| To whom correspondence should be addressed: Fumio Hanaoka, Graduate School of Frontier Biosciences, Osaka University, 1-3 Yamada-oka, Suita, Osaka 565-0871, Japan. Tel: +81–6–6879–7975, Fax: +81–6–6877–9382 Abbreviations: Ce, Caenorhabditis elegans; CPD, cyclobutane pyrimidine dimer; IPTG, isopropyl-1-thio-β-D-galactoside; NER, nucleotide excision repair; NGM, nematode growth medium; Pol, DNA polymerase; RNAi, RNA interference; TLS, translesion synthesis; XP, xeroderma pigmentosum. |
DNA can be damaged by a wide range of physical and chemical agents, both endogenous and environmental. To maintain the integrity of the genetic material, cells possess multiple pathways to repair various types of DNA damage (Friedberg et al., 1995a). Nucleotide excision repair (NER) is the main pathway involved in the removal of cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts produced by UV radiation, although CPDs are repaired fairly slowly by NER. Unrepaired damage can interfere with basic processes such as DNA replication and transcription and lead to mutations and cell death (Friedberg et al., 1995b).
In eukaryotes, another major pathway that circumvents such threats involves DNA polymerases that catalyze translesion synthesis (TLS) past DNA damage. Most TLS polymerases belong to the recently discovered Y-family (Ohmori et al., 2001). After UV irradiation, cultured cells from XP-V patients are defective in DNA replication and exhibit an increased mutation frequency with an altered spectrum (Lehmann et al., 1975; Maher et al., 1976; Wang et al., 1991, 1993; Waters et al., 1993; Stary et al., 2003). The XPV gene encodes human polη (Masutani et al., 1999a; Johnson et al.,1999) which bypasses CPDs by incorporating correct nucleotides (Masutani et al., 1999b, 2000; Johnson et al., 2000; Washington et al., 2001; Kusumoto et al., 2004; McCulloch et al., 2004), indicating that Polη is involved in the relatively accurate TLS pathway for bypass of UV-induced lesions in vivo and in vitro. In contrast, other TLS polymerases, such as Polι (Tisser et al., 2000a, 2000b; Frank et al., 2001), Polκ (Gerlach et al., 1999; Ogi et al., 1999; Ohashi et al., 2000a, 2000b), Rev1 (Nelson et al., 1996a; Lin et al., 1999a; Gibbs et al., 2000) and Rev3 with Rev7 (Polζ) (Lin et al.,1999b; Nelson et al., 1996b; Gibbs et al., 1998), are mutagenic polymerases for bypass of UV-induced lesions.
In XP-V patients, unrepaired UV-induced lesions are more likely to be bypassed by the mutagenic pathway, because the relatively accurate pathway is inactivated. As a result, XP-V patients are sensitive to sunlight and exhibit a concomitant increase in the incidence of cancer. However, the regulation of the TLS polymerases is still unclear (Kannouche and Stary, 2003; Fischhaber and Friedberg, 2005). The nematode C. elegans also has TLS polymerases such as Polη, Polκ, Rev1 (McDonald et al., 2001; Ohmori et al., 2001) and Rev3 (Lawrence, 2002), making this model multicellular organism an effective tool to explore DNA repair pathways relevant to TLS in vivo. However, at present little detailed information is available about C. elegans Y-family polymerases.
Here, we examined the sensitivity of C. elegans to UV radiation at various stages of embryogenesis using a feeding RNAi method. We show that suppression of Ce-POLH in early embryogenesis and gametogenesis stages confers hypersensitivity to UV radiation but that suppression at later stages confers only modest sensitivity. Our data suggest a possible role for Ce-Polη in damage tolerance during embryogenesis and gametogenesis.
Wild type N2 worms were grown on nematode growth medium (NGM) plates seeded with E. coli OP50 at 20°C (Sulston and Hodgkin, 1988).
To prepare the full-length coding region of F53A3.2, a DNA fragment containing the complete cDNA sequence was amplified by PCR from a C. elegans embryonic cDNA library, kindly provided by Dr. Hideyuki Okano (Department of Physiology; Keio University School of Medicine), using the following primers, Ce-POLH-1: 5'-GGT TAC TAG TAT GAA AAG AGT GAT CAG CCT CAT-3' and Ce-POLH-2: 5'-GGT TCC CGG GTT AGG GTT TCT TCT TTT TGA AAA-3', containing nucleotides 26349–26327 and 21212–21234 of the F53A3 cosmid clone (accession number: AF025460), respectively. PCR was performed as follows: initial denaturing at 94°C for 1 min, followed by six cycles of 94°C for 30 s, 51°C for 30 s, and 72°C for 1.5 min, in which the annealing temperature was decreased 1°C every two cycles until it reached 49°C; followed by 24 cycles of 94°C for 30 s, 47°C for 30 s, and 72°C for 1.5 min; with a final elongation at 72°C for 10 min. PCR products were loaded onto a 1% agarose gel and purified using the QIAEX II gel extraction kit (QIAGEN) according to the manufacturer’s instructions. Ce-POLD fragment was amplified by PCR using the following primers, Ce-POLD-F: 5'-GGT AAC TAG TAT GAC CTC CAA GCG TCC TG-3' and Ce-POLD-R: 5'-CGA TCC CGG GTG GCA GTG ATA GAA CTT TAG CAC G-3', containing nucleotides 18897–18915 and 20269–20246 of the F10C2 cosmid clone (accession number: Z81497), respectively. PCR was performed as follows: initial denaturing at 94°C for 1 min, followed by six cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 1.5 min, in which the annealing temperature was decreased 1°C every two cycles until it reached 52°C; followed by 24 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1.5 min; with a final elongation at 72°C for 10 min. PCR products were digested with Spe I and Sma I and cloned into pBlueScript SK (Stratagen). The cloned products were sequenced on both strands with a BigDye terminator cycle sequencing kit (Applied Biosystems). Multiple sequence alignments were compiled using Clustal W <www.ebi.ac.uk/clustalw/>.
Adult hermaphrodites were collected, suspended in 100 μl disruption buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, pH 8.0 and 0.02% lysozyme), quickly frozen at –80°C for 10 min, and incubated at 37°C for 3 h. Total RNA was extracted using the Total RNA Isolation kit (Macherey-Nagel, Inc.) according to the manufacturer’s instructions, and then detected by RT-PCR using the following primers, Ce-POLH-3: 5'-AAA GCG GCG ATT TAC GAT G-3', and Ce-POLH-4: 5'-TTT TGG TGT TGG ATT CGG C-3', corresponding to nucleotides 22460–22442 and 22311–22329 of the F53A3 cosmid clone, respectively. The same primers were used to amplify fragments from cDNA clone derived from C. elegans embryonic cDNA libraries. Amplified fragments were electrophoresed on a 15% acrylamide gel and visualized under UV light.
The vector L4440 (pPD129.36), which contains two convergent T7 RNA polymerase promoters in opposite orientation, and the bacterial strain HT115 (DE3) (W3110, rnc14::ΔTn10), which has an IPTG-inducible T7 RNA polymerase gene and is deficient in RNase III, were kindly provided by Dr. A. Fire (Carnegie Inst., Washington). RNAi induced by the feeding method was performed as described (Kamath et al., 2000; Timmons et al., 2001). Briefly, the plasmids described above were digested with Spe I and Sma I. Similarly digested PCR products were inserted between the two T7 RNA polymerase promoters of L4440 (pPD129.36) and the resulting plasmids were transformed into HT115 (DE3) cells. IPTG (1 mM) was added to transformed cells at log-phase and these were spread onto NGM agar plates containing 1 mM IPTG and 50 μg/ml ampicillin, which were incubated for 24 h at 20°C and then used as RNAi plates. As a control, HT115 (DE3) cells transformed by the L4440 vector were plated. Worms at the young adult stage were placed on these plates and transferred to fresh RNAi plates after 12 h feeding. To investigate the effects of Ce-POLH RNAi after UV irradiation, F1 eggs laid over the next 5 h and young adult F1 worms were irradiated with a germicidal lamp (254 nm) at doses of 0, 5, 10, 25, 50 J/m2. UV sensitivity was examined for the hatching abilities of the UV irradiated F1 eggs and F2 eggs laid by UV irradiated young adults. Hatching rates were scored 36 h after eggs were laid.
The absence of Ce-POLH transcripts following RNAi was examined by RT-PCR using the primers Ce-POLH-5: 5'-CGA ACT GCC GAG AAT CTG G-3' and Ce-POLH-6: 5'-GCG TGT CAA AAT TGT ACA GAA GTA AT-3', corresponding to nucleotides 23889–23871 and 21184–21209 of the F53A3 cosmid clone, respectively. As a control, ACT-1 transcripts were examined using the primers Ce-ACT-1-F: 5'-CGT GGT TAC TCT TTC ACC ACC ACC GCT G-3' and Ce-ACT-1-R: 5'-CAT TTA GAA GCA CTT GCG GTG AAC GAT GG-3', corresponding to nucleotides 29624–29651 and 30221–30193 of the T04C12 cosmid clone (accession number: Z81584), respectively. RT-PCR was performed as described (Hashmi et al., 2002). RNAi-treated adult hermaphrodites were collected, suspended in 100 μl disruption buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, pH 8.0 and 0.02% lysozyme), quickly frozen at –80°C for 10 min, and then incubated at 37°C for 3 h. RNA was extracted using the Total RNA Isolation kit (Macherey-Nagel, Inc.), and RT-PCR was carried out using the SuperScript One-Step RT-PCR System (Invitrogen) according to the manufacturer’s instructions. Each 50-μl reaction mixture was split into two tubes into which either Ce-POLH or Ce-ACT-1-specific primers were added. After 30–40 cycles of amplification, the RT-PCR products were separated on 1% agarose gels.
Information provided by the genomic sequencing consortium indicated that C. elegans has three Y-family DNA polymerases, Polη, Polκ and Rev1. Polη is encoded by the gene F53A3.2 and based on the genomic sequence was predicted to be composed of eight exons. This sequence was used to obtain Ce-POLH cDNA by PCR of embryonic cDNA libraries with primers that included the predicted start (Ce-POLH-1) and stop codons (Ce-POLH-2). Sequencing of the cDNA clone thus obtained (accession number: AB244413) revealed that it has a 57-bp deletion in predicted exon 7 with respect to the genomic sequence (Fig. 1B). To confirm whether Ce-POLH mRNA lacks these nucleotides, we performed RT-PCR with total RNA isolated from adult worms and primers flanking the deletion (Ce-POLH-3 and Ce-POLH-4). As shown in Fig. 1C, RT-PCR (lane 1) gave rise only to a 93-bp PCR product which lacks the 57-bp sequence found in genomic DNA, as did PCR (lane 2) of the cDNA clone obtained from the C. elegans embryonic cDNA library. The corresponding amino acids encoded by the 57-bp sequence are also absent in cDNA sequences of human and Drosophila melanogaster Polη proteins. In fact, this region includes a splice site consensus sequence. Taking these results together, it is likely that the Ce-POLH gene consists of nine exons (Fig. 1A) and encodes a protein of 584 amino acids which shares 30% and 26% identity with the human and Drosophila melanogaster Polη proteins, respectively (Fig. 1D).
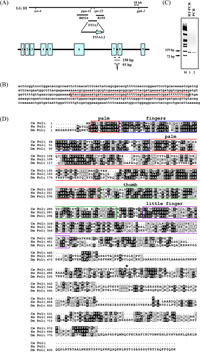 View Details | Fig. 1. Gene and domain structure of Ce-POLH. (A) Genomic map of linkage group III (LG III) in relation to F53A3.2 and the intron/exon structure of the Ce-POLH gene. The gene F53A3.2, which is located between the pqn-41 and rps-22 loci, consists of nine exons, instead of the eight exons previously assumed. Primer positions (arrow heads) used for RT-PCR and product sizes are indicated below the exon schematic. (B) Nucleotide sequence of exon 7 of the Ce-POLH gene. The 57 bases surrounded by red box are deleted in the cDNA clone derived from the cDNA library. (C) RT-PCR with total RNA from adult worms and PCR of the cDNA clone derived from the cDNA library. M, φX174 HaeIII DNA size marker. The figure is the negative image of an ethidium bromide-stained agarose gel. (D) A comparison of Ce-Polη (accession number: AB244413) with the human Polη (AB024313) and the Drosophila melanogaster Polη (AB049433) proteins. Identical and similar residues are shaded in dark and light gray, respectively. Palm (red), fingers (blue), thumb (green), and little finger (purple) domains are marked with boxes. The asterisk indicates the position of the 19 a.a. (57 bp) deletion. |
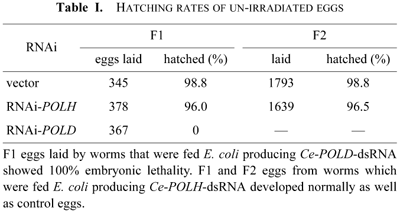
To investigate the role of Polη in C. elegans, Ce-POLH gene expression was suppressed by feeding worms with E. coli strains that contain plasmid vectors designed for the production of dsRNA by the bidirectional transcription of inserts (Timmons and Fire, 1998). Worms at the young adult stage were fed on E. coli producing dsRNA specific to Ce-POLH or control E. coli transformed by the vector alone. F1 embryos from these RNAi-treated worms were also fed on RNAi plates. To confirm the absence of Ce-POLH transcripts following RNAi treatment, RT-PCR using specific primers for Ce-POLH and for a control, ACT-1, which encodes the body wall actin gene, was carried out with total RNA prepared from RNAi-treated F1 adult worms. Fig. 2 shows that POLH mRNA is depleted in Polη-targeted adults (lane 3) as compared with control worms (lane 1), while ACT-1 mRNA is expressed in both control and treated animals (lanes 2 and 4). F1 and F2 progeny from worms which were fed E. coli producing Ce-POLH-dsRNA hatched and grew normally, but eggs laid by worms which were fed E. coli producing Ce-POLD-dsRNA did not hatch (Table I). These results suggest that the replicative DNA polymerase, Polδ, is essential for C. elegans embryogenesis, as expected, but that the TLS polymerase Polη is not.
 View Details | Fig. 2. RT-PCR of total RNA isolated from F1 adult worms treated by RNAi (Ce-POLH or control vector). PCR primers were specific to the endogenous Ce-POLH gene (Ce-POLH-5 and Ce-POLH-6) or to an internal control gene (Ce-ACT-1-F and Ce-ACT-1-R). Lanes 2 and 4, control Ce-ACT-1 gene mRNA. Lanes 1 and 3, Ce-POLH mRNA. M, 1 kb DNA size marker. The figure is a negative image of an ethidium bromide-stained agarose gel. |
The UV sensitivity of RNAi-treated worms was determined by monitoring the hatching rates of F2 embryos. The experimental scheme is shown in Fig. 3A. When previously laid eggs were UV-irradiated, Polη-targeted embryos were only modestly sensitive compared with untargeted embryos (Fig. 3B). In contrast, Polη-targeted F2 eggs laid 0–8 h after irradiation were clearly sensitive (Fig. 3C). In this case, eggs were irradiated while they were at the 32-blastomere stage and at the diakinesis-diplotene stages of oogenesis (Takanami et al., 2003). These results suggest that Polη significantly contributes to damage tolerance in C. elegans in early embryogenesis but less so in later stages. As early embryogenesis is characterized by high levels of replication, TLS by Polη may play an important role at this time. Interestingly, eggs laid 8–24 h after irradiation, which were exposed at the pachytene stage, also clearly showed UV sensitivity (Fig. 3D). To examine the contribution of Polη to damage tolerance in specific stages during embryogenesis in more detail, eggs at various developmental stages were irradiated and hatching rates were determined. The progression of gametogenesis and embryogenesis in C. elegans is conventionally classified into six periods (Fig. 4A) (Takanami et al., 2000). In control eggs, radiation sensitivity changed slowly throughout development, such that later-stage embryos were more sensitive than younger embryos (Fig. 4B). Periods II and III were the most UV-sensitive stages of Ce-POLH RNAi-treated eggs, but period I embryos were also somewhat sensitive (Fig. 4B and 4C). This result suggests that the genomic stability of fertilized eggs in early embryogenesis may depend on an accurate pathway using TLS to rapidly bypass DNA damage.
 View Details | Fig. 3. Effects of Ce-POLH RNAi on the UV sensitivity of hatching. (A) Schematic of RNAi experiments. Young adults were placed on RNAi plates that contained bacteria producing Ce-PolH-dsRNA. After 12 h feeding, the worms were transferred to fresh RNAi plates. F1 eggs laid over the next 5 h were used for experiments. Young F1 adults were transferred to fresh RNAi plates, and the hatching rates of F2 eggs irradiated with UV 0–5 h after laying, 0–8 h before laying, and 8–24 h before laying were determined. (B) F2 eggs laid over the course of 5 h by F1 worms were UV-irradiated and hatching rates were scored. (C) Young F1 adults were UV-irradiated, and the hatching rates of F2 eggs laid up to 8 h after irradiation were scored. (D) The hatching rates of F2 eggs laid during 8–24 h after irradiation were scored. Over 100 eggs were tested for each UV dosage in a single set of experiments, and each experiment was repeated three times. Error bars indicate standard deviations. Closed circles: control worms; closed triangles: Ce-POLH RNAi-treated worms. |
 View Details | Fig. 4. UV sensitivities at various embryonic stages. (A) Schematic of gametogenesis and early embryogenesis in a young gravid hermaphrodite. Gametogenesis and embryogenesis are conventionally classified into six periods as described in (Takanami et al., 2000). The eggs in period I (5 h after laying) contain ~100–550 nuclei and are at the stage from gastrulation to early morphogenesis. The eggs in period II (–0.5 h to 0 h after laying, with time 0 corresponding to the laying of the first egg) are at the early cleavage stage. The eggs in period III (–4 h to –0.5 h after laying) are at the stage from full grown oocytes and to fertilization and early cleavage. The eggs in period IV (–8 h to –4 h after laying) are at the oocyte to late pachytene stage. The eggs in period V (–16 h to –8 h after laying) and VI (–24 h to –16 h after laying) are at the pachytene nucleus stage. (B) UV sensitivity of eggs during periods I–VI. The survival of eggs laid during periods I–VI by a Ce-POLH RNAi hermaphrodite following UV irradiation of 10 J/m2. Closed circles, eggs from control worms; closed triangles, eggs from Ce-POLH RNAi worms. Independent triplicate experiments were carried out and the averages of scores from periods I–VI were 22, 3, 15, 18, 46 and 49 eggs per adult worm. Error bars indicate standard deviations. (C) Relative UV sensitivity of eggs during periods I–VI. The hatchability for eggs from Ce-POLH RNAi worms in Fig. 4B is expressed with respect to that for eggs from control worms in Fig. 4B as 100%. |
In this paper, we examined the roles of the C. elegans XPV/POLH homologue by the RNAi method. The embryos laid by worms treated by RNAi (Ce-POLH) were not significantly different from those of control worms, and larvae did not show any defects and further development into adults was normal. This result suggests that Ce-Polη is not essential under normal growth conditions. On the other hand, we showed that the down-regulation of Polη in C. elegans results in an increased sensitivity to UV radiation by several development and differentiation processes, including meiosis and embryogenesis. It has been reported that wild-type C. elegans embryos are significantly more resistant to the effects of UV radiation on DNA synthesis than are those of other organisms (Hartman and Nelson, 1998). For example, the UV dose required to reduce DNA synthesis by 50% in wild type C. elegans is 200 J/m2, a dose that in mammalian cells results in the rapid and permanent cessation of DNA synthesis (Hartman et al., 1991). NER is the main pathway involved in the removal of CPDs and (6-4) photoproducts induced by UV irradiation. However, in mammalian cultured cells, removal of (6-4) photoproducts is very fast, but CPD repair is much slower (Friedberg et al., 1995b). In contrast, the kinetics of repair of CPDs and (6-4) photoproducts in C. elegans from embryos to adults were similar to, if not slower than, that of CPDs in cultured human cells (Hartman et al., 1989). These data suggest that UV resistance in C. elegans embryos may depend on TLS, which rapidly bypasses DNA damage, in addition to the removal of damage by the NER system. Recently, it was reported that RNAi of the C. elegans homologues of human XPA (Park et al., 2002) and human XPF (Park et al., 2004), which participate in the NER pathway, increases the UV sensitivity of C. elegans embryos. It was also shown that down-regulation of CSB, which plays an important role in a NER subpathway, transcription-coupled excision repair, inhibits germ cell proliferation and enhances apoptosis after UV irradiation (Lee et al., 2002). However, embryos from worms in which Ce-POLH expression is suppressed are much more sensitive to UV irradiation than are embryos in which XPA (Park et al., 2002), XPF (Park et al., 2004) or CSB (Lee et al., 2002) is suppressed. For example, the hatching rate of eggs from Ce-POLH RNAi worms irradiated with UV (50 J/m2) was 61–64% lower than that of eggs from control worms (Fig. 3C and D), whereas the hatching efficiency of eggs from worms in which XPA (Park et al., 2002), XPF (Park et al., 2004) or CSB (Lee et al., 2002) was suppressed was only about 15% lower at the same UV dose. In rat male germ cells, intact early spermatocytes exhibit no or low NER activity, although cell extracts from early spermatocytes show high levels of incision activity. In contrast to somatic cells, little is known regarding the inhibition of the mutagenic effects of DNA damage in germ cells (Jansen et al., 2001). Rapidly proliferating germ cells and embryonic cells may be more dependent on Polη-mediated TLS than on NER in response to UV-induced DNA damage.
For various embryonic stages, we examined the effects of Ce-POLH RNAi on sensitivity to UV at a dose of 10 J/m2 (Fig. 4B). The most UV-sensitive stages were periods II and III, which include zygote formation, early cleavage, and establishment of embryonic axes, and the determination of somatic and germ-line founder cell fates after fertilization (Wood, 1988). In early C. elegans embryos, it was reported that the correct execution and timing of cell division is essential for proper cell fate patterning (Encalada et al., 2000). Conversely, embryos in period I, which covers the stage from gastrulation to early morphogenesis (Wood, 1988), were only moderately sensitive compared with controls (Fig. 3A, 4B and 4C), suggesting that Polη only modestly contributes to damage tolerance at this stage. Differences in these developmental stages may reflect distinct pathways that circumvent the effects of DNA damage. It was reported that Ce-rdh-51, the RAD51 homologue of C. elegans, contributes to damage tolerance after X-ray irradiation most largely in meiotic pachytene nuclei (Takanami et al., 2000). Although stage-specific activities of NER have not been elucidated, it may be possible that alternative DNA repair pathways express in a stage specific manner.
In summary, our observations suggest that Ce-Polη contributes to damage tolerance against UV irradiation to ensure the successful completion of embryogenesis, thus providings important insights into its role in DNA damage tolerance in germ and embryonic cells. C. elegans treated by RNAi is a good model system to investigate DNA repair in germ and embryonic cells. A drawback of this model system is that UV irradiation does not induce tumors in worms, regardless of the level of Ce-POLH expression. To further understand the role of Polη, we are studying XPV-deficient mice in detail.
We thank Dr. Hideyuki Okano for providing the C. elegans embryonic cDNA library and Dr. Andrew Fire (Carnegie Inst. Washington) for providing the bacterial strain HT115 (DE3) and the vector L4440 (pPD129.36). We are grateful to Dr. Yoshiaki Ohkuma, Dr. Masayuki Yokoi, and other members of Dr. Hanaoka’s laboratory at Osaka University for helpful discussions. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Human Frontier Science Program, and by Solution Oriented Research for Science and Technology (SORST) from the Japan Science and Technology Agency.
|