| To whom correspondence should be addressed: Chikashi Shimoda, Department of Biology, Graduate School of Science, Osaka City University, Sugimoto 3-3-138, Sumiyoshi-ku, Osaka 558-8585, Japan. Tel/Fax: +81–6–6605–2576 E-mail: shimoda@sci.osaka-cu.ac.jp Abbreviations: FSM, forespore membrane; SNARE, soluble NSF attachment protein receptor; SPB, spindle pole body. |
Type I myosin (myosin-I) is a highly conserved, actin-dependent motor protein that is involved in various membrane-associated cellular processes, including endocytosis and vesicle trafficking among others (reviewed by Mooseker and Cheney, 1995). The heavy chain of myosin-I of the fission yeast Schizosaccharomyces pombe, Myo1, encoded by the myo1+ gene, possesses features common to other eukaryotic myosin heavy chains (Lee et al., 2000; Toya et al., 2001). Myo1 shares 42% amino acid identity with the unconventional human myosin type 1F heavy chain (Strausberg et al., 2002; GenBank accession number, BC028071/AJ310570) and contains several conserved domains: namely, a head motor domain, an IQ domain, a tail homology domain and a C-terminal acidic domain (Lee et al., 2000). myo1Δ null mutants show sensitivity to high and low temperatures, and to high concentrations of KCl (Toya et al., 2001). Despite its dispensability for proliferation, Myo1 is essential for sexual reproduction, mating and sporulation (Toya et al., 2001), suggesting that it is more profoundly involved in sporulation.
Sporulation in fission yeast is a process of gametogenesis, because this morphogenetic process is accompanied by meiotic nuclear divisions (reviewed by Shimoda and Nakamura, 2004). Sporulation initiates with de novo assembly of double unit membranes, termed forespore membranes (FSMs), the inner membrane of which becomes the plasma membrane of newborn spores (reviewed by Shimoda, 2004). Formation of the FSM starts during meiosis-II on the cytoplasmic side of the spindle pole body (SPB) (Nakamura et al., 2001). The FSM expands to form cup-shaped structures that culminate in closed membrane compartments after engulfing the postmeiotic nucleus (Nakamura et al., 2001). Extension of the FSM depends on vesicle fusion mediated by the SNARE proteins Psy1 (Nakamura et al., 2001), Sec9 (Nakamura et al., 2005) and Syb1 (T. Yamaoka, unpublished). Meu14 has been identified as a novel protein that localizes to the front end of the growing FSM and is essential for its normal assembly (Okuzaki et al., 2003).
S. pombe possesses two calmodulin-coding genes, cam1+ (Takeda and Yamamoto, 1987; Moser et al., 1995; Moser et al., 1997) and cam2+ (Lord and Pollard, 2004). Calmodulin associates with the myosin-I heavy chain as a regulatory light chain (for review, Coluccio, 1997), and Myo1 of S. pombe is complexed with either Cam1 (Toya et al., 2001) or Cam2 (Lord and Pollard, 2004). Cam1 is essential for both vegetative growth and sporulation (Takeda and Yamamoto, 1987; Takeda et al., 1989); by contrast, the role of Cam2 has not been reported. A genome-wide DNA microarray analysis indicated that the mRNA level of cam2+ is increased about five-fold in meiosis (Mata et al., 2002). Taking these results together, we surmised that Myo1 and Cam2 may play important roles in sporulation. Here we report the subcellular localization of Myo1, Cam2 and F-actin during sexual reproduction, mating, meiosis and sporulation. Our results suggest that these proteins may be involved in spore morphogenesis.
The S. pombe strains used in this study are listed in Table I. Yeast-extract medium (YEA) and synthetic complete medium (MM+N) were used for growth. Malt-extract medium (MEA) and synthetic sporulation media (MM–N and SSA) were used for mating and sporulation. These media have been described by Egel and Egel-Mitani (1974), Gutz et al. (1974), and Moreno et al. (1990). S. pombe cells were grown and sporulated at 28°C, unless otherwise indicated. Synchronous meiosis was induced by adopting the temperature shift-up protocol using pat1-114ts homozygous diploid strains (Iino et al., 1995; Abe and Shimoda, 2000). Expression of nmt1 promoter-driven genes in the pREP1 vector (Maundrell, 1993) was controlled in the absence (turning on) or presence (turning off) of thiamine (20 μM).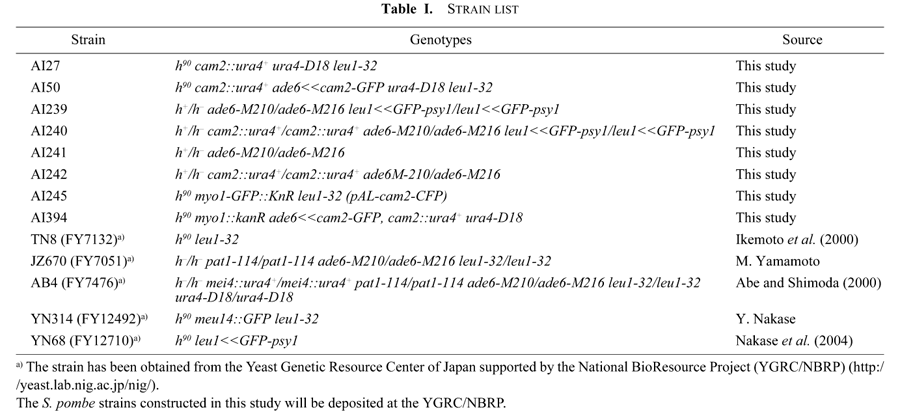
The plasmids used in this study are listed in Table II. Cam2 tagged with green fluorescent protein (GFP) was constructed in the multicopy plasmid pAL-KS (Tanaka et al., 2000). The cam2+ gene was amplified by polymerase chain reaction (PCR) using the primers 5'-AGCTCGTCGAC(SalI)GCGAAGCTCATAGACAGTTA-3' (forward) and 5'-GCTACGCGGCCGC(NotI)ATTTTGCCATGATTCTCTGTAC-3' (reverse). The PCR product was digested with SalI and NotI, and then ligated into pTN143 (Ikemoto et al., 2000) as a GFP fusion, yielding pAL(cam2-GFP). Plasmid pAL(cam2-CFP) was constructed in a similar manner, but a DNA fragment encoding the GFP derivative CFP was used instead of GFP. From pAL(cam2-GFP), a 3.0-kb ApaI-SacI fragment, containing the promoter and the protein-coding region of cam2+, as well as the GFP-coding gene and the nmt1 terminator region, was ligated into the pIA vector, yielding pIA(cam2-GFP). The same SalI-NotI fragment containing cam2+ was also ligated into the HA epitope-carrying vector pTN142, yielding pAL(cam2-HA). The 2.4-kb ApaI-SacI fragment derived from pAL(cam2-HA) was recloned into pBR(leu1) (Nakamura-Kubo et al., 2003), yielding pBR(cam2-HA).
The plasmid pIA(cam2-GFP) was linearized by cutting it at the single BamHI site located at the central region of the ade6+ gene, and then introduced into the homothallic haploid strain AI50. A few stable Ade+ transformants were inspected for accurate integration into the chromosomal ade6 locus by Southern blotting. The cam2+-HA allele was chromosomally integrated in a similar manner. Linearized pBR(cam2+-HA) was introduced into the recipient diploid strain JZ670. Stable transformants were obtained in which the cam2+-HA allele was integrated at the leu1 locus.
A 2.8-kb fragment containing the entire cam2+ ORF (429 base pairs) was amplified by PCR using the primers: 5'-AGCTCCTGCAG(PstI)GAGAACATTTTGACCATGGCT-3' (forward) and 5'-GTGCAGAGAGGGAGCAAATGGAGTT-3' (reverse). The amplified fragment was digested with PstI and SalI, and then cloned into pBluescript II-KS+ (Stratagene, La Jolla, CA, USA). A 0.3-kb EcoRV-HindIII fragment in the cam2 ORF was replaced by a ura4+-containing fragment. The resultant plasmid pAI106 was cut with BglII and SalI. The linear 2.8-kb BglII-SalI fragment containing cam2::ura4+ null allele was then introduced into strain AI27 and transformants were obtained. Replacement of the cam2+ allele with cam2::ura4+ allele was confirmed by genomic Southern hybridization (data not shown).
The recombinant DNA techniques used in this study followed standard methods (Sambrook et al., 1989). Genomic Southern hybridization and Northern hybridization were carried out according to our previously described procedures (Horie et al., 1998).
S. pombe cells were fixed according to the procedures of Hagan and Hyams using glutaraldehyde and paraformaldehyde (Hagan and Hyams, 1988). The Cam2-HA protein was visualized by indirect immunofluorescence microscopy using the rat anti-HA antibody 3F10 (Roche Diagnostics, Germany) and Alexa 594-conjugated goat anti-rat IgG (Molecular Probes, Eugene, OR, USA). For staining F-actin, cells were fixed with 2.9% formaldehyde and stained with rhodamine-conjugated phalloidin (Molecular Probes) at 100 ng/ml (Alfa et al., 1993). The nuclear chromatin region was stained with 4',6-diamidino-2-phenylindole (DAPI) at 1 μg/ml, or with Hoechst 33342 at 1 μg/ml in PBS buffer. Stained cells were observed under a fluorescence microscope (model BX51; Olympus Co., Tokyo, Japan). Fluorescent images were captured using a Cool SNAP CCD camera (Roper Scientific, San Diego, CA, USA).
The S. pombe major calmodulin, Cam1, has been reported to play an important role in sporulation (Takeda et al., 1989). We attempted to elucidate the potential role in sporulation of another calmodulin-like protein of S. pombe encoded by SPAC29A4.05 (also named cam2+; Lord and Pollard, 2004). The cam2+ gene has the capacity to encode a 16-kD protein comprising 144 amino acid residues. The cam2+ gene product showed marked homology to calmodulin: Cam2 shares 40%, 35% and 46% identity with S. pombe Cam1, S. cerevisiae Cmd1p and human vCam, respectively (Fig. 1). Calmodulins contain EF-hand motifs that constitute the Ca2+-binding sites. The canonical EF-hand is flanked by the acidic amino acid residues Asp (N-terminus) and Glu (C-terminus), which are thought to be important for Ca2+-binding activity (Geiser et al., 1991). None of four putative EF-hand motifs of Cam2 has a C-terminal Glu residue (Fig. 1), however, suggesting that it may not have Ca2+-binding activity. Unlike the major S. pombe calmodulin Cam1, Cam2 has not yet been analyzed. Therefore, here we studied the expression of Cam2, focusing on its potential role in meiosis and sporulation.
 View Details | Fig. 1. The cam2+ gene encodes a calmodulin-like protein. Shown is a comparison of the amino acid sequence of Cam2 with the calmodulin homologues from S. pombe (Cam1; Takeda and Yamamoto 1987), S. cerevisiae (Cmd1: Davis et al., 1986) and human (vCaM; Sasagawa et al., 1982). Identical amino acids are shown on a black background and similar ones are shaded. Putative EF-hand motifs are indicated by horizontal bars, and important acidic residues are indicated by asterisks. |
A genome-wide DNA microarray analysis of S. pombe previously showed that cam2+ transcripts increase approximately 5-fold during meiosis (Mata et al., 2002). The forkhead protein Mei4 is a major transcription factor that functions in meiosis and sporulation (Horie et al., 1998; Abe and Shimoda, 2000). We therefore examined whether the increased transcription of cam2+ is dependent on Mei4. Synchronous meiosis was induced in the pat1-114 homozygous diploid strain, harboring either mei4+ (JZ670) or mei4Δ (AB4), by raising the incubation temperature to 34°C. Progression of the meiotic divisions was monitored by visualizing nuclei with DAPI. The kinetics of the mei4+ strain is shown in Fig. 2B. The meiotic process of the mei4Δ strain was arrested at prophase-I (Horie et al., 1998; Abe and Shimoda, 2000). At hourly intervals, RNA samples were prepared for Northern blotting. As shown in Fig. 2A (mei4+, upper), the cam2+ gene was transcribed even in vegetative mei4+ cells (at 0 hr), and the level of cam2+ mRNA significantly increased from 7 hr to 9 hr, corresponding to the meiotic divisions. This increase was completely abolished in the mei4Δ strain (Fig. 2A, bottom), indicating that the increase in transcription of cam2+ during meiosis was Mei4-dependent.
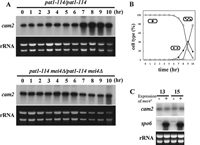 View Details | Fig. 2. Transcriptional regulation of the cam2+ gene during meiosis. (A) Changes in cam2+ mRNA levels in pat1-driven synchronous meiosis. Top, JZ670 (mei4+); bottom, AB4 (mei4Δ). Total RNA was analyzed by Northern blot hybridization. Equality of loading was roughly estimated by staining gels with ethidium bromide, which reveals rRNA. (B) Kinetics of meiotic nuclear divisions. Synchronous meiosis was induced using the JZ670 strain (mei4+/mei4+). Progression of meiosis was monitored by DAPI staining. Open circles, mononucleate cells; closed circles, binucleate cells; open squares, tri- or tetranucleate cells. About 500 cells were counted for each sample. (C) Effect of ectopic expression of mei4+ on cam2+ transcription. TN8 (h90) was transformed with either pREP1 or pREP1(mei4+) (Abe and Shimoda, 2000). The mei4+ gene was induced under the strong nmt1 promoter, which is derepressed in thiamine-free medium (expression, +) or repressed in thiamine-containing medium (expression, –). At 13 hr and 15 hr after transfer to MM+N medium without thiamine, RNA was prepared for Northern blotting. Samples were also probed for spo6+ as a positive control. Transcription of spo6+ is stimulated by ectopic expression of Mei4 (Horie et al., 1998). |
A known target gene of Mei4, spo6+ (Nakamura et al., 2000), was transcriptionally stimulated in vegetative cells by overproduction of Mei4 (Fig. 2C). It thus seemed unlikely that the upregulation of cam2+ transcription during meiosis was directly controlled by Mei4, because ectopic overexpression of Mei4 in vegetative cells did not enhance the transcription of cam2+ (Fig. 2C). In support of this notion, the FLEX motif, which is a Mei4 recognition site (Horie et al., 1998; Abe and Shimoda, 2000), is not found in the putative promoter region of cam2+.
Myo1 is not essential for growth but shows marked growth retardation at low (15°C) and high (36°C) temperatures (Toya et al., 2001). Intriguingly, Myo1 is crucial for spore morphogenesis following meiotic divisions (Toya et al., 2001). As Cam2 has been identified as a light chain of type I myosin (Lord and Pollard, 2004), we addressed the question of whether the cam2 null mutation exhibits a phenotype similar to myo1Δ. The cam2 deletion mutant (AI27; see Materials and Methods) was inspected for growth ability at temperatures between 15 and 37°C on YEA complete medium, as well as for cell morphology. No difference in these properties was found between the cam2Δ and cam2+ strains, indicating that the cam2+ gene is not essential for vegetative growth.
Next, we studied the kinetics of meiotic nuclear divisions in a cam2Δ homozygous diploid strain at both 28°C (suitable temperature for meiosis) and 34°C (marginally high temperature for meiosis). As conjugation was generally impeded at 34°C, the diploid strains were used in this experiment. The first and second meiotic divisions of cam2Δ (AI242) proceeded with kinetics similar to that of the isogenic wild-type strain (AI241) at 28°C (Fig. 3A). At 34°C, although the timing of first and second divisions did not differ between these strains, more than 20% of the cam2Δ cells did not enter meiosis-I (Fig. 3A). Furthermore, the cam2Δ cells were defective in sporulation at 34°C: only 15% of the cells developed four-spored asci, and the rest formed no spores or a reduced number of spores (1–3) in each cell (Fig. 3B). At 28°C, the cam2Δ cells sporulated normally (Fig. 3B). These observations suggest that cam2+ plays a role in meiosis and sporulation at high temperatures unfavorable for these processes.
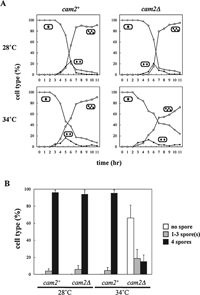 View Details | Fig. 3. Effect of cam2 disruption on meiosis and sporulation. (A) Kinetics of meiosis in the cam2Δ homozygous diploid strain. AI241 (cam2+) and AI242 (cam2Δ) were precultured overnight in liquid growth medium (MM+N), and then incubated with shaking at either 28 or 34°C in liquid sporulation medium (MM–N). To monitor the progression of meiosis, cells were stained with DAPI. For each sample, ~500 cells were counted. The results shown are based on one representative experiment. Open circles, mononucleate cells; closed circles, binucleate cells; open squares, tri- or tetranucleate cells. (B) cam2 disruption affects sporulation. Cells were stained as in (A). To induce sporulation, cells were incubated on SSA plates for 2 days at either 28°C or 34°C. Cells were classified into three categories: cells with no spores (open bars), cells containing 1–3 spore(s) (shaded bars), and cells containing four spores (closed bars). The mean±standard deviation (vertical bars) of three independent cultures is presented. |
The dynamic behavior of F-actin patches and cables has been well documented during both the cell cycle and the life cycle of S. pombe (Marks et al., 1986; Petersen et al., 1998; reviewed by Gachet et al., 2004). In addition, colocalization of Myo1 with F-actin during the life cycle has been reported (Toya et al., 2001). We therefore examined Cam2-GFP localization during vegetative growth and the sexual cycle. A single copy of the cam2-GFP fusion gene was integrated at the ade6 locus on chromosome III of the cam2Δ strain (AI50). The defects in sporulation at 34°C due to the cam2Δ mutation were recovered by a single copy of the cam2-GFP allele, indicating that this GFP-tagged version was fully functional (data not shown). AI50 cells were grown in defined complete medium at 30°C, then transferred to nitrogen-free medium (MM–N) and incubated at 28°C. After staining the DNA with Hoechst 33342, the fluorescent signals of Cam2-GFP were observed by fluorescence microscopy. In the log-phase culture, Cam2-GFP was predominantly localized to two distinct regions: the polar regions of interphase cells and the medial region of postmitotic cells (Fig. 4, a). Such localization in vegetative cells is similar to that of Myo1 (Toya et al., 2001) and F-actin (Marks et al., 1986). In fact, our double staining experiments indicated that Cam2/Myo1 and Cam2/F-actin were colocalized in most cells (Fig. 5A).
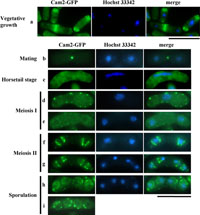 View Details | Fig. 4. Localization of Cam2-GFP during the life cycle of S. pombe. A homothallic haploid strain (AI50) carrying cam2-GFP was cultured in MM+N at 28°C for 16 hr (vegetative cells). For sexual reproduction, AI50 was cultured on MEA. Cells were sampled at different stages of mating, meiosis and sporulation. After staining cells with Hoechst 33342 to visualize nuclei, GFP signals were observed by fluorescence microscopy. Bars, 10 μm. |
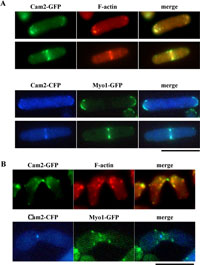 View Details | Fig. 5. Colocalization of Cam2 with F-actin and with Myo1. (A) Colocalization in vegetative cells. AI50 was cultured in MM+N at 28°C for 12 hr. Cells were then stained with rhodamine-conjugated phalloidin to visualize F-actin (top). The homothallic haploid strain AI345 carrying Myo1-GFP and Cam2-CFP was cultured on YEA medium (bottom). GFP images were obtained by using a filter set for YFP signals. Bars, 10 μm. (B) Colocalization of Cam2 and F-actin during conjugation. Cells were cultured on MEA for 16 hr and stained as in (A). Bar, 10 μm. |
In nitrogen-free medium, haploid cells of P or M mating-type extended projections towards their opposite mating-type cells in response to mating pheromones, and eventually fused to form a diploid zygote (reviewed by Yamamoto et al., 1997). The Cam2-GFP signal was detected as a single bright dot at the tips of conjugation processes. This fluorescence was maintained even after the P and M cells had fused (Fig. 4, b). F-actin patches and the Myo1 dots have been reported to accumulate in mating projections (Petersen et al., 1998; Toya et al., 2001). As shown in Fig. 5B, a punctuate signal from Cam2-GFP did not appear to overlap with either F-actin patches or Myo1, implying that Cam2 is a component of a structure other than F-actin patches. In support of this, the Cam2-GFP dots did not disappear in myo1Δ cells (Fig. 6A, b).
 View Details | Fig. 6. Dependency of Cam2 on Myo1 in sporulation. (A) Localization of Cam2 in myo1Δ cells. The AI394 strain (myo1Δ cam2-GFP) at different stages of the life cycle was observed under a fluorescence microscope after staining cells with Hoechst 33342 to visualize nuclei. Shown are vegetative cells cultured on YEA (a), conjugating cells cultured on SSA (b), and meiotic cells cultured on SSA (c). Bars, 5 μm. (B) Functional complementation of cam2Δ by overexpression of Myo1 or Myo1ΔIQ. AI339 (cam2Δ) was transformed with pSP1, pSP1(myo1) or pSP1(myo1ΔIQ). The transformants were incubated on SSA sporulation medium for 2 days at 34°C. Cells were classified into three categories: cells with no spores (open columns), cells containing 1–3 spore(s) (filled gray columns), and cells containing four spores (filled black columns). For each sample, ~500 cells were counted. The mean±standard deviation (vertical bars) of three independent transformants is presented. |
As the cells entered meiosis-I (Fig. 4, c–e), the Cam2-GFP dots dispersed into the peripheral regions and within the cytoplasm. When meiosis-II commenced, strong signals abruptly reappeared as broad rod- or ring-shaped structures at both sides of the nucleus (Fig. 4, f). After anaphase-II, the relative positions of Cam2-GFP and the nuclei changed: a pair of Cam2-GFP rods or rings were positioned between the divided sister nuclei (Fig. 4, g). After the spores formed, Cam2-GFP signals were seen as several dots in the peripheral region and in the cytoplasm of the newly formed spores (Fig. 4, h and i).
Next, we addressed whether the localization of Cam2 in vegetative and sporulating cells depends on Myo1 or not. Cam2-GFP was expressed in myo1Δ cells. In vegetative cells, the Cam2-GFP signals were dispersed over the nucleus and the cytoplasm (Fig. 6A, a). In meiotic cells, the characteristic localization of Cam2-GFP observed in the wild-type background was completely lost (Fig. 6A, c). Therefore, the subcellular localization of Cam2 is dependent on the type I myosin heavy chain Myo1, with the exception of its localization to mating projections (Fig. 6A, b).
The fact that the characteristic localization of Cam2 during meiosis-II is dependent upon Myo1 suggests the possibility that Cam2 functions through Myo1. To test this possibility, we studied whether the overexpression of Myo1ΔIQ (Myo1 lacking the IQ domain) could rescue the sporulation defects of cam2Δ at 34°C, because the deletion of IQ mimics the activated state (Toya et al., 2001). As Fig. 6B shows, Myo1ΔIQ significantly recovered the sporulation of cam2Δ and even wild-type Myo1 alleviated sporulation deficiency. This observation indicates further the collaboration of Myo1 and Cam2 in the sporulation process.
The FSM initiates near the nucleus in meiosis-II (Yoo et al., 1973; Nakamura et al., 2001). Our observation of Cam2 as rod- or ring-shaped structures near the nucleus during meiosis-II (Fig. 4, g) prompted us to test whether Cam2 is associated with the FSM at this stage. Psy1, a fission yeast homologue of the animal syntaxin 1A, is localized to the plasma membrane and the FSM (Nakamura et al., 2001). Meiotic cells expressing both CFP-Psy1 (as a marker of the FSM) and Cam2-GFP were examined. Fluorescence microscopy clearly showed that Cam2 preferentially localized to the extending rim of the cup-shaped FSM (Fig. 7A). This region of the FSM has been designated as the ‘leading edge’ of the FSM (Okuzaki et al., 2003). In addition, the Meu14 protein has been reported to be localized to the leading edge of the FSM (Okuzaki et al., 2003). Double staining of meiotic cells with Meu14-GFP and Cam2-HA indicated that these two proteins colocalized near the nucleus, although the signal of Cam2 was broad and extended slightly from the Meu14 ring (Fig. 7B). We conclude that Cam2 is localized to the leading edge region of the FSM.
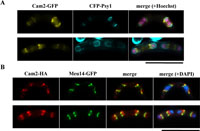 View Details | Fig. 7. Localization of Cam2 to the leading edge region of the FSM. (A) Fluorescence micrographs showing the spatial relationship between Cam2-GFP and the FSM. The homothallic haploid strain AI50 (cam2-GFP) was transformed with pREP1(CFP-psy1). The transformant was cultured in MM–N to induce meiosis and sporulation. Cells were stained with Hoechst 33342 (purple). Bars, 10 μm. (B) Colocalization of Cam2 with the leading edge marker protein Meu14. The homothallic haploid strain YN314 (meu14-GFP) was transformed with pAL(cam2-HA). The transformant was cultured on SSA plates to induce meiosis and sporulation. Fixed cells were stained with DAPI to visualize nuclei (blue). Cam2 was immunostained with anti-HA antibody, 3F10. Bars, 10 μm. |
Although localization of F-actin and Myo1 during meiosis and sporulation has been reported by other research groups, the relationship of these proteins to the FSM was not described (Petersen et al., 1998; Toya et al., 2001). The fluorescent signals of F-actin and Myo1 closely resemble those of Cam2 during meiosis-II; thus, it is likely that F-actin and Myo1 are also present at the leading edge region of the FSM. First, we studied the spatial relationship between Myo1 and Cam2 using a strain expressing Myo1-GFP and Cam2-CFP. As shown in Fig. 8A, both signals were largely colocalized at meiosis-I, meiosis-II and post-meiosis. We thus conclude that Myo1 is probably localized to the leading edge region of the FSM.
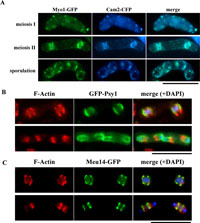 View Details | Fig. 8. Localization of Myo1 and F-actin to the leading edge region of the FSM. (A) Colocalization of Cam2 and Myo1 to the leading edge region. The homothallic haploid strain AI245, simultaneously expressing Myo1-GFP and Cam2-CFP, was sporulated on SSA plates. Bar, 10 μm. (B) Localization of F-actin to the open ends of the FSM. Cells were double-stained for F-actin and Psy1. The homothallic haploid strain YN68 (GFP-psy1) was cultured in MM–N. F-actin was stained with rhodamine-conjugated phalloidin. Bar, 10 μm. (C) Colocalization of F-actin with Meu14-GFP. The homothallic strain YN314 expressing meu14-GFP was sporulated on SSA. Bar, 10 μm. |
Next, we examined the localization of F-actin in spatial relation to the FSM. F-actin, as revealed by rhodamine-conjugated phalloidin in cells expressing GFP-Psy1, was shown to be present at the open ends of the cup-shaped FSMs during meiosis-II (Fig. 8B). Double staining of F-actin and Meu14-GFP indicated that both proteins largely overlap during assembly of the FSM (Fig. 8C). Similar to Cam2, the fluorescent signals from F-actin were rather broad and positioned slightly behind the Meu14 ring. Taken together, we conclude that F-actin and myosin-I (Myo1 and Cam2) are preferentially localized to the leading edge area of the FSM during meiosis-II.
The cam2Δ mutant is defective in spore formation at high temperature. We finally studied defects in sporulation at 34°C focusing on the FSM formation and the F-actin localization. As conjugation is inhibited at 34°C, heterozygous diploid strains were used in this experiments. The wild-type (AI239) and cam2Δ (AI240) strains expressing GFP-tagged Psy1were incubated in sporulation medium either at 28°C or 34°C. Fixed cells were stained with fluorescent phalloidin and DAPI to visualize F-actin and DNA, respectively. In the wild-type diploid strain, GFP-Psy1 signals were first observed at the plasma membrane of the mother cell before the completion of meiosis-I (data not shown); the signals then preferentially accumulated at the cup-shaped FSMs during meiosis-II (Fig. 9A) (Nakamura et al., 2001). At this stage, F-actin was present at the leading edge region (Fig. 9A). A closed compartment of the FSM containing a single nucleus was eventually formed (Fig. 9B, a). The cam2Δ cells showed aberrant FSM morphology and F-actin localization at 34°C. Only fragmented or anucleated FSMs were formed; and F-actin dots remained at peripheral regions of mother cells even during meiosis-II (Fig. 9A). The GFP-Psy1 fluorescent images gained from more than 500 tetranucleate mother cells that completed meiosis II were quantitatively analyzed (Fig. 9B, b). A majority of the wild-type cells (>90%) incubated at 34°C formed normal nucleated FSMs, but only 25% of cam2Δ cells did. Most of the remaining cam2Δ cells showed amorphous aggregates of GFP-Psy1 or formed anucleated FSM-like compartments. Most of the cam2Δ cells left the intense GFP-Psy1 signals at the mother cell plasma membrane. These facts suggest that the FSM formation was seriously impaired by the cam2Δ mutation at high temperature.
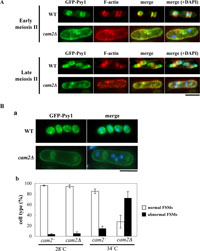 View Details | Fig. 9. Assembly of the FSM and localization of F-actin in cam2Δ at 34°C. (A) Formation of the FSMs and localization of F-actin during meiosis II. A wild-type (AI239) and a cam2Δ (AI240) diploid strains, expressing GFP-Psy1, were incubated on SSA plates for 16 hr at 34°C. After fixation, cells were stained with rhodamine-conjugated phalloidin and DAPI. Bars, 5 μm. (B) The cam2 disruption affects the FSM formation. A cam2+ strain (AI239) and a cam2Δ strain (AI240), both of which harbored the chromosomally integrated GFP-Psy1, were cultured on SSA plates for 2 days at either 28°C or 34°C. Fluorescent images of GFP-Psy1 (a) were observed, and cells were classified into normal (open columns) and aberrant (filled columns) cell types (b). Approximately 500 cells were counted for each sample. The mean value±standard deviation (vertical bars) of three independent cultures is presented. |
Calmodulin is a ubiquitous regulator that mediates calcium ion signaling in eukaryotes. S. pombe possesses two calmodulin-like proteins, Cam1 and Cam2 (Takeda and Yamamoto, 1987; Moser et al., 1995; Lord and Pollard, 2004). Cam1 is probably the major calmodulin in S. pombe, because its knockout results in lethality (Takeda et al., 1989), whereas the cam2 deletion mutant showed no detectable defects in cell multiplication (this study). Cam1 has been demonstrated to bind Ca2+ (Moser et al., 1995). The primary structure of Cam2 suggests that the acidic residues that are conserved in other calmodulins are missing in the putative Ca2+-binding sites of Cam2 (Fig. 1). Thus, the Ca2+-binding activity of Cam2 remains to be elucidated. Mutational analysis of cam1+ has indicated that it contains diverse functions not only in vegetative growth but also in sexual reproduction (Takeda and Yamamoto, 1987; Takeda et al., 1989). We found here that cam2Δ strains are defective in meiosis and sporulation at high temperature (Fig. 3). Assembly of the FSM was aberrant (Fig. 9).
Cam2 is capable of association with S. pombe type I myosin (Myo1), but not with type II myosin (Myo2) (Lord and Pollard, 2004). It remains possible that Cam2 might form a complex with type V myosins (Myo51/Myo5 and Myo52/Myo4). A single deletion of myo51 or myo52 showed no marked defects in sporulation (A. Itadani, unpublished results). Overexpression of Myo1 or Myo1ΔIQ in cam2Δ significantly alleviated sporulation defects. These facts suggest that the Myo1-Cam2 complex may play a rather specialized function in meiosis and sporulation.
As discussed below, a MyoI-Cam2 complex localizes to the growing front of the FSM, suggesting that myosin-I has a cellular function in assembly of the membrane compartment for spores. In fact, the FSM formation at high temperature is severely impaired by cam2 deletion (Fig. 9).
Cam2-GFP exhibited specific localization at the tips of mating projections (Fig. 4), the sites of cell elongation in response to mating pheromones. Notably, F-actin patches and Myo1 dots did not colocalize with Cam2 at this stage of the life cycle (Fig. 5B). This observation suggests that Cam2 can be activated independently of Myo1. Whereas heterothallic myo1Δ cells are almost completely sterile (Toya et al., 2001), mating efficiency was hardly affected by cam2 deletion. Thus, despite its localization at the mating projection, Cam2 is not required for conjugation under normal conditions.
Using tagged versions of Cam2, we have demonstrated that this protein localized to the future plasma membrane of newborn spores, called the FSM, and especially to the regions of the extending edge of the membrane (Fig. 7). The FSM grows by fusion with membrane vesicles probably derived from the endoplasmic reticulum and the Golgi apparatus (Nakase et al., 2001; Nakamura et al., 2001; Nakamura-Kubo et al., 2003; reviewed by Shimoda, 2004). This process is mediated by the two SNARE proteins Psy1, an S. pombe homologue of mammalian syntaxin-1A (Nakamura et al., 2001), and Sec9, a SNAP-25 homologue (Nakamura et al., 2005). The FSM is assembled into a nucleated membrane compartment via a cup-shaped intermediary form. Closure of the FSM is a critical step of spore morphogenesis, in which the leading edge may play an important role.
Meu14 was the first protein in S. pombe reported to constitute the ring-shaped structure at the leading edge of the FSM (Okuzaki et al., 2003), and the meu14Δ mutant forms aberrant FSMs (Okuzaki et al., 2003). Our study showed that type I myosin and F-actin also localize to the leading edge region (Fig. 4, Fig. 7 and Fig. 8). The fluorescent signals of Meu14 and F-actin partly overlapped and the visualized F-actin ring was broad as compared with Meu14 (cf. Fig. 8C). These observations strongly suggest that myosin-I and F-actin have pivotal roles in assembly of the FSM.
The budding yeast Saccharomyces cerevisiae essentially sporulates in a way similar to sporulation of S. pombe (reviewed by Neiman, 2005). Double unit membranes, named ‘prospore membranes’, which are equivalent to the FSM in S. pombe, are assembled during meiosis (Neiman, 1998). The leading edge of the prospore membrane is coated by several proteins identified as Don1, Ssp1 and Ady3 (Knop and Strasser, 2000; Moreno-Borchart et al., 2001). None of these proteins is homologous to Meu14 of S. pombe. The actin cytoskeleton is also implicated in S. cerevisiae sporulation, probably in spore maturation. The F-actin was neither localized to the prospore membranes, nor required for their formation in budding yeast (Taxis et al., 2006). Interestingly, despite the close resemblance of the sporulation mechanisms of fission and budding yeasts at a cellular level, the underlying pathways may differ at the molecular level. This observation may reflect a distant phylogenetic relationship between these two yeast species.
S. pombe type I myosin promotes actin assembly by the Arp2/3 complex, in conjunction with Wsp1 (Lee et al., 2000). Probably, the function of myosin-I in sporulation is, at least partly, to activate actin dynamics at the front end of the FSM. Inhibition of actin polymerization by latrunculin A severely impairs normal spore formation (Petersen et al., 1998). We can envisage several possible action mechanisms for F-actin associated with the FSM. First, F-actin polymerization might generate the pushing force that facilitates extension of the growing FSM. Second, a ring-shaped structure composed of F-actin/myosin-I/Meu14 might control morphogenesis of the FSM by binding the open end with hoops. Third, the opening of the cup-shaped FSM begins to close after encapsulating the postmeiotic nucleus, and this closing process might be mediated by myosin-I and F-actin, similar to contraction of the actomyosin ring on cytokinesis. These and other hypotheses remain to be tested by using mutants and specific inhibitors. The present study raises the important question of how the actin cytoskeleton controls assembly of the plasma membrane into nucleated membranous compartments.
We wish to thank Dr. M. Yamamoto (University of Tokyo), Dr. H. Nojima (Osaka University), Dr. D. Okuzaki (Osaka University) and the Yeast Genetic Resource Center Japan supported by the National BioResource Project (YGRC/NBRP; http://yeast.lab.nig.ac.jp/nig/) for strains. We also thank Dr. Y. Hiraoka (National Institute of Information and Communications Technology) and Dr. M. Yamamoto for plasmids. This study was supported by a Grant-in-Aid for Priority Area ‘Genome Biology’ from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
|