| To whom correspondence should be addressed: Anna Kurlandzka, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Pawinskiego 5A, 02-106 Warsaw, Poland. Tel: +48–22–592–1318, Fax: +48–22–658–4636 E-mail: ania218@poczta.ibb.waw.pl |
The cell wall of the yeast Saccharomyces cerevisiae participates in numerous biological processes, including resistance to osmotic stress, morphogenesis and budding. It is remodeled during cell growth, mating pheromone-induced changes of the cell shape, and nutrient-driven filamentation (Levin, 2005). In response to cell wall-damaging drugs the cell wall not only changes its shape but also its composition and this response is executed by the cell wall integrity pathway (Harrison et al., 2004; Levin, 2005).
Recently, numerous observations have accumulated which link the cell wall synthesis with the course of the cell cycle. For instance, it is known that Rho1p GTPase, a regulatory subunit of 1,3-β-glucan synthase, also regulates late stages of morphogenesis (Qadota et al., 1996; Levin, 2005). The targeting and activation of Rho1 is controlled by the polo-like kinase Cdc5 (Yoshida et al., 2006). This kinase has multiple functions in mitosis and cytokinesis by phosphorylating various substrates. Among other functions, Cdc5p regulates sister chromatid separation in yeast by phosphorylation of the cohesin subunit Scc1p and promotes mitotic exit (Alexandru et al., 2001). This set of observations couples chromatid separation with the final steps of the cell cycle and cell wall synthesis. Moreover, it has recently been found that cells defective in 1,3-β-glucan synthase (in which the synthesis of 1,3-β-glucan has stopped) arrest their growth at a stage before the separation of spindle pole bodies and spindle formation. It was postulated that a new checkpoint, termed the cell wall integrity checkpoint, ensures the coupling of cell wall synthesis and mitosis (Suzuki et al., 2004). Later it was shown that a mutant defective in the cell wall integrity checkpoint is in fact defective in the Arp1p subunit of the dynactin complex involved in nuclear migration (Igarashi et al., 2005). Deletion of ARP1 causes defects in both the cell wall integrity checkpoint and nuclear migration functions. It is assumed that the cell wall integrity checkpoint and nuclear migration involve distinct molecular functions of Arp1p, and that these two functions are separable in the Arp1p molecule. It is supposed that the molecular function of the cell wall integrity checkpoint may be independent of the dynein-based nuclear migration mechanism.
The examples described above indicate that there could be a link between the mechanisms regulating the cell wall biosynthesis, nuclear migration and chromosome segregation. In this report we describe another observation which supports this assumption, although we approach the issue from an opposite direction. We introduced an amino acid substitution F658G into the Irr1p/Scc3p cohesin, an element of the sister chromatid cohesion complex. This complex, responsible for chromosome segregation and genome stability, includes chromosomal ATPases Smc1p and Smc3p, the kleisin Mcd1p/Scc1p and Irr1p/Scc3p, the least studied component (for review, see Nasmyth, 2005; Nasmyth and Haering, 2005). The substitution is lethal in the haploid, and semi-dominant in the heterozygous diploid irr1-1/IRR1. We found that the diploid is disturbed in transmission of the nucleus from the mother to the daughter cell and has defects of cytokinesis. Moreover, the heterozygous diploid is sensitive to the cell wall-affecting compounds Calcofluor White and Congo Red, and shows disturbed chitin distribution. Since the overall biochemical composition of the cell wall is almost unchanged, this suggests that in the irr1-1/IRR1 strain cell wall assembly is impaired.
Standard molecular biology techniques for yeast and bacteria were used (Sambrook et al., 1989; Sherman et al., 1986). Yeast strains, isogenic with the strain W303, are listed in Table I. The deletion of IRR1 (ORF YIL026C) was constructed in our laboratory, the whole ORF was deleted and replaced by kanMX4. Escherichia coli XL1-Blue (Stratagene) was used for molecular manipulations. For drop-tests Calcofluor White (Sigma F-3397) was tested in concentrations from 0.5 mg/ml to 1.5 mg/ml, Congo Red (Sigma C-6767) from 10 μg/ml to 100 μg/ml, other reagents as indicated in the legend to Fig. 2. Site-directed mutagenesis was carried out using the Altered Sites in vitro mutagenesis system (Promega). To introduce the F658G substitution (TTC replaced by GGT) the oligonucleotide 5' TATCTGAACGTAGGTCACGGTCTGT 3' was used. By this manipulation the XmnI restriction site present in the original IRR1 sequence was replaced by a sequence which was not recognized by this enzyme, thus facilitating screening for the presence of the mutation.
To localize nuclei and GFP-tubulin in mitotic cells a Nikon Eclipse E800 fluorescence microscope with a 63x objective was used. DAPI (4',6'-diamino-2-phenylindole dichloride, Sigma) was used to stain DNA. Calcofluor White (0.5 mg/ml)-stained cell wall was observed in a Nikon Eclipse TE2000-E confocal microscope. Glycoproteins of the cell wall were stained with FITC-labeled concanavalin A (Sigma) and also observed in the confocal microscope.
For biochemical assays cells were grown in YPD medium to an OD600 of 1, collected and disrupted with glass beads. Cell walls were sedimented by 2600×g centrifugation and lyophilized. Chitin was determined using chitinase C (InterSpex Products) on whole cells according to Bulawa et al. (1986). Alkali-insoluble β-glucan levels were determined as described by Kapteyn et al. (1999). Briefly, alkali-soluble β-glucans were extracted three times in 3% (w/v) NaOH. The pellet was washed, suspended in 0.01 M Tris/HCl (pH 7.5) and digested with 1,3-β-glucanase (Zymolyase20T, ICN Biomedicals). After dialysis to water, 1,6-β-glucan was collected and quantified. Total alkali-insoluble glucan was measured as the hexose content before dialysis. The alkali-insoluble 1,3-β-glucan level was calculated by subtraction of the 1,6-β-glucan content from total glucan. The alkali-soluble 1,6-β-glucan fractions were dialyzed in water, lyophilized and dry samples were suspended in water. Serial dilutions were spotted on Hybond C-extra membrane (Amersham), probed with rabbit anti-1,6-β-glucan primary antibody and then with horseradish peroxidase-goat anti-rabbit secondary antibody (Amersham) (Kollar et al., 1997). The glucan signal was visualized by chemiluminescence, blots were scanned and quantified with the ImageQuant version 5.0 densitometry analysis software. The analysis of mannoproteins by confocal microscopy is described in the Microscopy section.
The cohesin Irr1p/Scc3p is the least studied element of the cohesion complex. It is assumed that the primary role of Irr1p consists in closing the cohesin ring and this protein is usually depicted as an element attached to the Mcd1/Scc1 kleisin subunit (for a review, see Nasmyth, 2005). However, it was also reported that the mammalian homologue STAG may act as a transcriptional co-activator (Lara-Pezzi et al., 2004). Moreover, our early results indicated that lowering the level of expression of IRR1 (after fusion with a regulatory catalase A gene promoter) affected colony formation on solid media (Kurlandzka et al., 1999).
Our main purpose was a systematic investigation of the role of various parts of the Irr1p cohesin since functional domains of this protein (or its homologues) are not known. To achieve this we introduced amino acid substitutions in parts of the Irr1p molecule which were deemed to be important as potential functional or structural sites. Altogether, we introduced six substitutions whose effects will be described in a separate paper. Here we report rather unexpected effects of one of those substitutions, F658G, which was introduced in this part of Irr1 cohesin which is evolutionarily conserved only among fungi.
To assay the effects of the mutations, variants of IRR1 were introduced on a centromeric plasmid into an irr1Δ::kanMX4/IRR1 hemizygous diploid, constructed in our laboratory. The substitution F658G (the allele named irr1-1) was introduced on the plasmid pAK11/3F-G (PIRR1-irr1-1-TIRR1 TRP1) to the irr1Δ/IRR1 hemizygote. Subsequently, sporulation/meiosis was induced, asci were dissected and phenotypes of viable spore-clones were analyzed. Forty asci were analyzed showing poor viability of spores. Only one or two spores from each tetrad germinated and none of the spore clones carried the irr1Δ::kanMX4 allele (kanamycin resistance, the marker of the disruption) and the plasmid (Trp+), which proved that the irr1-1 mutation is lethal in haploids. Wild-type haploids harboring the plasmid pAK11/3F-G were viable, although ca. 10% of cells had an aberrant morphology and/or defects in nuclear migration. In wild-type haploid yeast the percentage of morphologically aberrant cells did not exceed 3%.
To facilitate further experiments the irr1-1 gene was integrated into the TRP1 locus of the hemizygote irr1Δ/IRR1. In such strain numerous defects were observed, which were absent in irr1Δ/IRR1. Similar defects were seen in irr1Δ/IRR1 bearing the pAK11/3F-G plasmid. We observed cells with two nuclei in each cell and with misoriented short spindles. Such cells constituted ca. 15%–20% of all cells. Among them we also detected cells with disturbed cytokinesis, which are shown in Fig. 1 B.
 View Details | Fig. 1. Erratically dividing cells of the heterozygous irr1-1/IRR1 diploid have shortened and misoriented spindles. Mitotic spindle was visualized by GFP-α-tubulin, DNA was stained with DAPI, VIS-cells viewed in visible light. |
It has to be noticed that although the role of cohesin in the fidelity of chromosome segregation is well established, a direct link between this process and the process of nuclear migration has not been shown. Since the presence of a mutated copy of IRR1 caused aberrations in mitotic divisions, we assumed that they should lead to some growth phenotypes. Thus, IRR1/IRR1, irr1Δ/IRR1 and irr1-1/IRR1 were tested for sensitivity to various chemical, as recommended by Rieger et al. (1999). Growth was analyzed by plating drops of serial dilutions of cell suspensions onto solid media. Results of particular interest are summarized in Table II.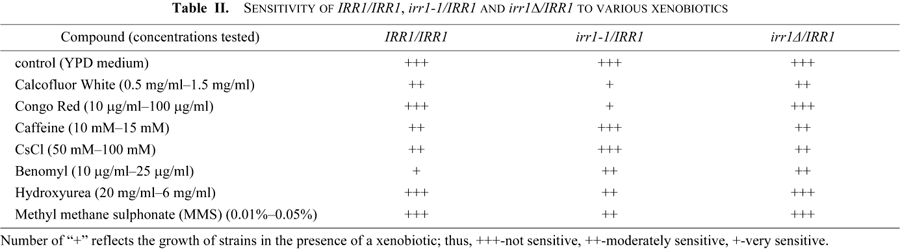
Since reports linking abnormalities in nuclear migration to cell wall defects have accumulated recently, in our tests we included Calcofluor White and Congo Red. Both compounds exert their effects through similar mechanisms leading to a loss of the cell wall integrity. Calcofluor White interacts specifically with chitin, whereas Congo Red interferes with both glucans and chitin (Roncero et al., 1985, 1988; Raclavsky et al., 1999). To our surprise, the diploid irr1-1/IRR1 exhibited sensitivity to both compounds even in the the lowest concentrations tested (0.5 mg/ml and 10 μg/ml, respectively). In Fig. 2 (central panel) we show an example of the test in which Calcofluor was used at 1 mg/ml and Congo at 40 μg/ml. We reasoned that the Calcofluor sensitivity could be associated with the lysis of cells which had the cell wall defect, but we found that addition of 1 M sorbitol to the Calcofluor-supplemented medium, which should prevent cell lysis, did not reverse the increased sensitivity of irr1-1/IRR1 to Calcofluor. We also found that irr1-1/IRR1 exhibits a decreased sensitivity to CsCl and caffeine compared to wild-type and moderately increased sesitivity to hydroxyurea, whereas irr1Δ/IRR1 behaves like the wild-type IRR1/IRR1 strain. None of the strains show sensitivity to UV light, actinomycin D, NaCl or divalent cations. The sensitivity to Calcofluor and Congo and the resistance to CsCl and caffeine were due to the presence of the mutated irr1-1 and not to a deficiency of wild-type IRR1 since the growth on media supplemented with these compounds of the hemizygous diploid irr1Δ/IRR1 was the same as the growth of the IRR1/IRR1 control. Moreover, both the nuclear migration and growth defects were dependent on the relative dosage of the irr1-1 allele, since they were less pronounced in an irr1-1/IRR1 strain additionally transformed with a plasmid bearing wild-type IRR1.
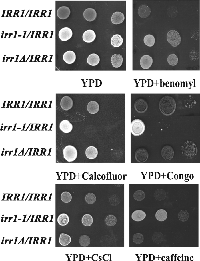 View Details | Fig. 2. The heterozygous diploid irr1-1/IRR1 exhibits increased sensitivity to Calcofluor White and Congo Red and moderately increased resistance to CsCl and caffeine. Liquid cell cultures were adjusted to a density (OD600) of 1. Growth was analyzed by plating 5-μl drops of 10-fold serial dilutions of cell suspensions onto solid media. In this example YPD plates were supplemented with benomyl (25 μg/ml), Calcofluor White (1 mg/ml), Congo Red (100 μg/ml), CsCl (0.075 M), and caffeine (10 mM). |
To check whether the sensitivity to Calcofluor White is associated with gross changes in the cell wall structure we performed confocal microscopy of Calcofluor-stained cells and of cells labeled with FITC-conjugated concanavalin A. Calcofluor stains chitin and concavalin A specifically stains mannans present in the cell wall (Tkacz et al., 1971). Comparing the images of the mutant and the control we found that the cell walls of irr1-1/IRR1 stained with Calcofluor are thicker but more dispersed compared to those of IRR1/IRR1 (Fig. 3, upper panel). Since the distribution of mannans in the mutant was the same as in the control (Fig. 3, lower panel), we supposed that the observed irregularities could be associated with changes in the content of chitin and 1,3-β- and 1,6-β-glucan. Levels of these compounds were directly measured in IRR1/IRR1 and irr1-1/IRR1. As we show in Table III, the content of chitin in the cell wall of the mutant is only slightly increased compared to the control value, and a slight decrease in 1,3-β-glucan and alkali-soluble 1,6-β-glucan contents can also be noticed. Thus, it is quite likely that the presence of mutated Irr1-1 protein mainly influences the distribution of chitin within the cell wall. The defects in chitin deposition concern 100% of irr1-1/IRR1 cells, both exhibiting and not exhibiting defects in nuclear migration and cytokinesis.
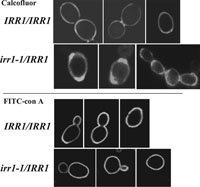 View Details | Fig. 3. Chitin is more dispersed in the cell wall of the irr1-1/IRR1 strain than in the cell wall of the wild-type, whereas distribution of mannoproteins remains unchanged. Confocal images of the cell wall stained for chitin with Calcofluor White (0.5 mg/ml) (upper panel) and for mannoproteins with FITC-conjugated concanavaline A (lower panel). |
Our results show that IRR1 affects regulation of the cell wall modeling although it is not clear how the cell wall structure is altered. Since in the irr1-1/IRR1 heterozygote we observed changes of chitin distribution associated with a significantly increased sensitivity to Calcofluor White and Congo Red, we assumed that the irr1-1 mutation may cause a defect in cell wall assembly. Recently, the cell wall assembly has been shown to be linked to the cell cycle, chromosome segregation and nuclear migration. It has been found that the cell wall synthesis is monitored by the cell wall integrity checkpoint which transduces signals through the dynactin complex (Arp1p, Jnm1p, Nip100p). It coordinates the cell wall integrity with spindle function, and regulates expression of M-phase cyclins (Suzuki et al., 2004; Igarashi et al., 2005). In consequence, mitosis can be affected as a result of blocking the synthesis of a major cell wall polysaccharide, 1,3-β-glucan.
It is not known whether such a checkpoint can function in the opposite direction, e.g. transducing a signal generated by aberrations in chromosome segregation to the cell wall assembly pathway. However, the phenotype of the irr1-1/IRR1 mutant implicates that the information between the cell wall and the mitotic spindle can be conveyed in both directions.
This work was supported by the Ministry of Science and Higher Education, grants 2 P04C01130 and PBZ-MIN-015/P05/2004.
|