| To whom correspondence should be addressed: Nobukazu Araki, Department of Histology and Cell Biology, School of Medicine, Kagawa University, Miki, Kagawa 761-0793, Japan. Fax: +81–87–891–2092 E-mail: naraki@med.kagawa-u.ac.jp Abbreviations:CLSM, confocal laser scanning microscope; DMSO, dimethylsulfoxide; EEA1, early endosomal autoantigen 1; EGF, epidermal growth factor; FDx, fluorescein dextran; 3-MA, 3-methlyadenine; PI3K, phosphoinositide 3-kinase; PtdIns(3)P, phosphatidylinositol(3)phosphate; RDx, rhodamine dextran; TRDx, Texas red dextran; YFP, yellow fluorescent protein. |
Macropinocytosis is a clathrin-independent, actin-dependent endocytosis which accounts for the bulk fluid-phase uptake from the extracellular environment. In macrophages and dendritic cells, macropinocytosis is constitutive and plays an important role in antigen presentation (Nobes and Marsh, 2000; Norbury et al., 1997; Norbury et al., 1995). It is known that macropinosomes formed in macrophages gradually mature and fuse with lysosomes (Racoosin and Swanson, 1993). However, in other cell types such as fibroblasts and epidermoid A431 cells, macropinocytosis is transiently observed shortly after stimulation with cytokines. Unlike macropinosomes formed in macrophages, macropinosomes formed in epidermal growth factor (EGF)-stimulated A431 cells do not fuse with lysosomes; instead the content of macropinosomes is recycled back to the extracellular fluid (Hamasaki et al., 2004; Hewlett et al., 1994; Swanson and Watts, 1995). Compared with the clathrin-dependent receptor-mediated endocytosis, the role and mechanism of macropinocytosis in these cell-types are less understood. Recently attention has been increasingly directed toward the molecular mechanism that regulates macropinocytosis, since some pathogenic bacteria such as Salmonella induce macropinocytosis to enter the host cells (Alpuche-Aranda et al., 1994; Terebiznik et al., 2002). It is also noteworthy that the bovine prion protein internalizes into mammalian cells mainly through macropinocytosis (Magzoub et al., 2006).
In the process of macropinocytosis, the formation of macropinosomes from cell surface ruffles is highly dependent on the actin cytoskeleton reorganization which is spatiotemporally coordinated by the Rho family GTPases and phosphoinositide signaling (Anton et al., 2003; Araki et al., 2003; Araki et al., 2000; Araki et al., 1996; Maniak, 2001). The molecular mechanism underlying subsequent maturation of macropinosomes mediated by membrane fusion and fission events is poorly understood. Better understanding of the molecular mechanism controlling macropinocytosis would be important for discovery of therapeutic targets for the prion and bacterial diseases infected through the macropinocytic pathway.
Phosphoinositides, phosphorylated inositol lipids, are important second messenger molecules being involved in a variety of distinct cell functions. In particular, the 3-phosphoinositides play a key role in the regulation of actin cytoskeleton and membrane trafficking during endocytosis. In mammalian cells, 3-phosphoinositides such as phosphatidylinositol(3)phosphate [PtdIns(3)P], phosphatidylinositol (3,4)bisphosphate [PtdIns(3,4)P2] and phosphatidylinositol (3,4,5)triphosphate [PtdIns(3,4,5)P3] are generated by different isoforms of phosphoinositide 3-kinase (PI3K); class I, II and III enzymes (Siddhanta et al., 1998; Stein and Waterfield, 2000; Vanhaesebroeck et al., 1997). The class I PI3K, which mainly generates PtdIns(3,4,5)P3 in vivo (Stein and Waterfield, 2000), is implicated in growth factor-induced signaling pathways leading to cell growth, cell cycle entry and cell survival (Toker and Cantley, 1997; Vanhaesebroeck et al., 1997; Vanhaesebroeck and Waterfield, 1999). It is also known that this class of PI3K is involved in the formation of phagosomes and macropinosomes through the regulation of membrane traffic and actin cytoskeleton (Araki et al., 1996; Cox et al., 1999; Vieira et al., 2001). Although the lipid product and the role of the class II PI3K in vivo remain to be established (Vanhaesebroeck et al., 1997; Wymann and Marone, 2005), its strong substrate preference for PtdIns and PtdIns(4) in vitro suggests that PtdIns(3)P and PtdIns(3,4)P2 might be the main lipid products of the class II PI3K (Brown and Shepherd, 2001). The class III PI3K is a mammalian homologue of the yeast VPS34 which solely generates PtdIns(3)P (Stein and Waterfield, 2000). The class III enzyme activity is required for the events in the later process of the endocytosis; early endosome fusion (Murray et al., 2002), internal vesicle formation within multivesicular endosomes (Futter et al., 2001), and phagosome maturation (Vieira et al., 2001).
Some attempts to clarify the nature of PI3K have been based on the use of its inhibitors such as wortmannin, LY294002 and 3-methyladenine (3-MA) (Blommaart et al., 1997; Chen and Wang, 2001a; Chen and Wang, 2001b; Hirosako et al., 2004; Petiot et al., 2000). Both wortmannin and LY294002 inhibit class I and class III PI3Ks (Corvera and Czech, 1998; Vanhaesebroeck et al., 1997), although class II PI3K is less sensitive to wortmannin (Domin et al., 1997). In addition, it is known that 3-MA inhibits class III PI3K activity (Caro et al., 1988; Futter et al., 2001; Hirosako et al., 2004; Petiot et al., 2000). A recent study suggested that the activity of class III PI3K might be specifically impaired by 3-MA, while class I PI3K activity was not much affected by 3-MA (Petiot et al., 2000).
During the process of early endosome homotypic fusion in receptor-mediated endocytosis, class III PI3K activity is shown to be crucial for the recruitment of early endosomal autoantigen 1 (EEA1) which has a FYVE finger domain. The FYVE finger domain interacts with PtdIns(3)P (Gaullier et al., 1999; Patki et al., 1997) and has been shown to be required for endosomal targeting of EEA1 (Mills et al., 1998; Stenmark et al., 1996). It was shown that EEA1 interacts with the GTP-bound form of Rab5 and that these two proteins are sufficient to mediate early endosomal fusion in vitro (Christoforidis et al., 1999b; Mills et al., 1998; Simonsen et al., 1998). EEA1 may act as a tethering protein that confers targeting specificity before the SNARE-dependent early endosomal fusion event (Christoforidis et al., 1999b). Recently, we have localized EEA1 on macropinosomes formed in EGF-stimulated A431 cells (Hamasaki et al., 2004). This fact implies that macropinocytosis and receptor-mediated endocytosis possibly share common molecular machinery at the endosome fusion step. However, it has not been determined whether or not the production of PtdIns(3)P controls the fusion of macropinosomes, because macropinosomes are not formed in the presence of wortmannin or LY294002. In this study, we employed 3-MA which may primarily inhibit class III PI3K to elucidate the role of PtdIns(3)P in macropinocytosis in EGF-stimulated A431 cells. We demonstrate here that PtdIns(3)P may be implicated in the EEA1-mediated fusion of macropinosomes in EGF-stimulated A431 cells, by using a specific probe for PtdIns(3)P and fluorescent fluid-phase probes for macropinocytosis.
Human recombinant epidermal growth factor (EGF), wortmannin, 3-methyladenine (3-MA), FITC-dextran (FDx, Mr 150 kD) and rhodamine-dextran (RDx, Mr 160 kD) were from Sigma Chemical (St Louis, MO, USA). Lysine-fixable FDx and Texas red dextran (TRDx, Mr 70 kD) were from Molecular Probes (Eugene, OR, USA). We used dextran probes larger than 70 kD as macropinocytic markers, since large fluid-phase probes more preferentially label large endocytic vacuoles by macropinocytosis than small endocytic vesicles by micropinocytosis (Araki, 2006; Araki et al., 1996). LY294002 was purchased from Calbiochem (San Diego, CA, USA). In each experiment, wortmannin, LY294002 and 3-MA were freshly diluted to desired concentrations. Rabbit polyclonal anti-cathepsin D antibody was kindly provided from Prof. Sadaki Yokota (Yamanashi University). Other reagents were purchased from Wako Pure Chemicals (Osaka, Japan), unless indicated.
The human epidermoid carcinoma A431 cells and RAW264 macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO-BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The cells were harvested from dishes, plated onto coverslips or 24-well dishes, and further incubated for 1 or 2 days. Before the experiments, the cells were serum-starved in the serum-free Ringer’s buffer (155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM NaH2PO4, 10 mM Hepes,10 mM glucose, and 0.5 mg/ml bovine serum albumin, pH 7.4) for 2–3 hours.
The serum-starved A431 cells were pretreated with 100 nM wortmannin, 50 μM LY294002 or 10 mM 3-MA in Ringer’s buffer during 15 min at 37°C. Control cells were pretreated with 0.1% dimethylsufoxide (DMSO), the final concentration of DMSO in the preparations treated with the inhibitors. Then, cells were pulse-labeled with 1.0 mg/ml FDx or RDx in Ringer’s buffer together with 100 ng/ml EGF for 5 min, and chased in Ringer’s buffer without the fluorescent probe for 0, 5, 30 or 60 min. For microscopy, the cells on coverslips were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer containing 6% sucrose for 30 min at room temperature, rinsed in phosphate buffer saline (PBS), and processed for immunocytochemistry or directly observed with a confocal laser scanning microscope (CLSM, BioRad Radiance 2100) as previously described (Hamasaki et al., 2004).
For the macropinosome fusion assay, cells were pulse-labeled with FDx for 5 min, briefly washed in Ringer’s buffer, and subsequently pulse-labeled with RDx for 5min. After chasing in Ringer’s buffer, cells were fixed and observed with the BioRad CLSM. Both images of FDx and RDx acquired by the lambda sequential scan mode without cross-talk between FDx and RDx were analyzed by MetaMorph imaging software. The macropinosome fusion rate was assessed by measuring colocalization of the two fluorescent probes using the MetaMorph software, and expressed as percentage of FDx-positive area overlapping RDx-positive area for all FDx-positive area.
A quantitative analysis of macropinocytic activity was carried out by spectrofluorometry as previously described (Araki, 2006; Araki et al., 1996). Briefly, the serum-starved cells were plated on a 24-well dish. After treatment with an inhibitor or vehicle (DMSO), cells were labeled with FDx in the presence of 100 ng/ml EGF for 5 min. Then, the dish was drained and rinsed twice by dipping in a 1000 ml beaker filled with ice-cold PBS plus 1 mg/ml BSA and once with PBS for 5 min each. Finally, the cells in the wells were lysed in a lysis buffer consisting of 0.1% Triton X-100 and 50 mM Tris, pH 8.5. The amount of fluorescence in the cell lysates was measured by a spectrofluorometer (Hitachi 650-40, Tokyo, Japan). Data were presented as mean±SD from triplicate determinants and analyzed by Student’s t-test.
To monitor the dynamics of PtdIns(3)P in living A431 cells after EGF-stimulation, we used an expression probe of fluorescent tandem FYVE domains specifically binds to PtdIns(3)P (Gillooly et al., 2000; Kutateladze et al., 1999). Plasmid cDNA encoding green fluorescent protein-fused tandem Hrs FYVE domains (2×FYVE), which was originally constructed in H. Stenmark Lab (Norwegian Radium Hospital), was subcloned into monomeric Citrine-C1 vector to create yellow fluorescent protein (YFP)-2×FYVE in J. A. Swanson Lab (University of Michigan Medical School). The cDNA was propagated in the competent cells DH5α and purified using NucleoBond EF (Machery-Nagel GmbH, Duren, Germany). A431 cells cultured on 25 mm circular coverslips were transfected with the cDNA by Fugene 6 (Roche, Basel, Switzerland). After 24–48 hours, the transfected cells were serum-starved in Ringer’s buffer and used for experiments. The circular coverslip was assembled into an Attofluor cell chamber (Molecular Probes) filled with Ringer’s buffer, and placed in a microincubator (Tokai Hit INU, Shizuoka, Japan) on the stage of an inverted microscope (Nikon TE300). Time-lapse images of phase-contrast microscopy and YFP fluorescence were acquired through a cooled CCD camera (Retiga EXi, QImaging, Burnaby, BC, Canada) using the MetaMorph Imaging System (Universal Imaging, West Chester, PA) and assembled into a movie file for supplemental video data.
A431 cells and RAW264 cells of which macropinosomes were labeled with lysine-fixable TRDx were fixed as described above, treated with 0.25% NH4Cl2 in PBS for 10 min, and subsequently with 0.25% Triton X-100, and 1% BSA in PBS (permeabilizing/blocking solution) for 10 min. Then, cells were incubated with mouse monoclonal anti-EEA1 antibody (1:250, BD Biosciences) or rabbit polyclonal anti-cathepsin D antibody (1:500) diluted in the permeabilizing/blocking solution for 90 min at room temperature. After rinsing PBS, the cells were incubated with Alexa 488-labeled anti-mouse IgG or anti-rabbit IgG (1:500 dilution) (Hamasaki et al., 2004). As negative controls, normal mouse IgG was substituted for the specific antibody at the same concentration. The specimen coverslips were mounted on glass slides using Fluoroguard (Bio-Rad Lab., Hercules, CA, USA) and observed with the CLMS.
To examine whether 3-MA shows any differences in the effect on macropinocytosis than two other PI3K inhibitors, wortmannin and LY294002, the serum-starved A431 cells were pretreated by wortmannin, LY294002 or 3-MA, and incubated with 1.0 mg/ml FDx for 5 min in the presence of 100 ng/ml EGF to allow macropinocytosis of FDx. Then, we quantified the fluid-phase uptake of FDx by macropinocytosis in the cell lysates by spectrofluorometry. Compared with that in control cells, the amount of FDx uptake was drastically reduced in wortmannin- or LY294002-treated cells (<50% of control). However, in 3-MA-treated cells, no significant reduction in the fluid-phase uptake of FDx was observed (Fig. 1). This result suggests that 3-MA does not impair the formation of macropinosomes induced by EGF stimulation. Also, this fact implies that the use of 3-MA enables us to elucidate the role of PtdIns(3)P in the process of macropinosome fusion.
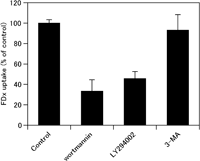 View Details | Fig. 1. Fluorometric quantitation of macropinocytic activity induced by EGF in A431 cells. Serum-starved A431 cells were pretreated with either 0.1% DMSO (control), 100 nM wortmannin, 50 μM LY294002, or 10 mM 3-methyadenine (3-MA), and stimulated with 100 ng/ml EGF in the presence of 1 mg/ml fluorescein dextran (FDx) for 5 min to allow macropinocytic uptake of the fluid-phase probe, FDx. The fluorescence of intracellularly accumulated FDx for 5-min incubation was measured with a spectrofluorometer. Compared with those in control cells, the uptake of FDx was greatly reduced in wortmannin- or LY294002-treated cells. However, the macropinocytic uptake of FDx was not significantly reduced by 3-MA treatment. The values represent the mean±s.d. of at least triplicate determinations. The results were reproducible in independent experiments. |
For further morphological observations, the drug-pretreated cells were similarly pulse-labeled with either of FDx or RDx for 5 min and chased in probe-free Ringer’s buffer for 0, 5 and 30 min. By confocal laser scanning microscopy, the nascent macropinosomes (<5 min age) varied in size (c.a. 0.2–5 μm) and shape, and were predominantly located in the peripheral region of the control cells (Fig. 2A). They appeared to be fused each other to form relatively large, round-shaped macropinosomes after 5-min chase (5–10 min age), and then reduced in number and size after 30- to 60-min chase (Fig. 2A). In wortmannin-treated cells, only a few macropinosomes were observed in the peripheral region even later than 5-min chase (Fig. 2B). In 3-MA-treated cells, macropinosomes labeled with RDx were formed in similar extent to control. However, most macropinosomes formed in 3-MA-treated cells were found to be relatively smaller in size even after 5-min chase (Fig. 2C). The presence of smaller macropinosomes may imply that the macropinosomes cannot tether and fuse with each other in 3-MA-treated cells.
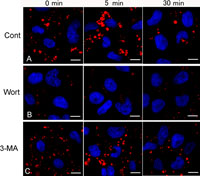 View Details | Fig. 2. Confocal laser scanning microscopy of macropinosomes-labeled with rhodamine dextran (RDx), a fluid-phase probe, in EGF-stimulated A431 cells. Serum-starved A431 cells were treated either with wortmannin or 3MA, pulsed with 100 ng/ml EGF and 1.0 mg/ml RDx for 5 min, and then chased in Ringer’s buffer for 0, 5 or 30 min. (A) In control cells, many macropinosomes labeled with RDx were observed in the cell periphery just after 5-min pulse with RDx. After 5-min chase, some macropinosomes gathered, and then gradually reduced in size and number with chasing times. (B) In wortmannin-treated cells, macropinosomes labeled with RDx were rarely observed. Macropinosomes observed on rare occasions were much smaller than control ones at any ages. (C) In 3-MA treated cells, macropinosomes labeled with RDx was observed in similar extend to control, but macropinosomes are located peripherally even after chasing for 5 min and 30 min. Cell nuclei were stained with DAPI (blue). Bars, 10 μm. |
Next, we visualized the dynamics of PtdIns(3)P in live A431 cells by expressing YFP-2×FYVE which specifically binds to PtdIns(3)P. In control cells expressing YFP-2×FYVE, the fluorescence of YFP was observed on the membrane of small vesicular structures, presumed to be early endosomes. The intracellular distribution of YFP-2×FYVE-labeled vesicles appeared to be similar to the localization of EEA1 that we have previously observed (Hamasaki et al., 2004). At 5–10 min after the EGF addition, YFP-2×FYVE was increasingly recruited to formed macropinosomes, although macropinocytic cups and very early macropinosomes were almost negative (Fig. 3, and Video 1). In a higher magnification movie of YFP-2×FYVE, we also found that YFP-2×FYVE-positive small vesicles and tubules moved toward and fused to nascent macropinosomes (Video 2). The macropinosomes acquired PtdIns(3)P frequently showed transient incomplete fusion and fission (kiss and run), and complete fusion among clustered macropinosomes (Videos 1 and 2). In the process of receptor-mediated endocytosis, PtdIns(3)P transiently appears on the membrane of early endosomes (Gillooly et al., 2000). However, in A431 cells, even after 30 or 60 min, 2×FYVE localized in the membrane of macropinosomes at the perinuclear region of the cells (Fig. 3). This suggests that the levels of PtdIns(3)P in the macropinosomal membrane are kept high as long as the macropinosome is present in the cells. After 3-MA-treatment, YFP-2×FYVE showed cytosolic punctate distribution throughout the cells, suggesting that 3-MA actually inhibits the PtdIns(3)P production. After EGF stimulation, many macropinosomes were formed in the 3-MA-treated cells. However, YFP-2×FYVE was not observed on the macropinosomal membranes at any stage (Fig. 4 and Video 3). It seemed that these macropinosomes rarely fused, and that they kept their size small (phase images in Fig. 4).
 View Details | Fig. 3. Live cell imaging of PtdIns(3)P by expressing YFP-2×FYVE in control A431 cells. Time-lapse images of A431 cells expressing YFP-2×FYVE were acquired by digital fluorescence microscopy using MetaMorph imaging system. (A) Phase contrast images are shown in the adjacent rows on the left of YFP images. Times after the EGF addition were indicated in minutes. Before EGF addition (0 min), YFP-2×FYVE was localized in small vesicles which are presumed to be early endosomes. After EGF additions, YFP-2×FYVE was increasingly recruited to macropinosomes which can be seen as phase-bright vacuoles in phase-contrast images. PtdIns(3)P was only faintly detected on just-closed macropinosomes (open arrows). Within a few minutes, nascent macropinosomes became PtdIns(3)P-positive, and keep PtdIns(3)P on their membranes as long as the macropinosomes persist in the cell (arrows in A). (B) The areas boxed by dotted line from selected frames were enlarged. It is noteworthy that YFP-2×FYVE-positive macropinosomes fused each other (arrows). The dynamics of PtdIns(3)P and the fusion of macropinosomes can be seen in the supplemental Videos 1 and 2 corresponding to Fig. 3A and B, respectively. At least five cells from three independent experiments were examined. Bar, 10 μm. |
 View Details | Fig. 4. Inhibition of the PtdIns(3)P production by 3-MA treatment. Serum-starved A431 cells expressing YFP-2×FYVE were treated with 3-MA for 15 min and stimulated with EGF. Time-lapse images were acquired by the same way as Fig. 3. The period of time after the EGF addition is indicated in minutes. Though many macropinosomes were formed by EGF stimulation, no production of PtdIns(3)P was observed on the macropinosomal membrane throughout the chase period (arrows). In phase-contrast microscopy, it was found that macropinosomes gathered but did not show complete fusion. A supplemental video is available (Video 3). These time-lapse images are representative from three independent experiments. Bar, 10 μm. |
In the process of receptor-mediated endocytosis, the product of class III PI3K, PtdIns(3)P, acts as a signal that recruits EEA1 to early endosomes (Gaullier et al., 1999; Lawe et al., 2002). Therefore, we attempted to ascertain whether the 3-MA treatment affects the localization of EEA1 of macropinosomes. The serum-starved A431 cells were pulsed with fixable TRDx and EGF for 5 min, followed by chase in Ringer’s buffer for 0, 5, 30 or 60 min. The fixed cells were immunostained for EEA1 using Alexa 488-labeled secondary antibody. In the control cells just after 5-min pulse with TRDx, only a small population of irregular-shaped macropinosomes was faintly positive for EEA1, but most were negative. After 5-min chase, most round-shaped macropinosomes gathered around the perinuclear region, tethered to each other, and tended to be more positive for EEA1 (Fig. 5A). Even after 30–60 min, the TRDx-labeled macropinosomes that remained in the cells were EEA1-positive (Fig. 5A). In contrast to control cells, wortmannin-treated cells showed a cytosolic distribution of EEA1 as small punctate structures in the cytoplasm (Fig. 5B). In 3-MA-treated cells, EEA1 was not recruited to macropinosomes at any age, and showed a cytosolic distribution (Fig. 5C). The localization of EEA1 in 3-MA-treated cells was very similar to that in wortmannin-treated cells (Fig. 5B, C), whereas TRDx-labeled macropinosomes were formed in 3-MA.
 View Details | Fig. 5. Redistribution of EEA1 in EGF-stimulated A431 cells after treatment of PI3K inhibitors. Cells pretreated with an indicated drug were incubated with Texas red dextran (TRDx; red) for 5 min in the presence of EGF, chased for indicated times and fixed. Then, cells were immunostained for EEA1 (green). (A) In control cells, the recruitment of EEA1 to macropinosomes was frequently observed after 5 min chase. Even after 30 or 60 min chase, macropinosomes labeled with TRDx were mostly positive for EEA1. (B) In wortmannin-treated cells, macropinosomes were scarcely seen, and EEA1 was diffusely distributed in the cytoplasm. (C) In 3-MA-treated cells, many macropinosomes labeled with TRDx were seen, but EEA1 was not associated with them throughout the entire chase time. Bars, 10 μm. |
Next, we examined the localization of EEA1 on macropinosomes in RAW264 macrophages in the presence or absence of 3-MA, in order to confirm that the effect of 3-MA on macropinocytosis and EEA1 is a common feature in other cell types. In control macrophages pulse-labeled with TRDx for 5 min, followed by 5-min chase (Fig. 6A), EEA1 localized on TRDx-loaded macropinosomes. After 30 min-chase, TRDx was observed in EEA1-negative compartments, presumably late endosomes or lysosomes. We have previously shown that 30-min chase of fluorescent dextran labels late endosomes or lysosomes which were positive for cathepsin D and Lamp proteins (Araki, 2006; Araki et al., 1996). Thus, the association of EEA1 with macropinosomes seems to be transient in macrophages. In 3-MA-treated macrophages (Fig. 6B), macropinosomes labeled with TRDx were scarcely immunolabeled for EEA1 after 5-min chase, suggesting that EEA1 recruitment to macropinosomes is inhibited by 3-MA. After 30-min chase, TRDx was transported to late endosomal or lysosomal compartments which were positive for cathepsin D in spite of the absence of EEA1 association (Fig. 6C).
 View Details | Fig. 6. Effect of 3-MA on the association of EEA1 with macropinosomes in RAW 264 macrophages. Control (A) and 3-MA-treated RAW264 cells (B) were pulse labeled with Texas red dextran (TRDx; red) for 5 min and chased in Ringer’s buffer for 5 min or 30 min. Then cells were fixed and immunostained for EEA1 (green). (A) After 5 min-chase, most macropinosomes-labeled with TRDx were positive for EEA1. After 30 min-chase, TRDx was seen in lysosome-like structures which were distinct compartments from EEA1-positive profiles. (B) TRDx-labeled macropinosomes were not immunolabeled for EEA1 in 3-MA-treated macrophages after 5-min chase. After 30-min chase, TRDx was transported to lysosome-like structures similar to control cells. (C) Colocalization of TRDx with cathepsin D, a late endosome/lysosome marker, in 3-MA-treated macrophages after 30-min chase. Even in the presence of 3-MA, TRDx was transported into late endosomes or lysosomes which were immunolabeled for anti-cathepsin D. Bars, 10 μm. |
To determine whether the production of PtdIns(3)P is required for the fusion of macropinosomes in vivo, we compared the rate of the macropinosome fusion between control and 3-MA-treated cells. A431 cells were first pulsed with FDx and EGF for 5 min. The FDx-labeled cells were briefly washed and subsequently pulsed with RDx for a further 5 min, followed by a chase in Ringer’s buffer for 0, 5 or 25 min. In control cells after 5-min chase in Ringer’s buffer (10-min chase for FDx-labeled macropinosomes), we could clearly find that some macropinosomes labeled with FDx were positive for RDx, suggesting that the FDx-labeled macropinosomes fused with RDx-labeled macropinosomes (Fig. 7A). At 30-min chase after FDx pulse-labeling, most of the FDx-labeled macropinosomes were positive for RDx (Fig. 7C). In contrast to control cells, FDx-labeled macropinosomes scarcely fused with RDx-labeled macropinosomes in 3-MA-treated cells after 10-min and 30-min chase (Fig. 7B, D). Using these images doubly pulse-labeled with FDx and RDx as described above, the rate of the macropinosome fusion was quantitatively image-analyzed by the colocalization measurement of FDx and RDx by MetaMorph imaging software. This quantitative image analysis revealed that the fusion percentage of FDx-labeled macropinosomes with RDx-labeled macropinosomes increased with chase time, and that 3-MA significantly inhibited the fusion of macropinosomes both at 10 min and 30 min after chasing (Fig. 8).
 View Details | Fig. 7. Fusion of macropinosomes is inhibited with treatment of 3-MA. Control (A, C) and 3-MA-treated A431 cells (B, D) were first pulse-labeled with FDx for 5 min, quickly rinsed and subsequently labeled with RDx for 5 min. Then, cells were chased in Ringer’s buffer for the indicated times, fixed with paraformaldehyde and observed by confocal laser microscopy. (A) In control cells at 10 min after FDx labeling, some macropinosomes labeled with FDx were positive for RDx (arrows), indicating that FDx-labeled macropinosomes fused with RDx-labeled macropinosomes. (B) In 3-MA-treated cells, FDx and RDx were present in distinct macropinosomal compartments. (C) In control cells after 30-min chase, most macropinosomes contained both FDx and RDx (arrows). (D) In 3-MA-treated cells after 30-min chase, FDx-positive compartments were still negative for RDx. Bars, 10 μm. |
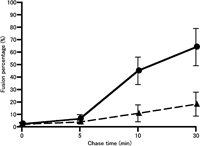 View Details | Fig. 8. Quantitative image analysis of the fusion rate of macropinosomes. Acquired images of FDx and RDx from the cells as shown in Fig. 7 were analyzed by the Measure Colocalization command of MetaMorph software to measure the area of overlap between the two fluorescent probes. The fusion rate was expressed as the percentage of FDx-positive area overlapping RDx-positive area for all FDx-positive area. At least 10 frames from each experiment were analyzed. (closed circle) control cells, (closed triangle) 3-MA-treated A431 cells. |
Both wortmannin and LY294002, inhibitors of PI3K, have been widely used as a useful tool to explore the role of PI3K in diverse cellular functions. However, these PI3K inhibitors cannot distinguish the role of different classes of PI3K, since wortmannin and LY294002 inhibit all classes of PI3K. Recently, 3-MA, which is known to be an inhibitor of the autophagic vacuole formation (Caro et al., 1988; Seglen and Gordon, 1982), has been shown to inhibit class III PI3K activity (Caro et al., 1988; Hirosako et al., 2004; Petiot et al., 2000). Although we could not completely rule out the possibility that 3-MA might inhibit other classes of PI3K, a study by Petiot et al. (2000) implied that 3-MA predominately inhibited the class III PI3K activity rather than the activity of the class I enzyme. Our study also showed that 3-MA did not inhibit the process of macropinosome formation which is sensitive to wortmannin and LY294002. More importantly, our study revealed that the production of PtdIns(3)P, which is visualized by YFP-2×FYVE, was greatly suppressed by 3-MA treatment. In addition, we recently observed that the product of class I PI3K, PtdIns(3,4,5)P3 visualized by YFP-Akt PH domain, was not abolished by the 3-MA treatment (submitted for publication), suggesting that 3-MA does not much affect the class I PI3K activity. Taken together, these results suggested that the initial phase of macropinocytosis, macropinosome formation, might be independent of 3-MA-sensitive PI3K activity, although it requires class I PI3K activity which is fairly resistant to 3-MA. Accordingly, 3-MA showing a unique specificity to PI3K classes allowed us to examine the role of PtdIns(3)P in the fusion process of macropinosomes formed in A431 cells after the EGF stimulation.
By live, digital fluorescence imaging of A431 cells expressing YFP-2×FYVE which specifically recognizes PtdIns(3)P, we found that the levels of PtdIns(3)P were remarkably elevated on the membrane of newly formed macropinosomes a few minutes after closure from macropinocytic cups. However, YFP-2×FYVE was not associated with the membranes of unclosed macropinocytic cups and very early macropinosomes within a minute. These findings suggested that PtdIns(3)P was locally produced on the membrane of newly formed macropinosomes. It is conceivable that the production of PtdIns(3)P may be mainly due to 3-MA-sensitive PI3K activity, although some PtdIns(3)P might be potentially produced by the actions PtdIns(5)- and PtdIns(4)-phosphatases from PtdIns (3,4)P2 and PtdIns(3,4,5)P3 (Martin, 1998). Also, we found that YFP-2×FYVE-positive small vesicles occasionally fused to nascent macropinosomes, suggesting that some of the PtdIns(3)P might be derived from other endomembranes. Immunofluorescence for EEA1, which has a FYVE domain, showed that EEA1 consistently localized on macropinosomes at 5 min after their formation. By contrast, in 3-MA-treated A431 cells, YFP-2×FYVE was scarcely associated with macropinosomes during the course of macropinocytosis. In 3-MA-treated cells, the recruitment of EEA1 to macropinosomes was abolished. Moreover, our quantitative fusion assay proved that the homotypic fusion between macropinosomes was inhibited by 3-MA. These results indicated that PtdIns(3)P is crucial for the localization and function of EEA1 at the fusion step of macropinosomes. In receptor-mediated endocytosis, PtdIns(3)P is produced on the membrane of early endosomes by class III PI3K which is recruited via activated Rab5:GTP (Christoforidis et al., 1999a). Concomitantly, EEA1 interacts with Rab5:GTP on the early endosome via its N-terminal Rab5-binding site, an adjacent domain of FYVE finger (Lawe et al., 2002; Simonsen et al., 1998; Stenmark et al., 1994). Lawe et al. (2002) have emphasized a temporal order of events in which endosome tethering is mediated by the interaction of EEA1 with PtdIns(3)P and subsequent endosome fusion is dependent on the interaction of EEA1 with Rab5. Molecular mechanisms of the EEA1 recruitment and macropinosome fusion seem to be the same as the case of early endosome fusion in the pathway of receptor-mediated endocytosis.
However, the pattern of dynamics of PtdIns(3)P and EEA1 throughout the time course in EGF-induced macropinocytosis seemed to be different from that in receptor-mediated endocytosis. In receptor-mediated endocytosis, PtdIns(3)P and EEA1 localized on early endosome, but not on late endosomes. PtdIns(3)P is removed from the limiting membrane of early endosomes by inward budding of the membrane to form multivesicular bodies (Gillooly et al., 2000). PtdIns(3)P on the intraluminal membranes of multivesicular bodies may be degraded or further modified by kinases such as PtdIns(3)P 5-kinase. In addition to PtdIns(3)P removal, Rab5:GTP hydrolysis to GDP may cause EEA1 to dissociate from the membrane (Simonsen et al., 1998). By contrast, in macropinocytosis observed in EGF-stimulated A431 cells, PtdIns(3)P and EEA1 persist on the membrane of macropinosomes as long as the macropinosomes are present in the cell. Although macropinosomes decrease in size and number with time, they remain in the perinuclear region for a hour, but never fuse with lysosomes nor acquire acid hydrolases in A431 cells (Hamasaki et al., 2004; Hewlett et al., 1994). The luminal content of macropinosomes in this cell type is extracellularly regurgitated by recycling pathways, although the significant role of the regurgitation is unknown (Hewlett et al., 1994; Swanson and Watts, 1995).
In constitutive macropinocytosis occurring in macrophages, we observed that macropinosomes were immunolabeled for EEA1, and that EEA1 recruitment to macropinosomes was inhibited by 3-MA. These findings indicated that the macropinosome fusion in macrophages is also mediated by EEA1 via PtdIns(3)P in common with EGF-stimulated A431 cells. However, the association of EEA1 with macropinosomes was transient in macrophages. After 30-min chase, the content of macropinosomes, TRDx, was transported to EEA1-negative, cathepsin D-positive late endosomes or lysosomes in macrophages (Fig. 6C). This result suggested that 3-MA did not affect the fusion of macropinosomes with late endosomes or lysosomes in macrophages. We have conducted an experiment to assess the effect on 3-MA on the homotypic fusion of macropinosomes in RAW264 macrophages by a similar way to the sequential pulse labeling fusion assay carried out in A431 cells using FDx and RDx. Unfortunately, we could not evaluate the direct effect on macropinosome homotypic fusion in macrophages, since both FDx and RDx were rapidly transported into late endosomes and lysosomes, and mixed in these compartments after 10–30-min chase (data not shown). Accordingly, we can show the direct effect of 3-MA on the homotypic fusion of macropinosomes only in a certain cell types such as A431 cells in which macropinosomes never fuse to late endosomes/lysosomes. Although we could not completely rule out the possibility that the effect of 3-MA on macropinosome fusion might be restricted to A431 cells, the effect of 3-MA on EEA1 recruitment to macropinosomes seems to be indicative of the role of PtdIns(3)P in the homotypic fusion of macropinosomes in macrophages.
Most recently, Kerr et al. (2006) showed that the majority of sorting nexin 5 (SNX5)-positive macropinosomes were positive for early endosomal markers such as Rab5 and EEA1 in HEK 293 cells-stimulated with EGF, although ~60% of SNX5-positive macropinosomes were positive for Rab7, a late endosomal marker, suggesting that at least some macropinosomes mature into late endosomes or lysosomes in HEK293 cells (Kerr et al., 2006). In Fcγ-receptor-mediated phagocytosis, class III PI3K activity appeared to direct the fusion of formed phagosomes with late endosomes/lysosomes, while phagosome formation requires class I PI3K activity (Vieira et al., 2001). Henry et al. (2004) showed that YFP-2×FYVE exhibited two patterns of phagosome labeling. Some phagosomes increase labeling for PtdIns(3)P quickly after phagosome closure and then lose the label within 20 min, similar to endosomes in receptor-mediated endocytosis. However, some phagosomes retain PtdIns(3)P for hours (Henry et al., 2004). The latter case seems to be analogous to the macropinocytosis that we observed in this study. Thus, it is likely that the dynamics of PtdIns(3)P and EEA1 may vary in each endocytic pathway and cell type, and the longer period association of EEA1 with macropinosomes formed in A431 cells may well be a cell-type specific feature.
Besides EEA1, a number of molecules with FYVE or PX domain which can bind to PtdIns(3)P are known (Ellson et al., 2002; Stenmark and Aasland, 1999; Teasdale et al., 2001). Further studies are required to determine the role of PtdIns(3)P in regulating the functions of other effector molecules during macropinocytosis and in other endocytic pathways.
We wish to thank Professor Joel A. Swanson of University of Michigan Medical School for providing the cDNA encoding YFP-2×FYVE. We are grateful to Y. Iwabu, K. Yokoi and T. Nakagawa for their skillful technical assistance. This study was supported by a Grant-in-Aid for Scientific Research B (2) 15390056 from the Japan Society for the Promotion of Science.
|