| To whom correspondence should be addressed: Hiroshi Kimura, Graduate School of Frontier Biosciences, Osaka University, 1-3 Yamadaoka, Suita, Osaka 565-0871, Japan. Tel: +81–6–6879–4623, Fax: +81–6–6879–4622 E-mail: hkimura@fbs.osaka-u.ac.jp Abbreviations: ChIP, chromatin immunoprecipitation; DAPI, 4,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ELISA, enzyme-linked immunosorbent assay; mAb, monoclonal antibody; RPL10, ribosomal protein L10; SAHF, senescence-associated heterochromatic foci; TSA. trichostatin A. |
In eukaryotic nuclei, DNA is packaged into nucleosomes, containing two copies of each core histone (i.e., H2A, H2B, H3, and H4). The post-translational modifications on these histones play critical roles in genome function, including the regulation of transcription and the maintenance of genome integrity (Jenuwein and Allis, 2001; Nightingale et al., 2006; Kouzarides, 2007). Acetylation and methylation of H3 are particularly important in epigenetic gene activation and silencing. Transcriptionally-active chromatin is generally associated with acetylation on lysine residues (like K9 and K14) and methylation on K4, whereas silent chromatin has methylation on K9. The status of lysine methylation can be mono, di, and tri, and the level of methylation is correlated with the degree of activation or silencing; for example, trimethyl-K9 is enriched in constitutive heterochromatin, and dimethyl-K9 in euchromatic silent chromatin, or facultative heterochromatin (Peters et al., 2003).
The development of antibodies recognizing site-specific modifications has allowed the detection of those modifications using various techniques including chromatin immunoprecipitation (ChIP), immunoblotting, and immunofluorescence (Turner et al., 1992; Peters et al., 2003). For example, ChIP has enabled the profiling of specific modifications at both single loci and throughout the genome (Wu et al., 2006; Bernstein et al., 2007). Recent whole genome analyses using ChIP and microarray, or high-throughput sequencing, have revealed the general view of histone modifications in relation to transcription activity (Bernstein et al., 2005; 2006; 2007; Barski et al., 2007; Heintzman et al., 2007; Mikkelsen et al., 2007).
The quality and reproducibility of these immunochemical analyses rely on antibody specificity, but most commercially-available antibodies are rabbit polyclonals and so the specificity can vary from lot to lot (Clayton et al., 2006). Moreover, each lot may not be extensively examined for cross-reactivity with other modifications. For example, enzyme-linked immunosorbent assay (ELISA) reveals that some commercially-available polyclonal antibodies are not very specific (e.g., see Fig. 1A and B). Therefore, we set out to develop a panel of monoclonal antibodies (mAbs) which are expected to retain their specificity over time. In this paper, we report mAbs directed against different (mono-, di-, or tri-) methylation of K4 and K9 and acetylation of K9 and K27. By using these mAbs for ChIP and immunoblotting, we showed that trimethyl-K4 was rather associated with acetyl-K27 than with dimethyl-K4 and acetyl-K9 within a few nucleosomes on active promoters. Furthermore, direct immunofluorescence analysis revealed that di- and tri-methylation on K4 was diminished during replicative senescence. These highly-reliable and fully-characterized mAbs may facilitate future epigenomic studies.
Human fibroblasts were kindly provided by Drs. M. Naito and S. Suzuki at the Department of Plastic and Reconstruction Surgery, Kyoto University Hospital (human baby skin fibroblasts) or purchased from American Type Culture Collection at the following passage number and population doubling (PDL). IMR-90: passage 11, PDL 24; MRC-5: passage 16, PDL 23; and WI-38: passage 14, PDL 23. hTERT-RPE1, telomerase-immortalized human female epithelial cells (Clontech), and HeLa cells were described previously (Chadwick and Willard, 2003; Kimura et al., 2006). Cells were grown in Dulbecco’s modified Eagle’s medium, high-glucose (Sigma) supplemented with 10 U/ml penicillin, 50 μg/ml streptomycin and 10% fetal calf serum.
Synthetic peptides (Sigma-Genosys; Table I) were coupled to keyhole limpet hemocyanin and used to immunize mice (Kimura et al., 1994); after generating hybridomas, clones were screened by ELISA using plates coated with the modified or unmodified peptide conjugated with bovine serum albumin. After recloning, supernatants from clones reacting with the specifically-modified peptide were used to probe blots prepared using HeLa total proteins lysed in SDS-gel loading buffer (Kimura et al., 2006), and the ones giving a single band at the size of histone H3 (e.g., see Fig. 3) were selected for immunofluorescence examination to see if they exhibited nuclear staining. Clones passing through these successive screens were further analyzed by ELISA against some related peptides listed in Table I, and the ones showing the highest specificity to the authentic peptides were selected. The isotype of each mAb was determined using a kit (Serotec; MMT1; see Table II). The specificity of rabbit polyclonal antibodies was also analyzed by ELISA using anti-dimethyl K4 (Upstate; 07–030) and anti-dimethyl K9 (Abcam; ab7312), which recognizes both dimethyl-K9 and dimethyl-K27 according to the data sheet and so designated as anti-dimethyl-K9/K27 in Fig. 1.
Hybridoma cells were routinely grown in GIT medium (Wako) and the supernatant from the confluent culture was mixed with sodium azide (0.02%) and stored at 4°C. For antibody purification, cells were grown in CD Hybridoma medium (Invitrogen) and the supernatant (250 ml) was passed through a HiTrap Protein G HP Sepharose column (1 ml; GE Healthcare). After eluting with glycine-HCl (pH 2.5), the buffer was immediately neutralized with Tris and then exchanged with PBS using HiTrap Desalting column (GE Healthcare). Purified antibodies were conjugated with a fluorescent dye using an Alexa Fluor monoclonal antibody labeling kit (Invitrogen) according to the manufacturer’s instructions.
HeLa cells (5–6×106 cells in a 90 mm dish) were cross-linked with 10 ml 1% formaldehyde (Electron Microscopy Sciences) in medium for 5 min at room temperature (25±2°C) and then incubated in 10 ml 200 mM glycine in medium for 5 min to quench reactive aldehydes. After rinsing cells with PBS, cells were immersed in 10 ml lysis buffer (10 mM Tris-HCl [pH 8.0], 10 mM NaCl, and 0.5% NP-40) for 10 min. The supernatant was discarded and 500 μl lysis buffer was added to harvest cells using a cell scraper. Cells were collected by centrifugation (1,000×g; 3 min; 4°C) and resuspended in 100 μl SDS lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, and 1% SDS). After mild rotation for 10 min at 4°C, 400 μl ChIP dilution buffer (50 mM Tris-HCl [pH 8.0], 167 mM NaCl, 1.1% Triton X-100, 0.11% sodium deoxycholate, and protease inhibitor cocktail [complete EDTA-free; Roche Diagnostics]) was added before sonication (Branson Sonifier 250 with microtip; 6 times for 12 sec; output level 1.2). After centrifugation to remove insoluble materials, 500 μl ChIP dilution buffer and 500 μl RIPA-150 mM NaCl (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, and protease inhibitor cocktail) were added to the supernatant to yield the input for ChIP. The size of fragmented chromatin, analyzed using an Agilent Bioanalyzer 2100, was ~300–1500 bp (median ~500 bp).
For cross-linked ChIP, Dynabeads (M-280 sheep anti-mouse IgG; Invitrogen; 40 μl original suspension) were washed with PBS, incubated with anti-histone mAb (10–50 μl hybridoma supernatant), anti-RNA polymerase II (10 μl; 8WG16; Covance), or normal mouse IgG (30 μg; Jackson Immunoresearch), in 500 μl RIPA-150 mM NaCl over 6 h at 4°C with rotation and washed twice with 1 ml RIPA-150 mM NaCl. [It should be noted that more reproducible results were obtained using anti-mouse IgG beads (instead of protein G beads) in combination with IgG1-class mAbs, probably because of the weak interaction between IgG1 and protein G in buffers used during ChIP.] An aliquot of ChIP input (500 μl) was incubated with IgG-bound Dynabeads overnight at 4°C with rotation. Beads were washed sequentially with 1 ml RIPA-150 mM NaCl, 1 ml RIPA-500 mM NaCl (50 mM Tris-HCl [pH 8.0], 500 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 0.1% sodium deoxycholate), and twice with 1 ml TE (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). After removing TE, beads were mixed with 200 μl direct elution buffer (10 mM Tris-HCl [pH 8.0], 300 mM NaCl, 5 mM EDTA, and 0.5% SDS) and incubated overnight at 65°C to reverse cross-linking. Samples were then treated with DNase-free RNase (Roche; 5 μg/ml; 37°C; 30 min) and proteinase K (Wako; 250 μg/ml; 55°C; 1 h). DNA was extracted sequentially with phenol, phenol-chloroform (1:1), and chloroform, and precipitated with ethanol with a carrier (Dr.genTLE; Takara; 3 μl). After centrifugation, the pellet was washed with 70% ethanol and finally dissolved in 40 μl TE.
Each sample (0.2 μl equivalent) was analyzed by real-time PCR (Qiagen; QuantiTect SYBR Green PCR kit) using a DNA Engine Opticon 2 (Bio-Rad). Results were presented as percentages of input DNA. Primer sequences were: GAPDH promoter F, 5'-TACTAGCGGTTTTACGGGCG-3'; GAPDH promoter R, 5'-TCGAACAGGAGGAGCAGAGAGCGA-3'; GAPDH coding F, 5'-GGCTCCCACCTTTCTCATCC-3'; GAPDH coding R, 5'-GGCCATCCACAGTCTTCTGG-3'; RPL10 promoter F, 5'-ACCCGTCTTCGACAGGACT-3'; RPL10 promoter R, 5'-GGAACGGAAGACGAGAACAG-3'; RPL10 4I5E F, 5'-CCCTGAGCTGGAGATAGTCG-3'; RPL10 4I5E R, 5'-GCCATCTTTGCCACAACTTT-3'; RPL10 6I7E F, 5'-CAGGCCTCCTGACTCAGTTC-3'; RPL10 6I7E R, 5'-TTTCAGCCACCATGTCTTCA-3'; beta-globin promoter F, 5'-GGGCTGAGGGTTTGAAGTCC-3'; and beta-globin promoter R, 5'-CATGGTGTCTGTTTGAGGTTGC-3'.
Nucleosomes were prepared according to previous papers (Kimura et al., 2006; Kanda et al., 1998) with modifications. Spinner-adapted HeLa cells (6×108 cells) were collected (360 g; 5 min; 4°C), washed with 100 ml ice-cold RSB (10 mM HEPES-NaOH [pH 7.4], 15 mM NaCl, 1.5 mM MgCl2), centrifuged (640 g; 10 min; 4°C), and resuspended in 50 ml ice-cold RSB containing 1% Triton X100 and protease inhibitor cocktail (Nacalai Tesque). After homogenization using Dounce homogenizer (tight pestle; 5 times), nuclei were collected (1,700 g; 15 min; 4°C), washed twice with 20 ml buffer A (15 mM HEPES-NaOH [pH 7.4], 15 mM NaCl, 60 mM KCl, 0.34 M sucrose, 0.5 mM spermine, 0.15 mM spermidine, 1 mM dithiothreitol, protease inhibitor cocktail; Nacalai Tesque), and resuspended in buffer A (10× volume of pellet; ~5×107 cells/ml; typically 10 ml buffer A was added to 1 ml pellet). Resuspended nuclei were aliquoted (1 ml), frozen in liquid nitrogen and stored at –80°C.
The nuclear suspension was thawed, and added with 1 μl 1 M CaCl2 (1 mM), 15 μl micrococcal nuclease (Sigma; 200 U/ml stock in 20 mM HEPES-NaOH [pH 7.4], 50 mM NaCl, 50% glycerol; 3 U/ml), and 0.2 μl Trichostatin A (Sigma; 0.5 mg/ml stock in DMSO; 100 ng/ml). The tube was incubated at 37°C for 2 h (mixing by inverting every 20 min). After adding 20 μl 0.5 M EDTA [pH 8.0] (10 mM) and centrifugation (10,000×g; 10 min; 4°C), the pellet was suspended in 450 μl 10 mM EDTA [pH 8.0]. After the addition of 50 μl 5 M NaCl (0.5 M), the tube was centrifuged (20,000×g; 5 min; 4°C) and the supernatant was collected.
For immunoprecipitation, 250 μl of nucleosome sample was mixed with 1 ml buffer B (20 mM Tris-HCl [pH 8.0], 5 mM EDTA, 500 mM NaCl, and 0.2% Tween 20) and 50 μl hybridoma supernatant, or control IgG (4 μg; Jackson Immunoresearch; 015-000-003). After incubating at 4°C overnight with rotation, 100 μl anti-mouse IgG Dynabeads (prewashed and suspended in buffer B) were added, and the mixture was further incubated for 3 h at 4°C. After washing 3 times with 1 ml buffer B and once with 1.25 ml buffer B without Tween 20, one fifth of the sample (250 μl) was transferred to a new tube for DNA analysis and the rest (1 ml) was used for protein analysis.
For DNA analysis, beads were collected, washed in TE, and resuspended in 100 μl TE containing 0.5% SDS. After the addition of proteinase K to give a final concentration of 200 μg/ml, the mixture was incubated at 55°C for 1 h. The input nucleosome preparation (1 μl) was also mixed with 100 μl TE containing 0.5% SDS and treated in the same way. DNA was purified using a QIAqick PCR Purification Kit (Qiagen), precipitated with ethanol, dissolved in 4 μl TE, and analyzed using an Agilent Bioanalyzer 2100 with a DNA Chip 7500.
For protein analysis, beads were collected and boiled in 100 μl 2× SDS-gel loading buffer for 10 min. Proteins (10 or 5 μl) were separated in 13% SDS-polyacrylamide gel, and stained with Coomassie or transferred to PVDF membranes (Pall) using a semi-dry blotting system (ATTO). Membranes were washed 3 times in TBST (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.05% Tween 20) for 5 min, blocked for 30 min in Blocking-One P (Nacalai Tesque; 30 min), and washed 3 times for 5 min in TBST. Membranes were incubated for 1 h at room temperature with mAbs (1:5–1:20 dilution of hybridoma supernatants) in Can-get-signal (Toyobo), washed 3 times for 10 min in TBST, incubated for 1 h with peroxidase-conjugated anti-mouse Ig (1:1,000; GE Healthcare) in Can-get-signal, and washed 3 times for 10 min in TBST. Signals were developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer) and detected using a LAS-3000 (Fujifilm). In some cases, antibodies were stripped using WB Stripping Solution Strong (Nacalai Tesque), before blotting with rabbit polyclonal antibody and peroxidase-conjugated anti-rabbit Ig (1:1,000; GE Healthcare). The following polyclonal antibodies were used: anti-hyperacetylated histone H4 Penta (1:1,000; Upstate; 06-946), anti-acetyl histone H4 Lys8 (1:1,000; Upstate; 06-760), and anti-histone H4 trimethyl K20 (1:2,000; Abcam; ab9053-100).
Cells were fixed, permeabilized, and blocked as described previously (Kimura et al., 2006), and then incubated in labeled mAbs (0.2–1 μg/ml) for 4 h at room temperature. After washing and staining with 4,6-diamidino-2-phenylidndole (DAPI), coverslips were mounted in Prolong Gold (Invitrogen) (Kimura et al., 2006). For Fig. 7, fluorescence images of single optical sections were collected using an Olympus FV1000 confocal microscope operated by the build-in software (ver 1.6) with a PlanSApo 60X (NA=1.35) objective lens (7× zoom; 640×640 pixels; 20 μs/pixel; 4 line Kalman; 12-bit; pinhole 120 μm). DAPI, Alexa488, Alexa555, and Alexa647 signals were acquired by sequential scanning using 405-, 488-, 543-, and 633-nm laser excitation combining with emission dichroic mirror/barrier filter SDM490/BA430-470, SDM560/BA505-525, SDM640/BA560-620, and none/BA650IF, respectively, with a main dichroic mirror DM405/488/543/633. The images were contrast stretched linearly and converted to 8-bit TIFF files using Photoshop ver 6.0 (Adobe) and assembled using Canvas 8 (Deneva). For Fig. 8, fluorescence images were collected using an ORCA-ER camera (Hamamatsu) operated by Metamorph ver 6.2 (Universal Imaging) equipped with an Olympus BX-81 microscope with a PlanApo 60X (NA 1.4; Fig. 8A), or a UPlanSApo 40X (NA 0.9; Fig. 8C), using U-MWU2, U-MNIBA2, U-MWIG2, and U-N41008 (HQ:Cy5) filter sets for DAPI, Alexa488, Alexa555, and Alexa647, respectively. Fluorescence intensities in nuclei were measured using Metamorph ver 6.2 (Universal Imaging) and the net intensities were calculated by subtracting the background. The numbers of nuclei in different intensity ranges were plotted using Excel (Microsoft).
We generated different mouse hybridoma clones producing mAbs directed against histone H3 peptides harboring specific modifications at K4, K9, and K27 (Table II). The specificity of these mAbs and commercial rabbit polyclonal antibodies was analyzed by ELSA (Figs. 1 and 2). For example, commercially-supplied anti-dimethyl K4 reacted with its target best, but cross-reacted almost equally with monomethyl-K4 and some extent with the other peptides (~3–27 fold difference; Fig. 1A). Similarly, a rabbit antibody that recognizes dimethyl-K9/K27 also partially reacted (~9–27 fold lower specificity) with other modifications including monomethyl- and trimethyl-K27 (Fig. 1B). In contrast, the newly-developed mAbs showed higher specificity. CMA306 reacted specifically with its target (monomethyl-K9), slightly with dimethyl-K9 (>27-fold difference), and hardly at all with all other peptides (Fig. 1C). CMA307 had a superior specificity reacting almost exclusively with its target, dimethyl-K9 (Fig. 1D). CMA308 reacted with its target (trimethyl-K9), and cross-reacted slightly with dimethyl-K9 and other K9 modifications but the difference was >27 fold (Fig. 1E). Comprehensive ELISA using various peptides (listed in Table I) indicated that each mAb specifically reacts with its authentic target, except CMA310 which cross-reacts with acetyl-K27 in addition to acetyl-K9 used as the immunogen (Fig. 2). The data indicated that CMA310 could be used as anti-acetyl K9/K27.
 View Details | Fig. 1. The specificity of antibodies evaluated by ELISA. The specificity of two commercially-available polyclonal (top row) and three newly-developed monoclonal antibodies (bottom row) was analyzed by ELISA using histone H3 peptides containing different modifications. Microtiter plates coated with the peptides (indicated on the top right; see Table I for the sequence) were incubated with 3-fold dilutions of each antibody (starting from 1:300 dilution of a commercially-supplied stock or 1:3 of a hybridoma culture supernatant). After incubation with peroxidase-conjugated secondary antibody and washing, the colorimetric signal of tetramethylbenzidine was detected by measuring the absorbance at 405 nm (Abs) using a plate reader. All 15 peptides listed on the top right were tested for all antibodies. (A) Rabbit polyclonal dimethyl-K4; (B) Rabbit polyclonal dimethyl-K9/K27; (C) Mouse monoclonal monomethyl-K9 (CMA306); (D) Mouse monoclonal dimethyl-K9 (CMA307); and (E) Mouse monoclonal trimethyl-K9 (CMA308). |
 View Details | Fig. 2. The specificity and occlusion by neighboring modification of mAbs. The specificity of each mAb and occlusion by neighboring modification were determined by ELISA as in Figure 1 using differently-modified histone H3 peptides (listed at the bottom; see Table 1 for the sequence). Microtiter plates coated with the indicated peptides were incubated with 3-fold dilutions of each mAb (starting from 1:300 dilution of a hybridoma culture supernatant). The peptides reacted with the individual mAb are indicated on the panel. |
As the reactivity of an antibody to specific modifications can be influenced by additional modifications around the target residue (Clayton et al., 2006), we also used peptides containing two modifications for ELISA; we found that some mAbs are indeed affected by phosphorylation of neighboring residues (Fig. 2). Modifications that allow or occlude mAb binding are summarized in Table II.
We then used immunoblotting analysis to examine if these mAbs react specifically with cellular histone H3. As shown in Fig. 3, all mAbs highlighted single bands at the size of histone H3 among HeLa total protein separated in SDS-polyacrylamide gels, suggesting that these mAbs react with the authentic cellular targets. As we shall see, immunoprecipitated histone H3 is indeed recognized by these mAbs by immunoblotting (see Fig. 6). The acetyl-specific mAbs were further evaluated using a histone deacetylatse inhibitor, trichostatin A (TSA). When cells were treated with TSA, the signal intensities remarkably increased within 15 min with mAbs directed against acetyl-K9 (CMA305), acetyl-K27 (CMA309), and acetyl-K9/K27 (CMA310), but not with the others in the same period (Fig. 3; data not shown). From these results, we concluded that the mAbs developed against synthetic peptides recognize cellular histone H3 harboring specific modifications.
 View Details | Fig. 3. Immunoblotting. HeLa cells were incubated without or with a histone deacetylase inhibitor, trichostatin A (TSA; 100 ng/ml), for 15 or 30 min, before lysis with SDS-gel loading buffer. The total protein was separated in 13% SDS-polyacrylamide gels and transferred to PVDF membranes, which were incubated with mAbs (hybridoma supernatants) at the following dilution and then peroxidase-conjugated anti-mouse Ig. Unmodified-K4 (CMA301), 1:200; monomethyl-K4 (CMA302), 1:20; dimethyl-K4 (CMA303), 1:50; trimethyl-K4 (CMA304), 1:20; monomethyl-K9 (CMA306), 1:20; dimethyl-K9 (CMA307), 1:20; trimethyl-K9 (CMA308), 1:20; acetyl-K9 (CMA305), 1:20; acetyl-K27 (CMA309), 1:100; and acetyl-K9/K27 (CMA310), 1:20. The positions of size standards are indicated on the left. |
We used these mAbs in ChIP analyses of HeLa chromatin. As the standard ChIP experiment in this field, chromatin was cross-linked with formaldehyde, and nucleosomes bearing a modification were selected using a specific antibody. The concentration of genomic loci enriched in the selected fraction was then determined by real-time PCR (Fig. 4). The results generally agree with recent genome-wide analyses (Barski et al., 2007; Heintzman et al., 2007); trimethyl-K4 and dimethyl-K4 were found on promoters and coding regions, respectively, in transcriptionally-active genes like glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein L10 (RPL10), whereas dimethyl-K9 and trimethyl-K9 were on inactive beta-globin gene (Fig. 4). More details are discussed together with the following ChIP-immunoblotting analysis.
 View Details | Fig. 4. Chromatin immunoprecipitation. After immunoprecipitation of cross-linked chromatin with the indicated antibodies, the enrichment of specific regions in the immunoprecipitated DNA was analyzed by real-time PCR and expressed as percentages of the input. (A) Schematic representation of analyzed gene loci and the positions of primers. Exons are indicated as boxes. (B) Real-time PCR. The averages with the standard deviations from 3 independent experiments are shown. Anti-RNA polymerase II (8WG16) and normal mouse IgG were used as positive and negative controls, respectively. |
To evaluate the association of one modification with another in the same or nearby nucleosomes, mAbs were also used for ChIP with native chromatin (O’Neill and Turner, 1995). After micrococcal nuclease treatment to generate short nucleosome strings mostly at the monomer and dimer levels (Fig. 5), histones containing one modification were immunoprecipitated. As shown in Fig. 5, mono- and di-nucleosomes were preferentially precipitated by mAbs recognizing modifications associated with active chromatin (e.g., dimethyl-K4 and acetyl-K9); in contrast, more polynucleosomes were recovered using antibodies recognizing inactive-chromatin (e.g., dimethyl- and trimethyl-K9). Such differences are probably caused by the preferential sensitivity to micrococcal nuclease of transcriptionally-active chromatin (compared to compact heterochromatin). It is also possible that antibody divalency coupled to modification density affects immunoprecipitation; divalency allows cross linking of adjacent nucleosomes, and this may be affected by modification density.
 View Details | Fig. 5. DNA analysis of immunoprecipitated chromatin. Nucleosomes prepared from HeLa nuclei were immunoprecipitated with the indicated mAb, and the size of DNA in the immunoprecipitates was analyzed by capillary gel electrophoresis. The numbers, 1 and 2, in ‘input’ indicate positions of mono- and di-nucleosomes, respectively; larger polysomes are also found in other samples. |
The presence of another modification in the immunoprecipitated fraction containing one modification was determined by immunoblotting (Fig. 6). Reassuringly, antibodies directed against one modification precipitated H3 containing the same modification (detected by blotting using the same antibody; Fig. 6B, arrows). The results of ChIP-immunoblotting analysis suggest several conclusions.
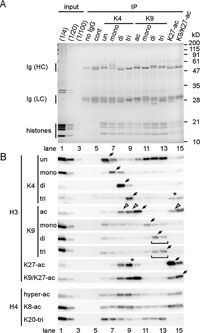 View Details | Fig. 6. Modifications on histone H3 in neighboring nucleosomes. Short nucleosomal strings mostly at the mono- and di-nucleosome levels (Fig. 5) were immunoprecipitated with the indicated mAb. The input (1/4, 1/20, and 1/100 dilutions) and immunoprecipitated materials were separated in 13% SDS-polyacrylamide gels, and stained with Coomassie (A) or blotted on to membranes before detection using the indicated antibodies (B). (A) Coomassie-stained gel. The positions of immunoglobulin heavy (HC) and light (LC) chains and core histones are indicated on the left, and of size standards on the right. (B) Immunoblots. Arrows indicate the signals of the target modifications immunoprecaipitated with the same antibody. Asterisks (lanes 9 and 14) indicate the mutual immunoprecipitation of trimethyl-K9 and acetyl-K27. The presence of acetyl-K9 in immunoprecipitates by dimethyl-K9, trimethyl-K9 and acetyl-K27 is indicated by open arrowheads. Square brackets indicate co-precipitation of dimethyl-K9 and trimethyl-K9. |
First, antibodies against one type of methylation on K4 immunoprecipitated only small amounts of nucleosomes bearing the other types of methylation on K4; this suggests that nucleosomes bearing these different modifications do not intermingle much within the few nucleosomes analyzed here (Fig. 6B, lanes 6–9). Instead, trimethyl-K4 was found more with acetyl-K27 than with dimethyl-K4 (compare lanes 14, asterisk, with lane 8 on trimethyl-K4 blot). These results are consistent with a ChIP analysis which showed that trimethyl-K4 and acetyl-K27 were enriched on the active GAPDH and RPL10 promoters, while dimethyl-K4 was found more in the coding regions (Fig. 4). Such transitions, from trimethyl to dimethyl and then to monomethyl, started near transcription start sites have been found in yeast (Pokholok et al., 2005) and in human cells (Barski et al., 2007; Heintzman et al., 2007), while substantial overlapping of dimethyl- and trimethyl-K4 was also observed (Bernstein et al., 2005; Kouskouti and Talianidis, 2005). This difference could be due to the size of nucleosome strings and/or the specificity of antibodies.
Second, acetyl-K9 is relatively broadly distributed through the promoter and coding region of active genes, as the modification was associated with the other markers found in active chromatin such as dimethyl-K4, trimethyl-K4 and acetyl-K27 (Fig. 6B, lanes 8, 9, and 14 on acetyl-K9 blot; open arrowheads). In contrast, the reciprocal immunoprecipitation using acetyl-K9 showed there was little dimethyl- and trimethyl-K4 in the selected fraction (Fig. 6B, lane 10). Real-time PCR also indicated that both the promoter and coding regions of GAPDH and RPL10 contained acetyl-K9 (Fig. 4). Whereas acetyl-K27 has not been studied so far, this modification appears to be more selective on active promoters compared to acetyl-K9 (Fig. 4).
Third, dimethyl- and trimethyl-K9 may intermingle in heterochromatin, as a substantial amount of dimethyl-K9 was observed in immunoprecipitates prepared using anti-trimethyl-K9, and vice versa (Fig. 6B, lanes 12 and 13 on dimethyl-K9 and trimethyl-K9 blots; square brackets). This apparent intermingling could, however, be overestimated due to longer (up to more than 10) nucleosomes precipitated by these antibodies (Fig. 5).
Fourth, acetylation and K20-trimethylation in histone H4 are associated with the active and inactive markers of H3, respectively, consistent with their localization in euchromatin (Turner et al., 1992) and heterochromatin (Sims et al., 2006).
To immunolabel different modifications both simultaneously and rapidly in fixed cells, mAbs were conjugated directly with different fluorescence dyes. In human XX (hTERT-RPE1) cells (Fig. 7), the modifications associated with transcriptionally-active chromatin, like dimethyl-K4, trimethyl-K4 and acetyl-K9, were excluded from DAPI-dense heterochromatin including the Barr body, which marks the inactive X chromosome enriched in dimethyl-K9. Although dimethyl-K4 and trimethyl-K4 were found on different nucleosomal strings at the monomer to dimer levels (Fig. 6), they were often seen together in the confocal microscope (Fig. 7); this apparent colocalization could result from the poor resolution of the fluorescent microscope (~200 nm in xy and ~900 nm in z-axis, where tens of nucleosomes can be present). Monomethyl-K4 and monomethyl-K9 were distributed more homogenously compared to their dimethyl- and trimethyl-forms (Fig. 7), which agrees with their broad distribution on adjacent nucleosomes (Fig. 6). Trimethyl-K9 was concentrated in DAPI-dense constitutive heterochromatin (Fig. 7). These results are essentially consistent with previous studies (Boggs et al., 2002; Chadwick and Willard, 2003; Peters et al., 2003).
 View Details | Fig. 7. Multicolor immunofluorescence with modification-specific mAbs. hTERT-RPE1 cells were fixed, immunolabeled with the indicated mAbs conjugated with Alexa dyes, and counter-stained with DAPI. Shown are single confocal sections (DAPI, Alexa488, Alexa555, Alexa647, and merge). Inset shows 2× magnified views of the boxed areas containing Barr bodies (inactive X chromosomes). Bar, 10 μm. |
Through the investigation of different cell lines using these labeled mAbs, we found that the signals of dimethyl-K4 and trimethyl-K4 appeared diminished in some cells in a population of human diploid fibroblasts IMR90 (Fig. 8A), whereas the levels of these modifications did not vary much in immortalized (hTERT-RPE1) or transformed (HeLa) cells (data not shown). The cell nuclei with less dimethyl-K4 and trimethyl-K4 were relatively bigger and often exhibited dotted DAPI-staining, appeared just like senescence-associated heterochromatic foci (SAHF) (Narita et al., 2003), where dimethyl-K9 and trimethyl-K9 were concentrated (Fig. 8A, arrows). Intensity measurements revealed that the signals of dimethyl-K4 and trimethyl-K4 were remarkably weak in individual nuclei with SAHF-like structure compared to the majority of those without such a structure (Fig. 8B). Other modifications were also slightly less intense in nuclei with SAHF-like structure (Fig. 8B); however, this might be caused, at least in part, by the enlargement of nuclear volume in senesced cells. These results prompted us to compare the levels of H3 modifications in human fibroblasts in early and late passages.
 View Details | Fig. 8. Modified histones in human diploid fibroblasts. Cells were fixed and immunolabled with the indicated mAbs conjugated with Alexa dyes and counter-stained with DAPI. (A and B) IMR90 cells at passage 16. (A) Fluorescence images. Four views of the same field of cells are shown (DAPI, Alexa488, Alexa555, and Alexa647; from the left to the right). Arrows indicate nuclei with SAHF-like structure. Bar, 10 μm. (B) Quantitative analysis. Fluorescence intensities in nuclei of individual cells in images like (A) were measured and the background was subtracted. Nuclei were separated into two categories judged by DAPI staining; one is the major fraction of relatively small nuclei without SAHF-like structure (no SAHF), and the other is bigger nuclei with SAHF-like structure (with SAHF). The numbers of nuclei in the two categories with intensities (arbitrary unit) of 0 to 200, 200 to 400, etc. are plotted. The range is adjusted dependently on the original intensities by each antibody to achieve wide dynamic range. Gray and black bars represent ‘no SAHF’ and ‘with SAHF’, respectively. The total number of analyzed nuclei (n) in each sample is as follows: K4 mono/di/tri-methyl ‘no SAHF’ (n=79), ‘with SAHF’ (n=23); K9/K27 acetyl ‘no SAHF’ (n=79), ‘with SAHF’ (n=21); and K9 mono/di/tri-methyl ‘no SAHF’ (n=87), ‘with SAHF’ (n=22). (C and D) MRC5 cells at passage 21 and 35. (C) Fluorescence images. Four views of the same field of cells are shown (DAPI, Alexa488, Alexa555, and Alexa647; from the left to the right). Bar, 10 μm. (D) Quantitative analysis. The numbers of nuclei with different intensity ranges are plotted as in (B). Gray and black bars represent passage 21 (p21) and 35 (p35), respectively (n=96). |
We analyzed several normal fibroblasts such as IMR90 and WI38 (XX; forming SAHF), MRC5 (XY; forming SAHF), and primary baby skin fibroblasts (XY, without SAHF). All cells tested here showed diminished dimethyl- and trimethyl-K4 signals in late passages (Fig. 8C; data not shown), suggesting that these modifications are decreased generally during replicative senescence. Intensity measurements again supported this conclusion. The intensity of dimethyl-K4 was drastically decreased in most cells in the late passage (Fig. 8D); about 58% (56 out of 96) of the cells had less than 25% fluorescence intensity of the average in the early passage. The levels of monomethyl-K4 and acetyl-K9/K27, as well as dimethyl-K9, were also decreased, but moderately, in late passages (Fig. 8, C and D; data not shown). As transcription activity still remains in senesced cells (Funayama et al., 2006), acetylation on K9/K27 may be associated with active genes.
K4 methylation is shown to mark promoters and/or enhancers of transcriptionally-active and potentially-active genes (Barski et al., 2007; Guenther et al., 2007; Heintzman et al., 2007). A loss of dimethyl- and trimethyl-K4 may be correlated with the commitment to senescence by losing marks on potentially-active genes including those involved in cell proliferation. In undifferentiated embryonic stem cells, many suppressed genes harbor both active (trimethyl-K4) and inactive (trimethyl-K27) marks (Bernstein et al., 2006; Guenther et al., 2007; Mikkelsen et al., 2007). Either mark can be erased to establish active or silent chromatin during differentiation. Such a bivalent regulation by K4 and K27 trimethylation is not restricted to embryonic stem cells but also found in somatic cells (Barski et al., 2007; Guenther et al., 2007), suggesting that K4 methylation may generally provide plasticity to chromatin. The rapid turnover of acetyl groups on H3 harboring methylated K4 (Hazzalin and Mahadevan, 2005) suggests that nucleosomes bearing methylated K4 are highly accessible to modifying enzymes (acetyltransferases and deacetylases) and transcription factors. In fact, promoters bearing trimethyl-K4 are often associated with RNA polymerase II on both the active and suppressed genes (Guenther et al., 2007). The plasticity of chromatin may be maintained by continuous unproductive transcriptional initiation from promoters bearing trimethyl-K4. As cellular senescence is an irreversible phenomenon, many suppressed genes might become permanently silent due to the general loss of dimethyl- and trimethyl-K4 in senesced cells. It is interesting to speculate that the levels of dimethyl- and trimethyl-K4 are correlated with the potency of cells; one extreme is pluripotent stem cells in which promoters of most coding genes are occupied with trimethyl-K4 (Guenther et al., 2007) and another extreme is senesced cells, lacking the potency to proliferate or differentiate, with diminished dimethyl- and trimethyl-K4.
Modification-specific antibodies have been powerful and versatile tools, and recent genome-wide ChIP analyses have provided global views of histone modifications on the complex genome in higher eukaryotes (Bernstein et al., 2005; 2006; Barski et al., 2007; Guenther et al., 2007; Heintzman et al., 2007; Mikkelsen et al., 2007; The ENCODE Project Consortium, 2007). We here showed that new mAbs directed against specific modifications on histone H3 are suitable for a variety of applications including ChIP and immunostaining. These highly-reliable and fully-characterized mAbs may be useful as standard reagents for analyzing epigenomic regulation during development and differentiation (Bernstein et al., 2007) and on healthy and diseased cells (Seligson et al., 2005; Jones and Baylin, 2007).
We thank Drs. M. Naito and S. Suzuki for primary human baby fibroblasts and P. R. Cook for valuable comments on the manuscript. This work was supported in part by Grants-in-aid, the Genome Network Project, and the Special Coordination Funds for Promoting Science and Technology, each from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.K.).
|