fig. 5c, e). Immunofluorescent microscopy at higher magnification revealed that immunoreactivity for Klotho protein was present at the cytoplasm of elongating spermatids, and it was absent in spermatogonia, primary spermatocytes, round spermatids, and sertoli cells (Fig. 5g, h). Thus, expression of Klotho protein in testis was highly restricted to germ cells at the late stage of spermatogenesis. Likewise, Klotho proteins in ovary were exclusively present in oocyte of Graafian follicle, the most mature follicle (Fig. 6e), but the immunoreactivity was absent or very weak at primary and secondary follicles (Fig. 6a, c). In the mature oocyte, Klotho proteins were mainly localized at the plasma membrane but also present in the cytosol as a speckled pattern (Fig. 6g).
 View Details | Fig. 5. Immunofluorescent micrograph of seminiferous tubules demonstrating localization of Klotho protein at mature germ cells. The sections were counterstained for DAPI to identify spermatogenic stages. Seminiferous tubules at stage IX (a, b), IV (c, d), VII (e–h) are shown. Immunoreactivity for Klotho protein was evident in the elongating spermatids. Arrowheads in (h) point to spermatogonia (1), spermatocyte at pachytene stage (2), round spermatid (3), and elongating spermatid (4). Bar represents 45.5 μm in (a–f), 10 μm in (g, h). |
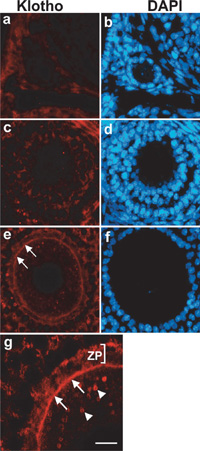 View Details | Fig. 6. Immunofluorescent micrograph showing expression of Klotho protein in ovary. Immunoreactivity for Klotho protein was absent or very weak in oocyte of primary (a, b), and secondary (c, d) follicles. In Graafian follicles (e–g), immunoreactivity for Klotho protein was detectable at the plasma membrane of the oocyte (arrows in e and g). At higher magnification, Klotho protein was also present in the cytosol as speckled pattern (arrowheads in g). ZP: zona pellucida. Bar represents 24 μm in (a–f), and 10 μm in (g). |
In this study we have demonstrated that Klotho protein was highly expressed at the choroid plexus in brain, where the protein was dominantly localized at the apical plasma membrane of the ependymal cells. By immunoelectron microscopy the antibody KM2076, which was raised against 55–261 amino acids of human Klotho protein, recognized the extracellular region of microvilli of the ependymal cells. This observation indicates that the putative extracellular domain of Klotho (Kuro-o et al., 1997) recognized by the antibodies is actually oriented extracellularly.
Despite the absence of Klotho protein at hippocampus, significant reduction of synapses was observed in kl-/- hippocampus (Fig. 3). In addition, cognition impairment in kl-/- mice was recently reported (Nagai et al., 2003). What causes these morphological and functional changes at the hippocampus? One possibility would be that Klotho proteins are secreted in the CSF and function as humoral factor. In fact, secreted Klotho protein was recently detected in the CSF (Imura et al., 2004). It is also conceivable that Klotho proteins enriched at the apical surface of ependymal cells, are cleaved and secreted in the CSF. Another possibility is that the function of choroid plexus in production of CSF and in transportation of calcium and phosphate ions into the CSF, is being disturbed by the lack of Klotho protein at the choroid plexus. Since CSF functions as extracellular fluid for the central nervous system, an unbalanced CSF might preferentially affect the hippocampus.
The type IIa Na/Pi cotransporters, which were normally localized both at the apical plasma membrane and in the cytoplasm of the proximal renal tubules in kidney, (Fig. 4c, e), were predominantly translocated to the apical plasma membrane in kl-/- mice (Fig. 4d, f). Thus, this is the first indication that the lack of Klotho protein causes a translocation of ion transporters. How does Klotho protein relate to the translocation of the type IIa Na/Pi cotransporters? The translocation of the cotransporters is also caused by parathyroid hormone (PTH) (Kempson et al., 1995; Lötscher et al., 1999), and PTH is down-regulated by serum Ca2+ level. Interestingly, Klotho protein is highly expressed in the chief cells in parathyroid and distal renal tubules in kidney (Kuro-o et al., 1997; Kato et al., 2000; Fig. 4 a and b in this study), which are implicated in PTH secretion and Ca2+ re-absorption respectively. Furthermore, in kl-/- mice, serum Ca2+ level is elevated (Yahata et al., 2003) and PTH is decreased (Yoshida et al., 2002; Tsujikawa et al., 2003). Thus, Klotho protein might regulate serum Ca2+ and PTH level, which in turn regulate the localization of the type IIa Na/Pi cotransporters at the proximal renal tubules. Considering the flow of primary urine from the proximal to distal renal tubules, and intracellular localization of Klotho protein in the distal renal tubules, it is unlikely that the Klotho protein at distal renal tubules functions as humoral factor. Feeding kl-/- mice with low phosphorus diet also induces translocation of the type IIa Na/Pi cotransporters from the cytosolic pool to the cell surface (Levi et al., 1994; Lötscher et al., 1997). It has been reported that kl-/- mice exhibit hyperphosphatemia (Yahata et al., 2003), and the phenotype is rescued by feeding kl-/- mice with low phosphorus diet (Morishita et al., 2001). Our finding that the type IIa Na/Pi cotransporters in kl-/- mice exclusively localize at the apical surface is consistent with the disorder of phosphate metabolism in the kl-/- mice.
In reproductive organs, Klotho protein was localized selectively in elongating spermatids in testis (Fig. 5) and at the plasma membrane of matured oocyte in Graafian follicle in ovary (Fig. 6e, g). In the kl-/- mouse, seminiferous tubules are extremely atrophic and spermatogenesis is arrested at pachytene stage, and the ovary lacks mature secondary or Graafian follicles (Kuro-o et al., 1997). Thus, while Klotho protein is present only in the most mature germ cells, maturation of germ cells in kl-/- mice is clearly impaired at earlier stages. This discrepancy can be attributed to the fact that the maturation of gonadal cells is regulated by gonadotrophic hormones released from anterior pituitary. Consistently, Klotho proteins are highly expressed in pituitary gland, and follicle-stimulating-hormone-producing cells in kl-/- mice are atrophic (Kuro-o et al., 1997). Furthermore, a recent finding that Klotho protein might function as an enzyme that catalyzes synthesis of steroid hormones (Tohyama et al., 2004) might additionally explain the discrepancy. Currently, intercellular signal released from matured germ cells is not known. Therefore, the target molecules of the Klotho protein in these organs remain to be elucidated.
In conclusion, Klotho protein not only functions as humoral factors to distant target organs, but also might be implicated in hormone release in hormone producing cells and indirectly regulate the target organs. Whether Klotho protein acts on the target directly as humoral factor, or indirectly via hormonal regulation, might depend on target organs. Due to its dual pathway of action, mutation of a single klotho gene may result in a variety of phenotypes.
We thank Prof. Y. Nabeshima (Kyoto University) for discussion and advice on this study. This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (to K. Takei).
|