| To whom correspondence should be addressed: Chikako Shingyoji, Department of Biological Sciences, Graduate School of Science, University of Tokyo, Hongo, Tokyo, 113-0033, Japan. Tel: +81–3–5841–4065, Fax: +81–3–5841–4385 E-mail: chikako@biol.s.u-tokyo.ac.jp |
Oscillation is a prominent feature of eukaryotic flagella. In sea urchin sperm, planar bending waves are formed at the base of the flagellum and propagate towards the distal end. The bending is caused by ATP-dependent, dynein-driven microtubule sliding (Summers and Gibbons, 1971; Shingyoji et al., 1977; Brokaw, 1991). Although all the dynein arms on the doublet microtubules of a flagellum are capable of moving the adjacent doublets (Sale and Satir, 1976), the speed and extent of microtubule sliding vary during a beat cycle according to the position in the flagellum and the phase of the bending cycle. In demembranated sea urchin sperm flagella, bending waves can be generated by ATP (Gibbons and Gibbons, 1972; Brokaw, 1975), and local application of ATP to any region of the demembranated flagellum induces a localized pair of bends or localized beating (Shingyoji et al., 1977). The local reactivation experiments corroborated the microtubule sliding model of ciliary and flagellar movement and identified some conditions for bend formation (Shingyoji et al., 1977; Takahashi et al., 1982), but are by themselves of limited use for further analysis of the mechanism of flagellar oscillation. In the sea urchin sperm flagella, bend formation in one direction is usually followed by bending in the opposite direction, and this cyclical formation of bends is always coupled with growth and propagation of the bends. These coupling phenomena make it difficult to analyze the factors governing the induction of flagellar oscillation. To overcome this difficulty a new method to control the timing of the induction of oscillatory movement was deemed necessary.
Previous studies have demonstrated that certain aspects of flagellar movement, e.g. beat frequency and wave initiation, can be modified by mechanical manipulation of the flagellum (Okuno and Hiramoto, 1976; Gibbons et al., 1987; Shingyoji et al., 1991; Shingyoji et al., 1995). It has also been shown that inactive dynein arms in the axonemes are activated by bending the doublet microtubules (Hayashibe et al., 1997; Morita and Shingyoji, 2004). The activity of the dynein arms thus appears to be modulated, in some cases enhanced, by the mechanical environment or mechanical “signals”. It seems probable that bending of the doublet microtubules constitutes an important factor in the self-regulatory feedback system underlying the flagellar oscillation (Morita and Shingyoji, 2004).
To analyze the factors governing flagellar oscillation, we studied the effect of bending demembranated, motionless flagella by micromanipulation in the presence of ATP at concentrations near or below the threshold for inducing spontaneous beating. By this method we were able not only to induce but also to control the timing of flagellar oscillation. Our results showed that the motile activity of dynein can be increased by the mechanical effect, thereby inducing oscillation even at very low ATP concentrations.
It has been demonstrated that ADP is involved in the regulation of flagellar motility and dynein activity (Kinoshita et al., 1995; Omoto et al., 1996; Yagi, 2000; Kikushima et al., 2004). Our study indicated that the presence of a very low concentration of ADP is essential for continuous beating at very low ATP concentrations. The difference in the species of dynein and/or the regulatory mechanism of the motile activity of dynein between the proximal and the more distal regions of the flagellum may also be an important factor determining the induction of flagellar oscillation.
Spermatozoa of the sea urchin, Pseudocentrotus depressus, were demembranated according to the previously described methods (Yoshimura and Shingyoji, 1999). Demembranation was stopped by adding reactivating solution without ATP. Demembranated sperm that showed 80% or higher reactivation rate at 200 μM ATP were used for experiments. All experiments at low ATP concentrations were performed within 10 min after the ATP addition. For experiments with ADP, ADP was added 15 sec before the addition of ATP. To reduce the concentration of ADP to as low as <0.1 μM (Shiroguchi and Toyoshima, 2001), we used an ATP regeneration system. For this experiment, we treated the demembranated sperm with 20 mM creatine phosphate and 0.2 mg/ml creatine kinase for 3 min on ice before the addition of ATP. For each condition we obtained the data from three to eleven separate experiments, in each of which one to three sperm flagella were usually studied. All experiments were carried out at room temperature (20–23°C).
A glass microneedle with the tip coated with 0.1% poly-L-lysine (P-1264, Sigma, U.S.A.) was mounted on a micromanipulator (WR-88, Narishige, Tokyo, Japan) and the head of a demembranated motionless sperm was attached to the tip. To bend the flagellar axoneme, the distal end of the axoneme was attached to another glass microneedle, which was then moved with respect to the head. To apply a mechanical pulse through the glass microneedle holding the sperm head, the manipulator was touched lightly by hand.
Flagellar responses to bending or a mechanical pulse were observed under an inverted microscope (TMD, Nikon, Tokyo, Japan) equipped with a dark-field condenser (Nikon, Tokyo, Japan) and a ×40 objective lens and recorded on video tape by using a CCD camera (Watec Neptune 100, Watec Co., Japan) with an image intensifier (DII-2050, Nakanishi Image Lab Inc., Tokyo, Japan) and a VHS videocassette recorder. The beat frequencies of flagella were measured by using a stroboscopic light source (Model 271C, Chadwick-Helmuth Co., U.S.A.). Recorded images of the flagella and the glass microneedles were traced from the monitor screen onto a transparent sheet by hand.
Fig. 1A shows the average reactivation rates, i.e. the percentage of motile sperm, at various ATP concentrations. As we did not use an ATP regeneration system, the sperm density was kept as low as possible so that the flagellar beat frequency at 3.0 μM ATP stayed almost constant for more than 10 min. Below 5.0 μM ATP, the rate of reactivation decreased with the ATP concentration. The wave propagation was also influenced by ATP. At 3.0 μM ATP, most of the beating sperm showed normal oscillatory waves with complete propagation along the length of the flagellum (Fig. 1B, left), while in others the bending waves were propagated only in the proximal and middle regions (Fig. 1B, right). The number of flagella showing incomplete propagation increased as the ATP concentration decreased. At 2.0 μM ATP, 5.8% of sperm were motile and all sperm became motionless at 1.5 μM ATP. The motionless flagella at 1.0–3.0 μM ATP were either straight or showed waves of very small amplitude. In contrast, the flagella in the absence of ATP had a clear bend or S-shaped bends showing so-called rigor waves (Gibbons and Gibbons, 1974).
 View Details | Fig. 1. Reactivation of sperm flagella at low ATP concentrations. (A) Average reactivation rates of demembranated flagella at 1.5–20 μM ATP. Each plot with a bar indicates mean±s.d. Numbers beside the plots show the total numbers of sperm observed. (B) Tracings of waveforms showing oscillatory beating with complete (left) and incomplete (right) propagation at 3.0 μM ATP. The sperm heads were attached to the glass surface. |
In the first series of experiments, we bent the flagellum of a motionless sperm by moving the distal end of the flagellum with respect to the head in the presence of 1.0–4.0 μM ATP. This induced a bend or bends in the flagellum (Fig. 2A and 2B). When the whole length of the flagellum was bent in one direction, a single bend was induced at the base of the flagellum (Fig. 2A). Such a bend, which was induced in 16 flagella, did not show further development. In contrast, when the flagellum was deformed so as to induce a pair of bends along the flagellum (Fig. 2B and 2C), the induced bends usually showed further development (Fig. 2D).
 View Details | Fig. 2. Effects of imposed bending on motionless flagella. (A) and (B) Schematic diagrams showing two ways of inducing bending in a motionless sperm whose head was held by the left microneedle. Bending the whole flagellum in one direction with the right microneedle induced a bend at the base of the flagellum (A), while pushing the distal end of the flagellum towards the head induced a pair of bends (indicated with open arrowheads) (B). (C) Video images showing the motionless flagellum at 2.5 μM ATP before (1) and during (2) mechanical deformation imposed as shown in B. The sperm head (indicated with a dotted line in 1) was held by the left microneedle (images overlapped). The tip of the flagellum was caught by the right microneedle. (D) Four types of flagellar response observed under imposed bending shown by sequential tracings from video images. The position of the flagellum (numbered with zero) and a glass microneedle before each mechanical deformation are indicated in red. Numbers indicate the sequence of tracings. In “beating”, changes of waveforms are shown only for the first of two or more cycles. Arrows indicate the direction of bend propagation. |
Induction of a pair of bends (which we call “bend formation”) was often followed either by propagation of the distal bend of the pair (“propagation”), or by growth and propagation of the proximal bend to accompany those of the distal bend (a “full cycle”), or by beating for two or more cycles (“beating”) (Supplemental Video 1), in the apparent order of development. All of the four types of response were induced at 2.0–3.0 μM ATP, but “beating” and “full cycle” were not induced at lower concentrations (Fig. 3A).
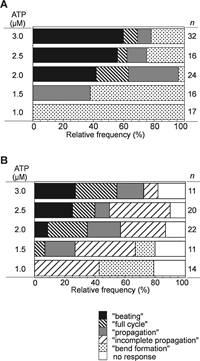 View Details | Fig. 3. Effects of mechanical manipulation on the frequency of occurrence of flagellar responses at low concentrations of ATP (1.0–3.0 μM). (A) Mechanical deformation imposed on motionless flagella induced four types of response: formation of a pair of bends, propagation of the distal bend of a pair of bends, growth and propagation of the proximal bend following the propagation of the distal bend, and beating for two or more cycles, which were named as “bend formation”, “propagation”, “full cycle, and “beating”, respectively. (B) A mechanical pulse applied to motionless flagella induced five types of response, which included propagation of the distal bend of a pair of bends for a part of the flagellum (“incomplete propagation”) in addition to the four types shown in (A). |
The flagellar response was observed only under imposed bending; removal of the mechanical constraint by detaching the microneedle from the tip of the flagellum terminated the responses at any moment, restoring the almost straight form of the flagellum (Supplemental Video 2). Induction of the four types of flagellar response by mechanical deformation was reproducible: applying the deformation again to the same flagella (n=27), induced the same response as the first one in 37% of them, while in 56% of them the responses were of the type a step more developed; for example, a “full cycle” in the first and “beating” in the second trial. The threshold concentration of ATP (2.0 μM) for induction of “beating” did not change between the first and the second trials. A microneedle, which was attached to the distal end of the flagellum, was moved at 3–41 μm/s for a distance of 2.2–34.9 μm, but the differences in these parameters did not affect the rates of occurrence of the four types of response.
The beat frequency of the mechanically induced “beating” increased with the ATP concentration like the frequency of spontaneous beating. However, the average beat frequencies of the mechanically induced “beating” at 2.0–3.0 μM ATP were slightly lower than those of the spontaneous beating (Table I): at 2.5 and 3.0 μM ATP the differences were statistically significant (Mann-Whitney U test, P<0.05).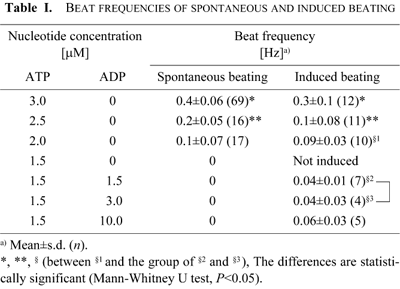
In the second series of experiments, we used only one microneedle to hold the sperm head, leaving free the distal end of the flagellum, and applied a mechanical pulse by touching the micromanipulator holding the microneedle. In many cases the pulse induced a pair of small bends near the base of the flagellum (Fig. 4A and 4B). In most cases the formation of bends (“bend formation”) was followed by one of the five types of flagellar response (Figs. 3B and 4C). Except for the cases in which the distal bend of the pair propagated for a part of the flagellum (“incomplete propagation”), the four types of response were similar to those induced by bending. All five types of flagellar response proceeded in the absence of a further mechanical pulse. This observation supports the idea that the flagellar response induced by the mechanical pulse involved the activity of dynein and was not a passive elastic response of the demembranated sperm.
 View Details | Fig. 4. Effects of a mechanical pulse on motionless flagella. (A) Schematic diagram showing the method of applying a mechanical pulse through the microneedle holding the sperm head. A light tap on the micromanipulator on which the microneedle was mounted induced a pair of small bends in the proximal region of the flagellum (indicated with two open arrowheads). (B) Sequential video images showing a demembranated sperm at 2.5 μM ATP before (1), during (2), and after (3) the application of a mechanical impulse. The distal bend of the pair of bends induced by the mechanical pulse was indicated with a white arrowhead. (C) Sequential tracings from the video images showing five types of flagellar response induced by the mechanical pulse. The position of the flagellum before each mechanical pulse was indicated in red (numbered with zero). The numbers show the sequence of tracings. Arrows indicate the direction of bend propagation. |
Fig. 3B summarizes the frequencies of occurrence of the five types of response induced by the mechanical pulse at different ATP concentrations. “Beating” was induced less frequently by a mechanical pulse than by bending (Fig. 3A), but the critical ATP concentration for inducing “beating” was the same in both methods (2.0 μM ATP) (Fig. 3A and 3B). The speed (8–24 μm/s) and the amplitude (1.3–2.5 μm) of the movement of the microneedle in the mechanical pulse did not affect the occurrence of the types of response.
Comparison of the pair of bends before and during their development into further flagellar responses for different types of responses showed that the position and angle of the distal bends were correlated with the types of the flagellar response, regardless of the ATP concentration. Table II summarizes the average position (distance from the base measured along the flagellum) and the average angle of the initially induced distal bends and the average angle of the maximally grown distal bends for each type of flagellar response induced at 1.0–3.0 μM ATP.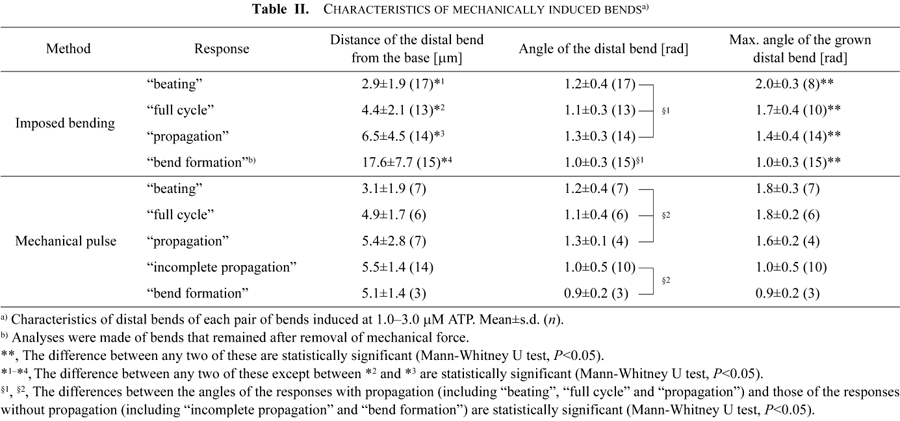
In the bending experiments, the more developed responses among the four types (beating>full cycle>propagation>bend formation) were observed when the first pair of bends was induced in a region closer to the flagellar base. The difference in the distance was statistically significant (*1–*4 in Table II; Mann-Whitney U test, P<0.05) between any two of the flagellar responses, except between the “full cycle” and the “propagation”. In the mechanical pulse experiments, “beating” was also induced when a pair of bends was formed closer to the base. It is interesting that “beating” and “full cycle” were developed from bends formed at similar positions (3.1 μm and 4.9 μm) in both the bending and the mechanical pulse experiments (Table II).
In both types of experiments, the bend angles of the distal bend did not differ much among the types of flagellar response, but when we compare the angles between the flagellar responses with and without propagation (§1 and §2 in Table II) it is clear that the responses with propagation grew from bends with larger angles (Mann-Whitney U test, P<0.05). The growth of the distal bends was also correlated with the type of response. In the bending experiments the larger the maximum bend angles, the more developed the type of response became (** in Table II; Mann-Whitney U test, P<0.05). More precisely, the threshold angles of the distal bends for the induction of “propagation”, “full cycle” and “beating” were about 1.4 rad, 1.7 rad and 2.0 rad, respectively. The proximal bend of the first pair of bends also grew up to 1.8±0.4 rad (n=10) for “full cycle” and 2.2±0.3 rad (n=8) for “beating”. There was no correlation between the curvature of the distal bend and the type of response.
Without ATP, bending the demembranated flagella in the presence of 1–100 μM ADP did not induce further flagellar responses. At 1.5 μM ATP, in the absence of ADP, the mechanically-induced pair of bends developed into “propagation” in 40% of the flagella (Fig. 5), while “full cycle” and “beating” did not appear. In the presence of both ADP and ATP at 1.5 μM, however, “full cycle” and “beating” as well as “propagation” were induced by imposed bending (Fig. 5). The position and the angle of the initial bends induced at 1.5 μM ATP with ADP could not be measured because the bends were not in the plane of focus.
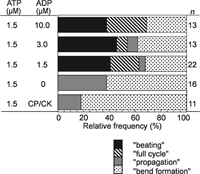 View Details | Fig. 5. Effects of the presence of ADP on the flagellar responses induced by imposed bending of motionless flagella at 1.5 μM ATP. In the presence of ADP “beating” and “full cycle” appeared. The concentration of ADP did not affect the rate of occurrence of these responses. CP/CK: in the presence of an ATP regeneration system, creatine phosphate and creatine kinase, instead of ADP. |
The frequencies of occurrence of the “full cycle” (20%) and “beating” (40%) were similar to those induced at 2.0 μM ATP without ADP (Fig. 3A). In contrast, at 1.5 μM ATP with 1.5–10 μM ADP the spontaneous beating, which was observed at 2.0 μM ATP, was never observed (Fig. 1A). The beat frequency as well as the frequencies of occurrence of “beating” at 1.5 μM ATP with ADP did not significantly change with the ADP concentration (1.5, 3.0 and 10 μM) (Table I). The average beat frequency at 1.5 μM ATP with 1.5 and 3.0 μM ADP (0.04 Hz) was significantly lower than that of the induced “beating” at 2.0 μM ATP (0.09 Hz) (Table I). These observations indicate that ADP does not function simply as a substrate of adenylate kinase to increase the ATP concentration. The presence of the ATP regeneration system (CP/CK) at 1.5 μM ATP did not change the frequency of occurrence of the responses but decreased the rate of occurrence of “propagation”. The results indicate that ADP may influence the motile activity of dynein so as to induce flagellar oscillation.
We found that ADP was also related to the stability of cyclical beating. As mentioned above, removal of the mechanical constraint by detaching the microneedle from the distal end of the flagellum terminated the flagellar response and the flagellum became straight. This was observed at 2.0–4.0 μM ATP. A typical example is shown in Fig. 6A (left) in which the “beating” induced by the imposed bending at 2.0 μM ATP (left top) stopped by detaching the right microneedle from the tip of the flagellum and the bends relaxed (left bottom). However, in the presence of 2.0 μM ADP in addition to ATP (center) the removal of the microneedle induced propagation of the last bend at more distal region of the flagellum (center bottom) before the stoppage of “beating” (center top). This was observed in one flagellum. We then examined the effect of the presence of both ADP and ATP at 2.5 μM. In three of the seven flagella studied flagella continued beating for 1–5 cycles after the removal of the microneedle (Fig. 6A right and 6B) (Supplemental Video 3).
 View Details | Fig. 6. Increase of the oscillatory ability of flagella by ADP. (A) Video images showing “beating” before and after the removal of mechanical manipulation. By detaching the right microneedle from the flagellum, “beating” stopped at 2.0 μM ATP (left), the last distal bend propagated at 2.0 μM ATP with ADP (center), and beating continued at 2.5 μM ATP with ADP (right). (B) Sequential tracings showing two examples of continuous beating after removal of the microneedle at 2.5 μM ATP with 2.5 μM ADP. The left tracings shown are from the same flagellum as shown in A (right). Numbers indicate the sequence of tracings. (C) Effects of ADP on the average beat frequency of spontaneously beating flagella at 3.0 μM ATP. Each plot indicates mean±s.d. (n=7–11). All average beat frequencies in the presence of ADP are significantly larger than the beat frequency in the absence of ADP (Mann-Whitney U test, P<0.05). |
The beat frequency of the mechanically induced “beating” in the presence of both ADP and ATP at 2.5 μM (0.3–0.4 Hz) was higher than the average beat frequency of the mechanically induced “beating” at 2.5 μM ATP without ADP (0.1 Hz). It seems likely that the continuous beating was achieved by an ADP-dependent increase of the oscillatory ability of the flagella. The beat frequency at 2.5 μM ATP in the presence of ADP (0.3–0.4 Hz) is near the maximum beat frequency of spontaneous beating without ADP (0.14–0.37 Hz). A similar increase of the beat frequency induced by ADP was also observed in the spontaneous beating at 3.0 μM ATP by an application of 1–20 μM ADP, but the beat frequencies in the presence of ADP did not significantly change with the ADP concentration (Fig. 6C). We should also note that even in the flagella that were beating at 0.3 Hz or higher under imposed bending at 3.0 μM ATP (Table I), continuous beating stopped on removal of the mechanical constraint. Thus, there was no direct correlation between the beat frequency and the stability of beating. We may conclude that the beat frequency does not by itself determine the stability of oscillation, and that the presence of ADP seems to be important for the regulation of the rate as well as the stable switching of the microtubule sliding during flagellar beating.
To determine whether the proximal region of flagella is important for the flagellar responses to mechanical perturbations, we studied the effect of bending the more distal region. To do this we attached both the head and a region of the flagellum just behind the head to the microneedle. The length of the attached part of the flagella was varied over the range 2–32 μm. As the average length of flagella was 40.7±2.6 μm (n=55), the length of the free region of the flagellum (L in Fig. 7A) that was experimentally bent was about 3–41 μm.
 View Details | Fig. 7. Effects of the length of the flagellum that can be deformed on the induced flagellar response. (A) The proximal part of the flagellum was attached to the microneedle (left) along with the sperm head (top). The free region of the flagellum (L) was bent by moving the right microneedle (bottom). (B) and (C) summarizes the rate of occurrence of the flagellar responses at 2.0 μM ATP (B) and 1.5 μM ATP with 1.5 μM ADP (C). The length of the free region, L, was measured as the distance measured along the flagellum between the tips of the left and the right microneedles. The whole lengths of the flagella used were about 41 μm. |
At 2.0 μM ATP, bending the whole flagella induced a pair of bends (“bend formation” in Fig. 7B). In most cases this was followed by “propagation” (35%), a “full cycle” (20%) or “beating” (35%), but preventing the movement of the proximal region decreased the frequency of development of these flagellar responses (Fig. 7B). The shorter the length of the free portion of the flagellum, the lower the frequency of occurrence of the more developed types of response. For the induction of “beating”, the proximal half or so of the flagellum was required.
We also tested the effect of preventing the movement of the proximal region in the presence of ADP. Fig. 7C shows the occurrence of the flagellar responses in the presence of 1.5 μM ATP and 1.5 μM ADP. Without prevention, some bends developed “beating” (Figs. 5 and 7C) and the percentage of the “beating” (40%) was similar to that observed in the whole flagella at 2.0 μM ATP (Fig. 7B). Preventing the movement of the proximal region for only 2–3 μm, however, reduced the percentage of “beating” to zero (Fig. 7C).
In this study we have shown that the demembranated flagella of sea urchin sperm are capable of beating by responding to imposed bending or a mechanical pulse at ATP concentrations around and below the threshold for spontaneous beating. We further identified several factors determining the induction of oscillation: 1) The critical (threshold) concentration of ATP is 2.0 μM; 2) the presence of ADP reduces the threshold to 1.5 μM ATP; 3) localized microtubule sliding in the flagellum to form a pair of bends is necessary; 4) the axial difference along the flagellum is important.
The threshold ATP concentration for the mechanically induced beating was 2.0 μM ATP (or 1.65 μM MgATP), which was similar to that for spontaneous beating. Four types of flagellar response were induced in motionless flagella in an ATP concentration-dependent manner and the beat frequency of the induced beating was also ATP-dependent. These observations suggest that the mechanically induced oscillation is caused by the activity of dynein under the same regulatory mechanism as the one that underlies normal oscillatory beating. The presence of a threshold ATP concentration for beating indicates that a certain proportion of dynein arms engaged in force production is required for the induction of microtubule sliding in the axoneme. The threshold ATP concentration for beating may be the minimum concentration of ATP necessary for the coordinated activity of dynein arms, which may involve a regulatory mechanism as well as force production of dyneins.
The ATP concentration required for the reactivation of beating is slightly higher in a reactivating solution containing KCl (8–10 μM) (Shingyoji et al., 1977) instead of K-acetate (2 μM in this study). It has been reported that the demembranated flagella show a higher beat frequency in a reactivating solution containing K-acetate instead of KCl (Gibbons et al., 1982). In general, acetate anions have a stronger stabilizing effect on the structure and function of proteins than chloride anions (Cacace et al., 1997); the different properties of these anions could affect the activation of dynein. The stability of dynein molecules would be important for the regulation of their motile function. Stabilizing ions may elevate the level of coordination between the dynein molecules, resulting in a decrease of the threshold ATP concentration for beating. It has been demonstrated that the presence of free Mg2+ and ATP4– at the mM level influences the dynein activity and decreases the beat frequency (Okuno and Brokaw, 1979). The concentrations of free Mg2+ (1.0 mM) and ATP4– (0.3–1.5 μM) in this study should not change over a broad range of ATP concentrations (1.5–10 μM). Therefore, we conclude that the stoppage of beating at a very low ATP concentration is caused by the lack of ATP: ATP above the threshold concentration is necessary for the coordinated activity of dynein arms to produce flagellar oscillation.
The importance of mechanical signals in the regulation of flagellar movement has been pointed out by previous studies, which suggested that the mechanical manipulation enhances the flagellar motility (Okuno and Hiramoto, 1976; Shingyoji et al., 1991; Shingyoji et al., 1995; Hayashibe et al., 1997; Morita and Shingyoji, 2004). In these studies, changes of the beat frequency and bend angle of beating flagella were induced by application of a continuous mechanical signal (imposed bending), while beating was achieved by a transient signal (mechanical pulse). Both continuous and transient signals were found to be useful to induce oscillatory beating in this study. The result indicates an important role of the mechanical signal in triggering the coordinated activity of dynein to produce microtubule sliding. Besides the increase of the dynein activity, imposed bending was effective to induce the switching of microtubule sliding necessary for the alternation of bending directions (Morita and Shingyoji, 2004). This seems to be related to the self-regulatory feedback system by which the motile activity of dynein is regulated to produce oscillatory movement. The present finding that the oscillatory beating can be induced in motionless flagella by imposed mechanical signals strongly supports the idea that mechanical signals are involved in the self-regulatory feedback system underlying flagellar movement.
ADP is known to play an important role in the regulation of the motile activity of dynein and flagellar movement (Lindemann and Rikmenspoel, 1972a, 1972b). ADP has been shown to be a factor enabling the non-motile Chlamydomonas flagella missing a part of the central pair or radial spokes to beat (Omoto et al., 1996). ADP also increases the velocity of microtubule sliding by isolated inner arm dynein (Yagi, 2000; Shiroguchi and Toyoshima, 2001; Kikushima et al., 2004). The motile activity of cytoplasmic dynein has been shown to be regulated by the condition of nucleotide binding (Kon et al., 2004). If this is also the case for axonemal dynein at low ATP, a very small amount of ADP can be important for the regulation of motile activity.
The present finding has shown that ADP is an activator of the motile function of dynein and strongly supports the above-mentioned studies: Responding to mechanical signals, demembranated flagella started oscillatory beating even at 1.5 μM ATP (or 1.2 μM MgATP) which was below the threshold, only when ADP was present. ADP possibly changes the structure and function of dynein to enable it to generate microtubule sliding in response to the mechanical signal. The beat frequency of the induced oscillation did not change over a wide range of ADP concentrations (1.5–10.0 μM ADP or 0.7–4.6 μM MgADP). The effect of ADP cannot be explained simply by an increase of the ATP concentration.
The induction of oscillation seems to be determined not directly by ATP but by a combination of ATP and ADP. The present observations appear to be closely related to the behavior of a monomeric inner arm dynein of Tetrahymena, whose ATPase activity and the microtubule translocation activity follow the Michaelis-Menten type kinetics only in the presence of a low concentration of ADP (Shiroguchi and Toyoshima, 2001). The mechanism by which ADP modulates the activity of dynein has not been elucidated, but a tempting possibility would be as follows: the mechanical signals accelerate the motile activity of dynein by increasing the binding of ADP to non-catalytic nucleotide binding sites of dynein. How ADP interacts with (or binds to) dynein under physiological ATP conditions is one of the next questions of interest.
Previous studies have shown that localized, cyclical formation of a pair of bends can be brought about by ATP iontophoresis in any region of the flagellum (Shingyoji et al., 1977; Takahashi et al., 1982). This demonstration shows that the ability to generate oscillation is distributed along the flagellum and not restricted to a specific region, e.g., at the flagellar base, and that local difference of the microtubule sliding along the flagellum is the basis for bend formation. In the present study, formation of a pair of bends by mechanical signals was required for further development including bidirectional bend formation (a “full cycle”) and oscillation (“beating”). The bending and the mechanical pulse used in this study induced similar responses. Dynein may have received different mechanical signals in these procedures, but the result indicates that the initial transient probably functioned as the signal to induce microtubule sliding to form a pair of bends.
Detailed analysis of the starting transients induced by an increase of pH (from 5.3 to 8.2) in live sperm or by an application of 0.2 mM ATP in demembranated sperm flagella have shown that the straight flagella begin to move by synchronous sliding, forming a basal bend, while flagella that contain a basal bend begin to move by metachronous sliding within that bend (Goldstein, 1979). When a sperm axoneme is subjected to bath application of ATP the synchronous sliding all along the flagellum would be followed by metachronous sliding at the basal bend, which would trigger continuous beating. Similar response, in which mechanically induced synchronous sliding triggers beating, has been reported in paralysed Chlamydomonas flagella missing the central pairs or radial spokes. Beating can be induced in these flagella when forced bending is applied so as to form a single bend at the base (Hayashibe et al., 1997). In the present study, mechanical deformation at a very low ATP concentration produced a basal bend probably by synchronous sliding (Fig. 2A) which, however, was not followed by metachronous sliding. In contrast, when a pair of bends was induced by bending, synchronous sliding occurred at the interbend region and metachronous sliding occurred at the bends. Furthermore, the present results indicate that the metachronous sliding at the distal bend of a pair of bends is important for the development of the bends, since in the “full cycle” and the “beating” the growth and propagation of the bend occurred first at the distal bend and then at the proximal bend. This result indicates a possible role of the metachronous sliding at the distal bend in regulating the dynein activity at the more proximal bend.
The ability of generating oscillation has also been demonstrated to be distributed along the flagella by laser-beam amputation experiments (Goldstein et al., 1970) and by locally inhibiting microtubule sliding with an inhibitor (Fujimura and Okuno, 2006). These observations have suggested that beating can occur if the microtubules are tied together at one fixed end of the flagellum. This “basal anchoring model” is also supported by computer simulation (Brokaw, 1986) and by theoretical models (Lindemann, 1994; Lindemann and Kanous, 1995). In the present study, a microneedle was attached to the proximal region of the flagellum which prevented bending movement, but probably did not completely inhibit microtubule sliding, in this region. We found that mechanical induction of a pair of bends in the middle region of the flagellum produces “beating”, indicating that the fixed basal end is not essential for flagellar oscillation. It seems that the formation of a pair of bends, which results from spatial differences in microtubule sliding along the flagellum is essential. However, oscillatory movement was not induced in the distal half of the flagellum even when a pair of bends is induced by mechanical deformation. Thus, induction of microtubule sliding to form a pair of bends is not enough for the initiation of oscillation.
The regional difference along the flagellum is consistent with the incomplete wave propagation observed in the distal region at a low ATP concentration, such as 3 μM ATP. The observed difference between the proximal and the distal regions may be caused by a difference in the types of dynein arms along the flagellum (Piperno and Ramanis, 1991) although there is no clear evidence supporting this idea in sea urchin sperm. It is also possible that the spatial difference is caused by differences in structures other than dynein, e.g. the central pair/radial spoke complex, which are important for the regulation of dynein activity.
The mechanical deformation (or imposed bending) used in this study is a useful method for inducing stable, reproducible responses. To remove the mechanical signal, however, the microneedle attached to the distal end of the flagellum should be detached, which inevitably gives a new mechanical signal to the flagellum. The presence of a small amount of ADP (1.5–10.0 μM ADP or 0.7–4.6 μM MgADP), however, enables the flagellum to beat even during and after the removal of the microneedle. The mechanism of activation of dynein by ADP is not understood. If ADP behaves as a positive regulator of dynein activity, a possible interpretation is that the mechanical signal activates the motile function of dynein through the interaction of ADP with the dynein arms.
Whether mechanisms similar to what we have found under low ATP conditions operate in live sperm or in reactivated sperm flagella under high ATP conditions remains to be elucidated. In all cases, axial differences along the flagellum should be considered as a factor regulating the activity of dynein during flagellar oscillation. This difference would generate a spatial difference in microtubule sliding along the flagellum to cause bend formation. Formation of bends in distal regions will trigger microtubule sliding in more proximal regions, leading to further formation of bends (Morita and Shingyoji, 2004) and thus initiating flagellar oscillation.
We would like to express our gratitude to Professor Keiichi Takahashi for discussion and improving of the manuscript. This work was supported by Grant-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government (C.S.).
|