| To whom correspondence should be addressed: Takenori Yamada, Department of Physics (Biophysics Section), Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. Tel: +81–3–5228–8228, Fax: +81–3–5261–1023 E-mail: yamada@rs.kagu.tus.ac.jp Abbreviations: AFM, atomic force microscope; EGTA, ethyleneglycol(bis) tetraacetic acid; MHC, myosin heavy chain; PTR, phalloidin-tetramethyl-rhodamine; TRITC, tetramethylrhodamine-5-(and-6)-isothiocyanate. |
Skeletal muscle fibers are composed of bundles of myofibrils in which series of sarcomeres run from end to end along the fiber axis. In each sarcomere (Fig. 1), the contractile force is produced by the interaction between the myosin heads and the actin filaments in the overlap region of thin actin filaments and thick myosin filaments (Squire, 1981). In order for muscle fibers to stably develop the contractile force, the molecular assembly of each sarcomere in myofibrils should not collapse during contraction so that the myosin heads extruded from the thick filaments can specifically interact with the actin filaments. It is known that the sarcomere striations of myofibrils are kept uniform during contraction and relaxation cycles (Yang et al., 1998; Wakayama and Yamada, 2000), and, under specific conditions, during changes in oscillation (Yasuda et al., 1996b). X-ray studies of skeletal muscle fibers indicate that the lattice structures and the periodicity of actomyosin filaments components are well preserved in relaxed and contracting muscle fibers (Huxley and Brown, 1967; Wakabayashi and Yagi, 1999). Thus both the overall structures and the molecular configurations of actomyosin lattice structures are kept intact in dynamic fashion in muscle fibers under various physiological conditions. On the other hand, it is known that muscle fibers are split by extensive contractions. Further, in myofibril preparations, the sarcomere striation becomes irregular after repeated contractions, and local damage to sarcomeres develops to cause the eventual splitting of myofibrils (Bartoo et al., 1993; Wakayama and Yamada, 2000). However it is not known in detail how strong the sarcomere structure is relative to the mechanical stress produced by the contractile force.
 View Details | Fig. 1. Schematic sarcomere structures of skeletal muscle. (A) Longitudinal section. M and Z represent M-line and Z-disk respectively. (B) Transverse section at the overlap region of thin and thick filaments. Large and small spots respectively represent thick myosin filaments and thin actin filaments. |
Recently it has become possible to examine the mechanical properties of cellular components at the molecular level by employing submicromanipulation techniques (Kojima et al., 1994; Miyata et al., 1996; Yoshikawa et al., 1999; Nishizaka et al., 2000; Nyland and Maughan, 2000; Li et al., 2002). In the present studies, the mechanical strength of sarcomere structures of skeletal muscle was studied by rupturing peripheral structures of single myofibrils by use of optical tweezers (Block, 1990) and atomic force microscope (AFM) (Heckl and Marti, 1998).
The experimental chamber for AFM experiments was a trough cut in a silicone rubber plate glued on a coverslip (10×5 mm2 and 3 mm in thickness). On the other hand, the experimental chamber for optical tweezers experiments was a flow chamber composed of a pair of spacers of 100 μm in thickness placed in parallel at the distance of 5 mm on a coverslip (Wakayama et al., 2002). The chamber was covered with another coverslip during experiments. Before each experiment, the coverslip of the experimental chambers was treated with 3 M KOH solution, rinsed with distilled water, and then treated with aminosilane following the method of Idilis et al. (2000). During experiments, the experimental chamber was fastened to a built-in stage for the optical microscope which could be moved in 3D directions with an accuracy of ca. 1 nm with piezo units (P753-11C, Physik Instrumente, Germany) driven by piezo drivers (E-409.C3/E-503.00, Physik Instrumente, Germany). All measurements were made at room temperature (24–26°C).
The care of the animals and the experimental protocol were approved by the Animal Care and Use Committee of Tokyo University of Science. Single myofibrils were prepared from glycerinated fibers of rabbit psoas muscle by homogenization (Yoshikawa et al., 1999). Myofibrils were attached to the bottom coverslip by putting a drop of a suspension of myofibrils in a relaxing solution (133 mM K+-propionate, 5 mM MgCl2, 5 mM ATP, 10 mM ethyleneglycol(bis)tetraacetic acid (EGTA), 20 mM imidazole, pH 7.0) onto the coverslip. During the experiments, the experimental chamber was filled with a relaxing solution. These myofibrils are called relaxed myofibril preparations, and had a sarcomere length of about 2.2 μm and a diameter of about 1 μm. Rigor myofibrils were prepared from relaxed myofibril preparations by replacing the bathing solution with a rigor solution (143 mM K+-propionate, 5 mM MgCl2, 10 mM EGTA, 20 mM imidazole, pH 7.0). Trypsin- and calpain-treatments of myofibrils were made as detailed in Akiyama et al. (2006). Trypsin-treated myofibrils were prepared by incubating rigor myofibril preparations for 20 min in the experimental chamber filled with the rigor solution containing 0.25 μg/ml of trypsin. Calpain-treated myofibrils were prepared by incubating rigor myofibril preparations for 20 min in the experimental chamber filled with a solution of 50 mM K+-propionate, 10 mM CaCl2, 20 mM imidazole (pH 7.0) containing 0.15 mg/ml calpain. After the experimental chamber was well rinsed with the rigor solution, it was filled with fresh rigor solution during experiments.
SDS-PAGE analysis of myofibril preparations was made following the method of Kimura et al. (1992) as detailed in Akiyama et al. (2006). Myofibril preparations were dissolved in an SDS solution and an aliquot of the solution was applied to a gradient gel (2.5–12%). After the electrophoresis, gels were stained with Coomassie Brilliant blue for analysis.
Fluorescent actin filaments were prepared by incubating actin filaments with phalloidin-tetramethyl-rhodamine (PTR) (Wakayama et al., 2002). Fluorescent myosin filaments were prepared as follows. Native myosin and myosin rod were prepared by the method of Margossian and Lowey (1982). Myosin rod was fluorescently labeled with tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC) following the method of Nagashima (1986). Fluorescent myosin filaments were prepared by mixing solutions of native myosin and of fluorescent myosin rod in 0.3 M KCl, 5 mM MgCl2, 0.1 mM CaCl2, 25 mM HEPES (pH 7.0) and diluting the KCl concentration stepwise to 0.1 M to copolymerize myosin filaments and myosin rods.
To utilize the specific binding of α-actinin, a major component of Z-disk, with actin filaments (Miyata et al., 1996), α-actinin-coated beads were prepared following the method of Yasuda et al. (1996a). A suspension of carboxylated-polystyrene microbeads (1.9 μm in diameter; Polysciences, Warrington, PA, USA) was diluted to 1% (v/v) in 0.2 M Na+-borate (pH 8.5) containing 0.19 mg/ml α-actinin and 5 μg/ml BSA. After incubating the mixture for 15 min, beads were washed with the rigor solution by centrifugation and used for experiments.
Optical tweezers experiments were made as detailed in Wakayama et al. (2002). An optical tweezers (Sigma-Koki, Saitama, Japan) used was installed in an inverted optical microscope (IX70; Olympus, Tokyo, Japan). A light beam emitted from a Nd:YVO4 laser (1.06 μm in wavelength; Spectra Physics, Mountain View, CA, USA) was guided through the optical microscope and focused on the experimental chamber via an ocular lens (x100 oil, Apo. NA=1.35; Olympus, Tokyo, Japan). Beads were captured at the focus and moved in 3D directions by use of a motor-driven Galvano-mirror system (Sigma-Koki, Saitama, Japan). Beads were observed with a CCD-camera (C-3077; Hamamatsu Photonics, Shizuoka, Japan), and their images stored on a videotape recorder (AG-7355; Panasonic, Osaka, Japan). The xy-positions of beads were determined on a computer (Macintosh, Apple, USA) by employing a VHS-PC converter (LG-3, Scion, Tokyo, Japan) and a computer program (NIH Image, USA). The spring constant and the maximal trapping force of the optical tweezers were estimated, following the method of Block et al. (1989), to be 0.1–0.2 pN/nm and about 70–80 pN, respectively.
AFM experiments were made by use of an AFM (NV-2500; Olympus, Tokyo) incorporated in another inverted optical microscope (IX-70; Olympus, Tokyo, Japan) (Yoshikawa et al., 1999). AFM cantilevers having an aminosilanated whisker-tip were prepared as follows. A small amount of ZnO whiskers was put into a solution of 5% (v/v) aminopropyldimethylethoxysilane in 93% (v/v) ethanol. After incubating it for about 15 min, the whiskers were rinsed with ethanol and then with distilled water, and dried before use. The aminosilanated ZnO whisker was glued to the tip of cantilever (OMCL-TR400PSA-HW; Olympus, Tokyo, Japan; the resonance frequency of 11 kHz and the elastic constant of 20 pN/nm) with epoxy resin by use of micromanipulators (Narishige, Tokyo, Japan) under optical microscope. ZnO whiskers glued at the tip of cantilever were positively charged and firmly adhered to α-actinin-coated beads.
In the present experiments, sarcomere structures of myofibrils were ruptured by detaching beads attached to the surface of myofibrils by use of the AFM and optical tweezers. After capturing beads by use of either AFM or optical tweezers, the detachment of beads was made by retracting myofibrils from the beads by moving the experimental chambers. In the AFM experiments, myofibrils were moved downwards so that beads were retracted in the vertical direction from the focal plane. By contrast, in the optical tweezers experiments, myofibrils were moved horizontally so that beads were retracted perpendicularly from the surface of myofibrils in the focal plane of the optical microscope. These experimental systems can be simplified by a mechanical-equivalent model as shown in Fig. 2A, where a filamentous component at the surface of myofibrils is picked up by a spring. Note that the figure does not represent the actual retraction direction in the optical tweezers experiments. As the myofibril is retracted downward (X), the spring is elongated (ΔY) producing a force-distance curve which is a function of the force F (=kΔY) and the distance of retraction X where k is the elastic constant of the spring. When the selected component produces ruptures at sites A and B as shown in the right panel of Fig. 2A, for example, the corresponding rupture signals appear in the force-distance curve at a and b, respectively (Fig. 2B). The elastic constant of the component that was picked up can be calculated from the slope of force-distance curves. When the spring gets broken during the above procedure, or when beads were bound to myofibrils with greater strength than the trapping force, the force-distance curves become out of scale as shown by the dotted line. Myofibrils were retracted at a velocity of 0.1–0.3 μm/s, comparable to slow shortening velocities of muscle fibers (Squire, 1981), taking into consideration that rupture forces depend on the velocity of retraction (Hummer and Szabo, 2003).
 View Details | Fig. 2. Experimental protocols of rupturing myofibrils. (A) A simplified mechanical-equivalent model showing the rupture experiment. With capturing beads attached to myofibrils by use of either AFM or optical tweezers, myofibrils were retracted from the beads in a vertical direction in AFM experiments and in a horizontal direction in optical tweezers experiments. See the text for details. (B) A schematic force-distance curve showing rupture signals at a and b associated with ruptures at A and B in myofibril. The rupture starts and is finalized at s and f, respectively. The dotted trace occurs when the rupture is interrupted at i. For further details, see the text. |
Trypsin, calpain, α-actinin, BSA, ATP, and PTR were purchased from Sigma Chemicals (St. Louis, MO, USA), and TRITC from Molecular Probes (Eugene, OR, USA). Other chemicals were of analytical grade and purchased from Wako Chemicals (Osaka, Japan).
To establish the experimental approaches used in the present studies, first we examined whether or not actin filaments were exposed to the surface of single myofibrils. This was verified by utilizing the specific binding between actin and myosin filaments in a rigor state (Squire, 1997). After myofibrils were attached to the bottom coverslip of the experimental chamber, a rigor solution containing fluorescent myosin filaments was introduced into the experimental chamber. Under fluorescence optical microscope, it was observed that the fluorescent myosin filaments were attached to the surface of myofibrils in parallel to the fiber axis (Fig. 3A). In separate experiments, a rigor solution containing fluorescent actin filaments in place of fluorescent myosin filaments was introduced into the experimental chamber. It was observed that the fluorescent actin filaments were similarly attached to the surface of myofibrils in parallel to the fiber axis (data not shown). These results strongly suggest that thick myosin filaments as well as thin actin filaments are exposed to the surface of single myofibrils as schematically depicted in Fig. 1.
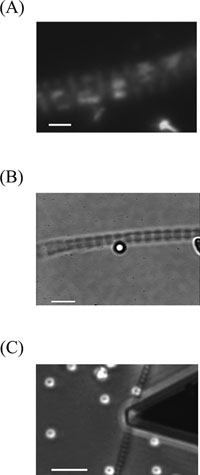 View Details | Fig. 3. Optical microscope images of myofibrils immobilized to coverslip. (A) Fluorescent myosin filaments attached to the surface of a myofibril. Bar: 2 μm. (B) A bead attached to the surface of a myofibril. Bar: 5 μm. (C) A whisker-tip cantilever positioned over a bead (not visible) attached to the surface of a myofibril. Bar: 10 μm. |
A suspension of α-actinin-coated beads (ca. 100 beads/ml) in a rigor solution was introduced into the experimental chamber, to the bottom of which myofibrils were attached. After washing with a rigor solution, it was observed that the beads were attached to the surface of myofibrils as can be seen in Fig. 3B. As α-actinin specifically binds to actin filaments, this strongly suggests that the beads were attached to the actin filaments exposed on the surface of myofibrils via α-actinin molecules bound to the surface of the beads.
Next it was examined how strongly actin filaments were bound to α-actinin-coated beads. A suspension of α-actinin-coated beads was introduced into the experimental chamber so as to make beads attach themselves to the bottom coverslip at an appropriate density. Then a rigor solution containing fluorescent actin filaments was introduced into the experimental chamber. It was occasionally observed under fluorescent optical microscope that single actin filaments simultaneously bridged two neighboring beads attached to the bottom coverslip. One such bead was captured by the tip of an AFM cantilever and moved away from the other bead. Both α-actinin-coated beads attached to the tip of AFM cantilevers and those attached to coverslip never got detached during experiments. The actin filaments were stretched and eventually severed into two filaments, in which each end of the filament remained attached to the beads in all the cases examined. This indicates that the binding strength between actin filaments and α-actinin-coated beads is greater than the tensile force to split single actin filaments, 250–550 pN (Tsuda et al., 1996).
The above experimental results strongly suggested that actin filaments exposed to the surface of myofibrils would be peeled off from the bulk structures of myofibrils by detaching the α-actinin-coated beads attached to the surface of single myofibrils. In the following experiments, these detachment experiments were made for various myofibril preparations; i.e., rigor and relaxed myofibrils, and trypsin- and calpain-treated myofibrils in rigor states. After capturing one of the beads attached to single myofibrils by use of either the AFM (Fig. 3C) or optical tweezers, the bead was pushed once to the surface of myofibrils, with a force of about 1 nN and about 2–3 pN for the AFM and optical tweezers experiments, respectively. By this step, it was shown that the beads stably adhered to the surface of myofibrils more strongly in the AFM experiments. After the bead was captured in a fixed position, the myofibril was retracted from the beads by moving the stage of optical microscope either vertically in the AFM experiments or horizontally in the optical tweezers experiments. It was found that myofibrils always remained attached to the bottom coverslip of the experimental chamber. In the AFM experiments, the beads attached to the myofibrils were completely detached from them. In the optical tweezers experiments, however, the beads attached to myofibrils were not detached from the myofibrils except for those attached to calpain-treated myofibrils. The latter beads were completely detached from myofibrils.
In force-distance curves obtained by these detachment experiments (20–30 experiments for each myofibril preparation), characteristic signals coming from ruptures of myofibril components were clearly observed as elastic components were picked up. Typical force-distance curves thus obtained for various myofibril preparations are shown in Fig. 4. In the AFM experiments (Fig. 4A), it can be seen that elastic components were picked up, the elastic constant of which was 0.2–3 pN/nm for intact, trypsin- and calpain-treated rigor myofibrils, and 0.15–0.6 pN/nm for intact relaxed myofibrils. Small ruptures of 50–100 pN frequently took place at 200–300 nm intervals for intact, trypsin- and calpain-treated myofibrils. Corresponding ruptures took place only occasionally for relaxed myofibrils. Ruptures were completed after retracting the myofibrils about 2 μm from the beads, with the maximal rupture forces of 900–1600 pN needed for rigor and relaxed myofibrils, about 500 pN for trypsin-treated rigor myofibrils, and about 150 pN for calpain-treated rigor myofibrils. For the AFM experiments the magnitude of the force required for detachment was 719.7±278.7 (n=23, SEM) for rigor myofibrils, 463.4± 248.7 (n=30, SEM) for relaxed myofibrils, 353.1±199.1 (n=23, SEM) for trypsin-treated rigor myofibrils, and 175.6±6.6 (n=17, SEM) for calpain-treated rigor myofibrils. For the optical tweezers experiments it was 38.1±14.6 (n=63, SEM) for calpain-treated rigor myofibrils while beads could never be detached from other myofibril preparations.
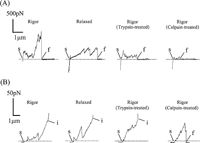 View Details | Fig. 4. Typical force-distance curves for various myofibril preparations. (A) AFM experiments and (B) optical tweezers experiments. (From left to right) Rigor, relaxed, and rigor myofibrils after trypsin- and calpain-treatments. Ruptures start and are finalized at s and f, respectively, and interrupted at i. |
In the optical tweezers experiments (Fig. 4B), the elastic components were similarly picked up, the elastic constant of which was 0.03–0.06 pN/nm for rigor, relaxed myofibrils, and for trypsin-treated and calpain-treated myofibrils. Many small ruptures of 2–10 pN (indicated by arrowheads) took place at intervals of about 300 nm for intact myofibrils and for trypsin- and calpain-treated myofibrils in a rigor state. No comparable ruptures but occasional ruptures of about 50 pN took place for myofibrils in a relaxed state. For intact and trypsin-treated myofibrils, however, the force-distance curves went off the scale when the tensile force needed to retract the myofibrils about 3 μm from beads reached 70–80 pN. Under these situations, the beads went out of the trapping range of the optical tweezers, indicating that beads were bound to myofibrils via elastic components with a mechanical strength greater than the trapping force of the optical tweezers. For the calpain-treated myofibrils, on the other hand, the overall ruptures were completed at a maximal rupture force of about 50 pN when myofibrils were retracted to about 3 μm from beads.
Typical SDS-PAGE patterns of intact, trypsin-treated and calpain-treated myofibrils are shown in Fig. 5. It can be seen that connectin and nebulin were severely digested by the trypsin treatments of myofibrils but that α-actinin was not digested. α-Actinin, connectin and nebulin were severely digested by the calpain treatments. Myosin heavy chains (MHC) and actin molecules were not digested by the trypsin and calpain treatments under the present proteolytic conditions. These results are consistent with the results reported by others (Reddy et al., 1975; Astier et al., 1993; Helmes et al., 1996; Akiyama et al., 2006).
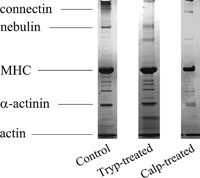 View Details | Fig. 5. SDS-PAGE patterns of control, trypsin-treated, and calpain-treated myofibrils. The bands for MHC, actin, connectin, nebulin, and α-actinin are indicated on the left. |
The results of the present experiments clearly indicate that peripheral sarcomere components of myofibrils were ruptured by detaching α-actinin-coated beads attached to the surface of single myofibrils. It has been reported that (1) Z-disks were the most rigid structures in the transverse stiffness distributions along myofibrils (Yoshikawa et al., 1999; Wakayama et al., 2000), and (2) by forcefully over-stretching rigor muscle fibers, overall sarcomere structures were completely disrupted while thick filaments and Z-disks remained almost undamaged (Suzuki and Sugi, 1983). These results suggest that Z-disk and A-bands are mechanically stronger compared with other sarcomere components. Taking these results into consideration, we analyzed the characteristic ruptures produced in various myofibril preparations as above and here discuss the mechanical strength of sarcomere structures.
Notably, in all the above experiments, the ruptures of myofibrils were completed, or interrupted, at 2–3 μm after myofibrils were retracted from beads attached to myofibrils. In our previous experiments (Yamada et al., 2003) in which myofibrils were severely ruptured by the tip of the AFM cantilever, the overall ruptures were also completed at 1–2 μm of the retraction of AFM cantilever from immobilized myofibrils. As the sarcomere spacing of the myofibril preparations is about 2.2 μm, these results strongly suggest that each sarcomere structure has relatively weak loci, and that the ruptures tend to take place at these loci. As Z-disks and A-bands are mechanically stronger than other sarcomere components, this assumption is in accord with the observations that myofibrils prepared by homogenizing muscle fibers have both ends with edges cut nearly perpendicular to the fiber axis at I-bands (data not shown).
Both the tensile forces required for detaching beads from myofibrils and the elastic constants of picked-up components were several times greater in magnitude for the AFM experiments than for the optical tweezers experiments (Fig. 4). This is in accord with the starting conditions of the above detachment experiments in which beads were pressed to myofibril preparations with greater force for the AFM experiments. Based on the surface geometry of the beads (1.9 μm in diameter) and myofibrils (about 1 μm in diameter) and the transverse Young’s modulus of myofibrils (Yoshikawa et al., 1999), the number of actin filaments picked up by beads was calculated to be approximately 10–20 actin filaments in the AFM experiments and 3–5 actin filaments in the optical tweezers experiments.
The number of actin filaments picked up by beads was estimated based on the rupture force to detach beads from myofibrils. The force to detach single actin filaments from myofibrils was assumed to be the minimal force to detach beads by use of AFM as beads could never be detached from intact myofibrils once beads were attached to the surface of myofibrils in the optical tweezers experiments. The minimal force to detach beads thus obtained was 120.3±22.6 pN (n=17). The number of actin filaments picked up by beads was then calculated by dividing the force required for detachment of beads from myofibrils by this number (120.3 pN); i.e., about 8 for rigor myofibrils, about 6 for relaxed myofibrils, and about 5 for trypsin-treated myofibrils. These values are consistent with those estimated based on the geometric analysis made above. As the elastic constant of components picked up by beads, 0.03–3 pN/nm, was significantly smaller than that of single actin filaments, 35–65 pN/nm (Kojima et al., 1994; Liu and Pollack, 2002), we may assume that elastic filaments like connectin filaments, the elastic constant of 1–4 pN/nm (Forbes and Wang, 2004), would have been picked up by beads together with actin filaments.
In the optical tweezers experiments, beads attached to myofibrils were not detached from myofibrils except for those attached to calpain-treated myofibrils. This indicates that actin filaments picked up by beads were connected to calpain-sensitive component(s) bound to the bulk structures of myofibrils with the mechanical strength greater than the trapping force of the optical tweezers, 70–80 pN. As α-actinin is readily digested by the calpain treatments (Fig. 5), it is suggested that actin filaments are anchored to Z-disks with mechanical strengths greater than 70 pN. This assumption is consistent with the results of the AFM experiments in which the rupture forces for calpain-treated myofibrils were significantly smaller compared with those for intact myofibrils. Notably the mechanical strength of the binding between thin actin filaments and Z-disk estimated above is far greater than the rupture force between single actin filaments and α-actinin, 1–44 pN (Miyata et al., 1996). As the Z-disk is composed of α-actinin networks decorated with other components (Jushou et al., 2005), we may assume that these components contribute to strengthening the binding between the thin actin filaments and the Z-disks, as suggested by Akiyama et al. (2006).
Many ruptures of 5–20 pN were consistently observed in the optical tweezers experiments (Fig. 4B) for intact, trypsin-treated, calpain-treated myofibrils in a rigor state but no corresponding ruptures were observed for myofibrils in a relaxed state. As all myosin heads were attached to and detached from actin filaments in rigor and relaxed myofibrils, respectively (Cooke and Franks, 1980; Lovell et al., 1981) and as the tensile force required to break a rigor complex is about 15 pN (Nishizaka et al., 2000), these 5–20 pN ruptures may have come from breakings of the rigor complexes formed in myofibrils. Similarly, in the AFM experiments (Fig. 4A), 50–100 pN ruptures took place for intact, trypsin-treated and calpain-treated myofibrils but no corresponding ruptures were observed for intact myofibrils in a relaxed state. The differences in rupture patterns between rigor and relaxed myofibrils would derive from whether or not massive breakings of rigor complexes took place in myofibril preparations.
Ruptures greater than 200 pN were occasionally observed in the AFM experiments (Fig. 4A) for rigor and relaxed myofibrils. These would be either ruptures of thin actin filaments from Z-disks and/or ruptures of Z-disk components as comparable ruptures were not observed for calpain-treated myofibrils. Alternatively, they could be due to the splitting of actin filaments, 250–550 pN (Tsuda et al., 1996), and/or intramolecular ruptures of connectin filaments, about 200 pN (Li et al., 2002). Notably, in the AFM experiments, the ruptures that took place in trypsin-treated and calpain-treated myofibrils were significantly smaller in magnitude than those that took place in intact myofibrils in rigor and relaxed states (Fig. 4A). As connectin had been severely digested in trypsin- and calpain-treated myofibrils (Fig. 5), these results suggest that connectin filaments somehow contributed to strengthening the sarcomere structures.
Based on the above considerations, we estimated that the protein components contributing to the strength of various myofibril preparations were as follows. In rigor myofibril preparations, actin filaments bound to Z-disks as well as thick myosin filaments bundled by M-lines would be strengthened by the binding of myosin heads to actin filaments, and connectin filaments bridging Z-disk and thick filaments also contributed to the strengthening. In relaxed myofibrils, however, the strengthening by the binding of myosin heads to thin filaments was absent with other factors similarly contributing as in rigor myofibrils. In trypsin-treated rigor myofibrils, the potential strengthening by connectin linking between Z-disks and thick filaments would be lost. In calpain-treated rigor myofibrils, the potential strengthening by connectin and that by the junction between Z-disk and thin filaments would also be lost. However in trypsin- and calpain-treated rigor myofibrils, the structure of their actomyosin filaments system would be maintained by the rigor complexes formed.
Considering the sarcomere structures shown in Fig. 1, we originally expected that ruptures of myofibrils would show certain kinds of patterns derived from the regular arrangements of actin and myosin filaments and other sarcomere components. Unfortunately we could not find any such regular rupture patterns in the present experiments. The possible reasons would be that (1) the actin filaments located at the very surface of myofibril preparations could be partly damaged during preparations, and (2) the rupture force of breaking rigor complexes depends on the mutual configurations of the complex formed (Tsuda et al., 1996) as actin filaments are incorporated in thick filaments array in complicated steric configurations. As these points are of interest and importance, further detailed studies are required. In any case, the various ruptures that took place in myofibril preparations examined in the present studies could basically be explained by the sarcomere model depicted in Fig. 1.
In contracting muscle, the force produced by the actomyosin system would deform the molecular architecture of sarcomeres of muscle fibers, including the lattice structures of actomyosin filaments. As the contractile force of 2–5 pN is produced per myosin head (Finer et al., 1994; Ishijima et al., 1994; Miyata et al., 1995) and one thin filament interacts with 70–80 myosin molecules per half sarcomere (Squire, 1981), each single actin filament will produce the force of about 200 pN in maximally contracting muscle fibers. In the above experiments, characteristic ruptures took place at the tensile forces of 2–500 pN in various myofibril preparations in which small ruptures of 10–50 pN were frequently observed and ruptures in the order of 100 pN were occasionally observed. Thus the sarcomere structure is stabilized by many molecular components having relatively weak mechanical strengths including the attachment of myosin heads to thin actin filaments. Notably the force to detach single actin filaments from myofibrils estimated above (120.3 pN) is significantly smaller than the force to be sustained by single actin filaments in maximally contracting muscle fibers. This suggests that the junction between actin filaments and Z-disk is more readily ruptured when actin filaments are picked up in a direction perpendicular to the fiber axis than when they are pulled in parallel to the fiber axis.
As ruptures of sarcomere structures took place at tensile forces comparable in magnitude to the force produced in the actomyosin system, the mechanical strength of the molecular assembly of sarcomeres would be marginal to sustain the contractile force produced. Thus the molecular assembly of sarcomere structure could be damaged by extensive contractions, and glycerinated muscles become mechanically weak possibly as essential component(s) strengthening the sarcomere structure were damaged or removed during the preparations.
The present submicromanipulation techniques could be applied to study the mechanical strength of various cellular organelles, especially those having actin filaments as components.
We wish to thank O. Itoh and Y. Noguchi for their technical assistance. This work was supported in part by a grant from the Ministry of Education, Science, Sport and Culture of Japan to T. Y. (Grant for Fundamental Research C(2)-12680660).
|