| To whom correspondence should be addressed: Mamoru Isemura, Graduate School of Nutritional and Environmental Sciences, University of Shizuoka, Yada, Shizuoka 422-8526, Japan. Tel: +81–54–264–5824, Fax: +81–54–264–5824 E-mail: isemura@u-shizuoka-ken.ac.jp Abbreviations: ATRA, all-trans-retinoic acid; AP-1, activator protein-1; ELISA, enzyme-linked immunosorbent assay; IL-2, interleukin-2; IL-2R, interleukin-2 receptor; PCR, polymerase chain reaction; RT, reverse transcription; PHA, phytohemagglutinin; PKC, protein kinase C; RAR, retinoic acid receptor; TPA, 12-O-tetradecanoylphorbol-13-acetate. |
Antigenic stimulation of T cells initiates a complex series of intracellular signaling pathways that target and activate different cytokine genes. In vivo, T-lymphocytes are activated by the interaction between T-cell receptor and antigens displayed on the surface of antigen-presenting cells to trigger a cascade that produces interleukin-2 (IL-2) resulting in cell proliferation. The activation of T-cells can be mimicked in vitro with various stimulants including anti-CD3 antibodies, anti-CD28 antibodies, phytohemagglutinin (PHA), 12-O-tetradecanoylphorbol-13-acetate (TPA), and ionomycin (Manger et al., 1985; Ertesvag et al., 2002).
The human T-cell lines HUT-78 and Jurkat have been used extensively to study the T-cell activation mechanism because these cells produce IL-2 in response to various stimulants. HUT-78 cells are considered to be the more activated phenotype as compared to Jurkat cells (Manger et al., 1985; Manger et al., 1986; Kelleher et al., 1988). Although in some cases one stimulant alone has been used to trigger the production of IL-2 (Kanda and Watanabe, 2001), a combination of two stimulants has usually been utilized (Wiskocil et al., 1985; Manger et al., 1986; Hardy et al., 1987; Ioannides et al., 1990; Felli et al., 1991; Calvo et al., 1992; Kloth et al., 1994; Bao et al., 2006). For example, a combination of TPA and PHA has been used to achieve a high level of IL-2 production (Wiskocil et al., 1985; Manger et al., 1986; Hardy et al., 1987; Ioannides et al., 1990; Calvo et al., 1992; Kloth et al., 1994; Bao et al., 2006).
Vitamin A plays an important role in the regulation of immune function, but the mechanisms whereby the vitamin regulates T-cell biology are poorly defined (Engeldal et al., 2006). For example, all-trans-retinoic acid (ATRA), a biologically active metabolite of vitamin A, has been shown to enhance IL-2 secretion in human T-cells stimulated with TPA, but the mechanism remains largely unknown, although the involvement of retinoic acid receptor (RAR) has been suggested (Ertesvag et al., 2002; Engeldal et al., 2004). These findings, however, appear to be in contrast to those reported by Felli et al. (1991), who found that retinoic acid down-regulated IL-2 mRNA expression in Jurkat cells stimulated by a combination of TPA and the calcium ionophore A23187.
To obtain further information on the immunomodulatory activity of vitamin A, we examined here how ATRA influences the expression of IL-2 in HUT-78 cells stimulated with either TPA or PHA. We also examined the effects of ATRA on gene expression of protein factors which influence the gene expression and protein synthesis of IL-2. These include activator protein-1 (AP-1), protein kinase C (PKC), and IL-2 receptor (IL-2R) for which the following respective effects can be expected.
Since the IL-2 promoter contains AP-1 binding sites (Felli et al., 1991) and since the AP-1 complex is composed of the protein products of the c-fos and c-jun genes, the expression level of these genes will affect IL-2 production. Among PKC isoenzymes, PKC-beta has a critical role in IL-2 synthesis and proliferation in stimulated human lymphocytes (Szamel et al., 1993). ATRA has been suggested to augment IL-2 mRNA production with a possible paracrine effect on IL-2Rα expression in T cells derived from adenoidal tissues (Ballow et al., 1997).
ATRA and TPA were obtained from Wako Pure Chemical Industries Ltd., Osaka, Japan. PHA (PHA-P) was from Sigma-Aldrich. Alamar blue was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Human HUT-78 cells were purchased from Dainippon-Sumitomo Pharmaceutical Co. Ltd. (Osaka, Japan) and cultured in 10% fetal bovine serum in RPMI 1640 medium containing 50 U/ml penicillin, 50 μg/ml streptomycin, and 2.5 μg/ml amphotericin B at 37°C under a 5% CO2 atmosphere.
The proliferation of cells was evaluated by the Alamar blue assay as described previously (Shoji et al., 1999). Briefly, cells (2×104) in plastic 48-well multidishes were cultured with 200 μl of the culture medium in the absence or presence of test samples for 24 h, and an aliquot of Alamar blue solution (20 μl) was added. After incubation for 3 h at 37°C, fluorescence was measured with excitation at 560 nm and emission at 590 nm.
Cells (4×105) were cultured with 1 ml of the culture medium in the absence or presence of test samples for 24 h. An aliquot (50 μl) of the supernatant of the culture medium was used to determine the IL-2 protein concentration using an Amersham High Sensitivity Interleukin-2 Human, ELISA Biotrak System (GE Healthcare UK Ltd., Buckinghamshire, UK).
Total RNA was extracted from cells cultured for 24 h under various conditions and mRNA was prepared using a QIAamp RNA Blood Mini Kit (Qiagen Ltd., Tokyo, Japan) according to the manufacturer’s directions. To prevent possible contamination, samples were treated with deoxyribonuclease (RT-grade, Wako Pure Chemical Industries Ltd.) as recommended by the manufacturer (Suzuki et al., 2005). Amplified DNA was subjected to electrophoresis in 2% agarose, stained with SYBR Green I (Molecular Probes Ltd., Eugene, OR, USA), and imaged and calculated using a FluorImager (Molecular Dynamics Tokyo Ltd., Tokyo, Japan) as described previously (Suzuki et al., 2005). RT-PCR was performed using the Thermal Cycler Dice (TaKaRa Bio., Tokyo, Japan). Primers used for RT-PCR are listed in Table I.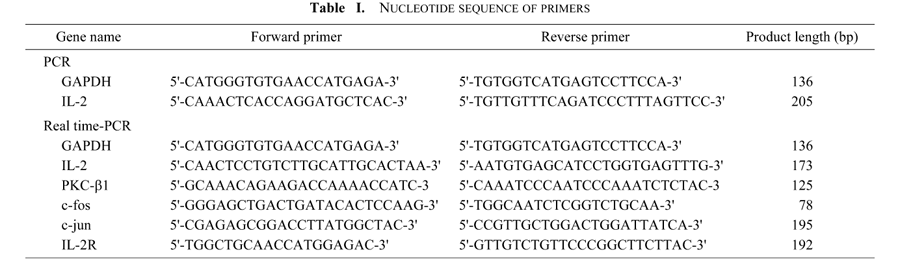
Quantitative real time-PCR was performed using total RNA extracted from cells cultured for 8 h or 24 h and the Thermal Cycler Dice Real Time System (TaKaRa Bio.) according to the manufacturer’s instructions. Primers used are listed in Table I.
When HUT-78 cells were cultured in the presence of PHA, IL-2 protein was detected in the culture medium by the ELISA, while no appreciable amount of IL-2 was detected without stimulation with PHA. The production was dependent on the PHA concentration with the greatest effect at 5 μg/ml (Fig. 1). Therefore, we used PHA at 5 μg/ml in the subsequent experiments.
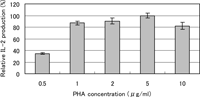 View Details | Fig. 1. Effect of PHA on IL-2 production by HUT-78 cells. The cells were incubated with PHA at the concentration indicated for 24 h, and the concentration of IL-2 in the culture medium was determined by ELISA. The results from three determinations are expressed relative to the value at 5 μg/ml of PHA (100%). Without PHA stimulation, the value was less than 1%. |
In the present experiments, HUT-78 cells were stimulated with TPA at 10 ng/ml as reported previously (Kelleher et al., 1988). Consistent with previous findings in human peripheral blood lymphocytes (Ertesvag et al., 2002; Engeldahl et al., 2004), IL-2 production in HUT-78 cells was stimulated by TPA (Fig. 2), while no appreciable amount of IL-2 was detected without stimulation. The addition of 1 μM ATRA enhanced the effect of TPA in HUT-78 cells, being consistent with the previous finding that the TPA-stimulated IL-2 production was enhanced by 1 μM ATRA in human peripheral blood lymphocytes (Estesvag et al., 2002). In contrast, PHA-stimulated production of IL-2 was suppressed in the presence of 1 μM ATRA. ATRA itself had only a marginal effect on IL-2 production.
 View Details | Fig. 2. Effect of ATRA on IL-2 production by HUT-78 cells stimulated with either TPA or PHA. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 24 h, and the concentration of IL-2 in the culture medium was determined by ELISA. Without stimulation with either TPA or PHA, the value was less than 6 pg/ml of the minimum sensitivity of the method. The results are from three determinations. |
In the ELISA determining the IL-2 concentration in the culture medium, the value depends on the number of the IL-2 producing cell. To examine the possibility that ATRA affected the cell growth differently, the relative number of cells after culturing for 24 h under each set of conditions was determined by the Alamar blue assay (Fig. 3). We found no significant difference in fluorescence intensity among these culture conditions, indicating that 1 μM ATRA did not affect the growth of HUT-78 cells under the conditions used.
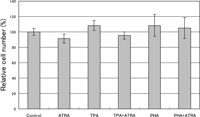 View Details | Fig. 3. Effect of ATRA on proliferation of HUT-78 cells stimulated with either TPA or PHA. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 24 h, and the relative cell number was determined by an Alamar blue assay. The results from three determinations are expressed relative to the value for the control (100%). |
The results of RT-PCR indicated that both TPA and PHA stimulated the gene expression of IL-2 in HUT-78 cells. ATRA enhanced the gene expression mediated by TPA, but suppressed that mediated by PHA (Fig. 4). A semiquantitative analysis using a FluorImager showed that ATRA caused a 1.59-fold up-regulation of the TPA-mediated gene expression and that the expression level in the cells stimulated with PHA in the presence of ATRA was suppressed to 85% of that in the cells stimulated with PHA alone.
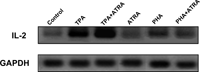 View Details | Fig. 4. Effect of ATRA on IL-2 gene expression in HUT-78 cells stimulated with either TPA or PHA as examined by RT-PCR. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 24 h, and the gene expression was examined by RT-PCR. The results were visualized using a FluorImager. |
To confirm the findings from the RT-PCR using RNA from 24 h-cultured cells, we carried out a real-time PCR (Fig. 5). The results for the 24 h-cultured cells were consistent with the findings from RT-PCR. In the case of 8 h of stimulation by TPA, ATRA slightly suppressed the gene expression of IL-2. The stimulative effect of TPA was diminished by 24 h and ATRA appeared to prevent this decrease. In the case of PHA, the stimulative effect was maintained through 24 h, and the suppressive effect of ATRA was also maintained.
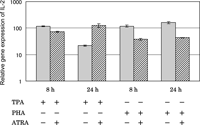 View Details | Fig. 5. Effect of ATRA on IL-2 gene expression in HUT-78 cells stimulated with either TPA or PHA as examined by real time-PCR. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 8 or 24 h, and the gene expression level was determined by real time-PCR. The results are expressed relative to the level in untreated cells (control). Data are from one experiment representative of three. |
The effects of ATRA on TPA- and PHA-stimulated PKC-β1 gene expression were examined by real-time PCR (Fig. 6). TPA up-regulated the gene expression of PKC-β1 and ATRA augmented TPA’s effect. In contrast, PHA down-regulated the gene expression of PKC-β1. Although ATRA reduced PHA’s effect, the expression level of the cells stimulated with PHA in the presence of ATRA still remained below that of the unstimulated cells. ATRA itself caused up-regulation of PKC-β1 gene expression. Time-dependent increases in these effects were observed in all cases.
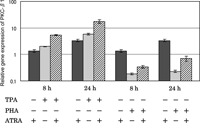 View Details | Fig. 6. Effect of ATRA on PKC-β1 gene expression in HUT-78 cells stimulated with either TPA or PHA as examined by real time-PCR. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 8 or 24 h, and the gene expression level was determined by real time-PCR. The results are expressed relative to the level in untreated cells (control). Data are from one experiment representative of three. |
When the effects of ATRA on TPA- and PHA-stimulated c-fos gene expression were examined by real-time PCR, a marked difference was detected (Fig. 7). ATRA did not inhibit the up-regulating effect of TPA, but inhibited greatly the activity of PHA that caused up-regulation of the gene expression of c-fos. ATRA alone did not affect the gene expression of c-fos.
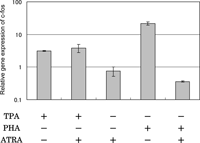 View Details | Fig. 7. Effect of ATRA on c-fos gene expression in HUT-78 cells stimulated with either TPA or PHA as examined by real time-PCR. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 24 h, and the gene expression level was determined by real time-PCR. The results are expressed relative to the level in untreated cells (control). Data are from one experiment representative of three. |
The results of real-time PCR indicated PHA caused a 7.3-fold up-regulation of the gene expression of c-jun, but that ATRA suppressed this up-regulation by 63%. ATRA alone did not affect the gene expression of c-jun. The c-jun expression level in HUT-78 cells treated with TPA in the presence of ATRA was similar to that in control cells. The up-regulation of c-jun gene expression by PHA was consistent with the finding in activated lymphocytes reported by DePalma et al. (1998).
The results of real-time PCR indicated that ATRA suppressed the TPA- and PHA-mediated IL-2R gene expression to a similar degree and the effect was slight (Fig. 8).
 View Details | Fig. 8. Effect of ATRA on IL-2R gene expression in HUT-78 cells stimulated with either TPA or PHA as examined by real time-PCR. The cells were incubated with either 10 ng/ml TPA or 5 μg PHA in the presence or absence of 1 μM ATRA for 24 h, and the gene expression level was determined by real time-PCR. The results are expressed relative to the level in untreated cells (control). Data are from one experiment representative of three. |
The present study clearly showed that PHA alone was capable of stimulating the production of IL-2 (Figs. 1 and 2) with the up-regulation of IL-2 gene expression in HUT-78 cells (Figs. 4 and 5), a hitherto undescribed finding. Many studies have used a combination of two stimulants such as TPA and PHA to obtain maximal production of IL-2. Indeed, we observed that the TPA-stimulated production of IL-2 was further increased in the presence of PHA, but only by 10% (data not shown). It appears that TPA and PHA each stimulates T-lymphocytes through a different pathway in view of the fact that TPA alone cannot induce IL-2 production whereas PHA can do so in Jurkat cells, a cell line with a less activated phenotype as compared to HUT-78 cells. Studies using a sole stimulant would provide clearer clues than a combination of stimulants to clarify the mechanism whereby the activated T-cells produce IL-2.
ATRA has been reported to cause an increase in TPA-stimulated IL-2 production in human peripheral blood lymphocytes (Estesvag et al., 2002; Engedal et al., 2004). The present finding (Fig. 2) is consistent with such results, confirming the usefulness of HUT-78 as a model cell line for examining regulatory mechanisms operating in the production of IL-2. In addition, the present results provided evidence for the changes in the IL-2 gene expression conforming to those in the protein production (Figs. 4 and 5).
These findings appear to be in contrast to those reported by Felli et al. who showed that retinoic acid decreased IL-2 mRNA levels and the transcriptional activity of the IL-2 promoter via a process that involves RAR. The differences in the experimental conditions may explain the different effects of ATRA, since they used Jurkat cells activated by a combination of TPA and ionomycin.
When the effect of ATRA on PHA-induced IL-2 protein production in HUT-78 cells was examined, ATRA was found to rather inhibit the action of PHA (Fig. 2). The results of RT-PCR and real-time PCR showed the inhibitory effect of ATRA on the up-regulation exerted by PHA (Figs. 4 and 5), consistent with the effect on the protein production (Fig. 2).
The action of ATRA was not attributable to an effect on cell growth, which would affect the IL-2 concentration in the culture medium, since there was no change in the relative number of HUT-78 cells treated with TPA in the presence and absence of ATRA (Fig. 3). This was also true for the PHA-treated cells (Fig. 3).
Previously, Dreikhausen et al. (2003) reported a highly specific function of PKC-β for regulation of the gene expression and secretion of IL-2 in Jurkat cells. In HUT-78 cells, it was demonstrated that the expression of PKC-β was critical to the export of IL-2 molecules from the cells (Long et al., 2001).
In the present study, it was demonstrated that TPA up-regulated the gene expression of PKC-β1 and that this was further augmented by ATRA (Fig. 6). In contrast, PHA down-regulated the gene expression of PKC-β1 and the expression level of the cells stimulated with PHA remained below that of the unstimulated cells even in the presence of ATRA. Thus, augmentation of the gene expression of PKC-β1 by ATRA in the case of the TPA treatment may explain, at least partly, the increases in the enhanced gene expression of IL-2 (Figs. 4 and 5) and the amount of IL-2 protein in the culture medium (Fig. 2) of HUT-78 cells stimulated with a combination of TPA and ATRA as compared to the treatment with TPA only. The up-regulating effect of ATRA on gene expression of PKC-β1 may be compatible with the finding that the protein and mRNA levels of PKC-β1 and PKC-β2 became elevated in HL-60 cells when treated with retinoic acid (Nakashima et al., 1996–1997).
The reason why ATRA stimulated TPA-dependent up-regulation of PKC-β1 gene expression is not clear at the moment. This may be related to the possibility that ATRA increased an intracellular concentration of calcium, since it was demonstrated that retinoic acid caused a significant increase in the calcium concentration in chondrocytes (Sanchez and Wilkins, 2004) and since the increase in calcium influx was demonstrated in HL-60 cells treated with retinoic acid (Rephaeli et al., 1990). This increase would lead to up-regulation of PKC-β1 in view of the finding that the level of PKC-β mRNA was elevated in gonadotroph-derived αT3-1 cells treated with ionomycin which increases intracellular concentration of calcium (Shraga-Levine et al., 1994). This effect of ATRA appears to contribute to further increase in the level of PKC-β1 mRNA up-regulated with TPA, but seems to be insufficient for the PKC-β1 gene expression down-regulated with PHA to reach a higher level than the control level.
TPA can induce an accumulation of the c-fos and c-myc transcripts in normal human peripheral blood lymphocytes (Grausz et al., 1986). Accumulation of mRNA for the c-fos gene was also reported in TPA-treated Jurkat cells (Makover et al., 1991). In human mononuclear cells, the proto-oncogenes c-fos and c-myc were induced to express by either PHA or TPA alone (Granelli-Piperno et al., 1986). Adult lymphocytes were reported to show an increase in the mRNA expression of c-fos and c-jun on stimulation with PHA (DePalma et al., 1998).
The present findings that the c-fos gene expression was up-regulated by each of TPA and PHA (Fig. 7) is consistent with these previous findings. The up-regulation of c-fos gene expression by each of TPA and PHA appears to contribute to the up-regulated gene expression of IL-2 (Figs. 4 and 5), since the IL-2 promoter contains the AP-1 binding site (Felli et al., 1991).
ATRA transcriptionally suppressed the expression of the c-fos gene but not that of the c-jun gene in tumor necrosis factor-alpha-treated osteoblastic cells and appeared to act as a potent negative regulator for AP-1 binding activity in the cells (Hanazawa et al., 1994).
In HUT-78 cells, negative regulation by ATRA was found only in the case of activation with PHA (Fig. 7). It is likely that ATRA reduced the protein production and gene expression of IL-2 up-regulated by PHA (Figs. 2 and 5) through negative actions on the c-fos (Fig. 7) and c-jun gene expression which would lead to repression of the activity of AP-1 which interacts with the IL-2 promoter (Felli et al., 1991).
The mechanism by which ATRA inhibits PHA-induced c-fos expression is not known at present. Previously Li et al. (1994) demonstrated that retinoic acid inhibited the increase in the c-fos mRNA level stimulated with insulin-like growth factor I in breast carcinoma cells. These authors suggested that retinoic acid inhibited the insulin-like growth factor I-mediated increase in the c-fos mRNA level by decreasing c-fos mRNA stability. This possibility should be examined in a future study.
Previously, it was shown that the cell surface IL-2R expression was enhanced in PHA-activated peripheral blood mononuclear cells as compared to unstimulated cells (Su et al., 2003). Treating Jurkat cells with TPA induced expression of the gene for IL-2Rα (Makeover et al., 1991). Therefore, we examined the effects of ATRA on the expression of IL-2R in HUT-78 cells stimulated with either TPA or PHA by real-time PCR. The results indicated that TPA and PHA up-regulated the expression and that ATRA slightly suppressed both these effects to a similar degree (Fig. 8). The up-regulation by ATRA itself was only marginal.
Although Ballow et al. (1997) have suggested that ATRA can augment IL-2 mRNA production with a possible paracrine effect on IL-2Rα expression in T cells derived from adenoidal tissues, the present results indicated that the effect of ATRA on IL-2R gene expression is unrelated to the different effects on the production of IL-2 between HUT-78 cells stimulated with TPA and those stimulated with PHA.
In spite of the importance of vitamin A in the human immune system (Stephensen, 2001; Ross, 2007) and the central role of IL-2 in the proliferation of T-lymphocytes (Felli et al., 1991), there have been only a limited number of reports examining the effect of vitamin A on in vivo IL-2 expression. Carman and Hayes (1991) have shown that the secretion of IL-2 by T-lymphocytes from vitamin A-deficient mice was equivalent to that by those from vitamin A-sufficient mice. Seguin-Devaux et al. (2005) demonstrated that ATRA increased the gene expression and protein production of IL-2 in the rat liver without significantly affecting the concentration of IL-2 in plasma.
Obviously, further studies are needed to reveal the in vivo effects of vitamin A and its metabolites and molecular mechanism underlying vitamin A’s action.
|