| To whom correspondence should be addressed: Kazuhisa Nakayama, Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Tel/Fax: +81–75–753–4527/4557 E-mail: kazunaka@pharm.kyoto-u.ac.jp Abbreviations: EGF, epidermal growth factor; ESCRT, endosomal sorting complex required for transport; GAT, GGA and Tom1; GGA, Golgi-localizing, γ-adaptin ear homology domain, Arf-binding protein; MVB, multivesicular body; Tom1L1, target of Myb 1-like 1; UEV, ubiquitin E2 variant; VHS, Vps27p/Hrs/Stam. |
In eukaryotic cells, proteins internalized from the plasma membrane are transported to early endosomes from which they are either recycled back via recycling endosomes or delivered to degradative compartments (Sorkin and von Zastrow, 2002; von Zastrow and Sorkin, 2007). Plasma membrane proteins that are destined for degradation, such as the epidermal growth factor (EGF) receptor, undergo ubiquitination and are delivered to late endosomal compartments, referred to as multivesicular bodies (MVBs), which in turn fuse with lysosomes. The limiting membranes of MVBs are invaginated to form intralumenal vesicles, which sequester the ubiquitinated proteins from the cytoplasmic milieu. A series of ESCRT (endosomal sorting complex required for transport) protein complexes are involved in the sorting of the ubiquitinated proteins into the intralumenal vesicles (Piper and Katzmann, 2007; Slagsvold et al., 2006; Williams and Urbé, 2007). On the limiting membrane, the ubiquitinated proteins are first recognized by the Hrs-Stam complex (ESCRT-0) and transferred to Tsg101, a critical component of the ESCRT-I complex, through an interaction between a Pro-Thr-Ala-Pro (PTAP) sequence of Hrs and the UEV (ubiquitin E2 variant) domain of Tsg101. The association of Hrs and Tsg101 leads to recruitment of ESCRT-I and downstream ESCRT-II and -III onto the limiting membrane and to subsequent invagination of the membrane to sequester the ubiquitinated cargo molecules. The ESCRT machinery has also been shown to be required for egress of enveloped viruses, such as HIV-1, from host cells by recognizing signals in viral Gag proteins (reviewed in Martin-Serrano, 2007; Morita and Sundquist, 2004; Slagsvold et al., 2006), and most recently shown to be implicated in cytokinesis through transiently associating with the midbody (Carlton and Martin-Serrano, 2007; Morita et al., 2007).
Tom1L1 (target of Myb 1-like 1; also known as Srcasm) and related proteins, Tom1 and Tom1L2, constitute a family of proteins that share an N-terminal VHS (Vps27p/Hrs/Stam) domain and a following GAT (GGA and Tom1) domain (Katoh et al., 2004). The GAT domain of both the GGA (Golgi-localizing, γ-adaptin ear domain homology, Arf-binding protein) and Tom1 family proteins was shown to bind ubiquitin (Bonifacino, 2004; Nakayama and Wakatsuki, 2003), suggesting their implication in sorting of ubiquitinated proteins to the MVB pathway (Katoh et al., 2006; Katoh et al., 2004; Puertollano and Bonifacino, 2004; Scott et al., 2004; Shiba et al., 2004; Yamakami et al., 2003); for review, see Hurley et al., 2006; Nakayama and Wakatsuki, 2003; Piper and Luzio, 2007. Recently, Puertollano reported that, by using a yeast two-hybrid system, Tom1L1 is able to interact with Tsg101 through a PTAP sequence located near the N-terminus of the GAT domain (see Fig. 1A), although she did not address the physiological relevance of the interaction (Puertollano, 2005).
 View Details | Fig. 1. Pull-down assays for Tom1L1 interaction with the Tsg101-UEV domain. (A) Schematic representation of the domain organization of human Tom1L1. Relative positions of the PTAP and PSAP sequences are indicated. (B) GST fusion proteins of the VHS domain, GAT domain and C-terminal region of Tom1L1 and Tom1 were used to pull down lysates from E. coli cells expressing the His6+T7-tagged Tsg101-UEV domain. (C) GST fusion proteins of full-length Tom1L1 and its mutants indicated were used to pull down lysates from E. coli cells expressing the His6+T7-tagged Tsg101-UEV domain. (D) GST fusion proteins of the GAT domain and C-terminal region of Tom1L1, and their mutants indicated were brought to pull down lysates from E. coli cells expressing the His6+T7-tagged Tsg101-UEV domain or its M95A mutant. In the experiments shown in (B)–(D), a half of the pull-down materials were subjected to immunoblot analysis using anti-T7-tag antibody. In the lysate lanes, 10% (B and C) or 2% (D) of the input sample was loaded. The other half of the materials were electrophoresed and stained with Coomassie Brilliant Blue (shown below the immunoblot data). |
In the present study, to obtain a clue to the role of Tom1L1 in the MVB biogenesis, we set out to characterize the Tom1L1-Tsg101 interaction. We found that not only the PTAP sequence in the GAT domain but also a PSAP sequence in the C-terminal region of Tom1L1 is responsible for its interaction with the UEV domain of Tsg101 and competes with the HIV-1 Gag protein for the Tsg101 interaction. Furthermore, we showed that, by means of Tsg101, Tom1L1 is associated not only with endosomes but also with the midbody during cytokinesis.
Construction of expression vectors for the VHS domain (amino acid residues 1–154), the GAT domain (residues 143–303) and the C-terminal region (residues 297–476) of human Tom1L1 was described previously (Katoh et al., 2006; Katoh et al., 2004). An expression vector for mCherry-tagged Tom1L1 was constructed by subcloning of the entire coding sequence of the human Tom1L1 cDNA into the pcDNA3.1-mCherry vector (Shaner et al., 2005) (a kind gift from Dr. Roger Tsien, UCSD). An expression vector for GFP-Tsg101 was constructed by subcloning of a cDNA fragment covering the entire coding sequence of human Tsg101 into the pEGFP-C1 vector (Clontech). An expression vector for HIV-1 Gag with a C-terminally Myc-tag was a kind gift from Dr. Nobuyuki Tanaka (Miyagi Cancer Center Research Institute, Japan). For pull-down assays, a cDNA fragment for the Tsg101-UEV domain (amino acid residues 1–145) was subcloned into the pET28a vector (Novagen). Amino acid substitutions were introduced into the cDNAs using a QuikChange XL Site-directed mutagenesis kit (Stratagene).
The following antibodies were used in the present study: monoclonal mouse anti-β-tubulin antibody (Chemicon); monoclonal mouse anti-T7-tag antibody (Novagen); monoclonal rat anti-HA antibody, 3F10 (Roche Diagnostics); polyclonal rabbit anti-MKLP1 antibody and monoclonal mouse anti-Myc antibody, 9E10 (Santa Cruz Biotechnology); AlexaFluor488-, AlexaFluor555- and AlexaFluor647-conjugated secondary antibodies (Molecular Probes); and Cy3-, Cy5- and horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories).
Pull down assays using GST-fusion proteins of the domain of Tom1L1 or Tom1 and lysate from Escherichia coli cells expressing the His6+T7-tagged wild type or mutant Tsg101-UEV domain were performed as described previously (Katoh et al., 2006; Katoh et al., 2004).
DNA transfection and immunofluorescence analysis were performed as described previously (Katoh et al., 2006; Katoh et al., 2004; Shin et al., 2004).
In an attempt to explore the potential role of Tom1L1 in the MVB pathway, we examined whether Tom1L1 is able to interact with Tsg101, since Tom1L1 has potential Tsg101-binding sequences; a PTAP sequence (amino acid residues 179–182) in the GAT domain and an equivalent PSAP sequence (residues 310–313) in the C-terminal region (schematically shown in Fig. 1A). A GST pull-down assay using GST-fusion proteins of Tom1L1 fragments and lysates of E. coli cells expressing the His6+T7-tagged Tsg101-UEV domain showed that not only the GAT domain (lane 4) but also the C-terminal region (lane 5) of Tom1L1 pulled down the UEV domain (Fig. 1B). Full length Tom1L1 also pulled down the UEV domain (Fig. 1C, lane 3). In contrast to Tom1L1, none of the Tom1 domains examined pulled down the UEV domain (Fig. 1B, lanes 7–9), consistent with the fact that there is no P(T/S)AP sequence in the Tom1 protein.
These results were rather unexpected, since Puertollano recently detected an interaction of Tsg101 with the VHS+ GAT domain but not the C-terminal region of Tom1L1 using a yeast two-hybrid system (Puertollano, 2005). In order to address this apparent discrepancy and to examine whether the PTAP and PSAP sequences are responsible for the interaction, we performed a pull-down assay using full-length Tom1L1 with replacement of the two Pro residues by Ala residues either in the PTAP sequence within the GAT domain (ATAA) or in the PSAP sequence within the C-terminal region (ASAA), or with the replacement in both the sequences (ATAA+ASAA) (schematically shown in Fig. 1A). As shown in Fig. 1C, mutations of both the PTAP and PSAP sequences abolished the Tom1L1 interaction with the UEV domain (lane 6), whereas a mutation of either sequence had a minor effect on the interaction (lanes 4 and 5). The results shown in Fig. 1, B and C, together indicate that both the PTAP sequence in the GAT domain and the PSAP sequence in the C-terminal region are able to interact independently with the Tsg101-UEV domain.
Previous biochemical and X-ray crystallographic studies indicated that the Met95 residue in the Tsg101-UEV domain is crucial for its interaction with P(T/S)AP sequences by making extensive intermolecular hydrophobic interactions with Pro and Ala in the sequences (Pornillos et al., 2002), and substitution of Met95 by Ala (M95A) extremely reduced the UEV interaction with P(T/S)AP sequences (Lu et al., 2003; Pornillos et al., 2002). We therefore carried out a GST pull-down assay using an E. coli cell lysate expressing the UEV(M95A) mutant. As shown in Fig. 1D, the M95A mutation abolished the UEV interaction with both the GAT domain and the C-terminal region (lanes 7 and 8). These data support the above conclusion that the Tsg101-UEV domain is able to recognize both the PTAP and PSAP sequences in the Tom1L1 protein.
In an attempt to examine the interaction between Tsg101 and Tom1L1 in the cell, we then performed immunofluorescence analyses. When expressed alone in HeLa cells, GFP-Tsg101 was localized on punctate endosomal structures distributed throughout the cytoplasm (Fig. 2A), while HA-tagged Tom1L1 was predominantly cytosolic (Fig. 2C) as described previously (Katoh et al., 2006). In striking contrast, when GFP-Tsg101 and HA-Tom1L1 were coexpressed, the latter was found to become localized on the punctate Tsg101-positive structures (Fig. 2, D–D”), indicating that the Tsg101-Tom1L1 interaction occurs in the cell.
 View Details | Fig. 2. Tsg101 recruits Tom1L1 onto endosomal structures. HeLa cells were transfected with an expression vector for GFP-Tsg101 (A), GFP-Tsg101(M95A) (B), or HA-Tom1L1 (C), or co-transfected with vectors for either GFP-tagged Tsg101 (D–G) or Tsg101(M95A) (H) and either HA-tagged Tom1L1 (D’ and H’), Tom1L1(ATAA) (E’), Tom1L1(ASAA) (F’), or Tom1L1(ATAA+ASAA) (G’). The cells were stained with anti-HA antibody. Merged images are shown in D”–H”. |
We then addressed whether the Tom1L1 colocalization with Tsg101 is based on a specific interaction between them, since it was possible that overexpression of Tsg101, like that of Hrs (Komada et al., 1997), might disturb normal endosomal trafficking and induce aberrant enlarged endosomes, where Tom1L1 could be accumulated non-specifically. To this end, we exploited the P(T/S)AP mutants of Tom1L1 and the Tsg101(M95A) mutant. When either the PTAP sequence in the GAT domain (ATAA) or the PSAP sequence in the C-terminal region (ASAA) was mutated, a small but significant fraction of HA-Tom1L1 appeared to remain colocalized with GFP-Tsg101, although a large fraction was cytosolic (Fig. 2, E–E” and F–F”, respectively). These results suggest that Tom1L1 having either of the PTAP and PSAP sequences partially retains its ability to associate with Tsg101 in the cell, being in agreement with the pull-down results (Fig. 1C). On the contrary, the colocalization of Tom1L1 with Tsg101 was no longer observed when both the PTAP and PSAP sequences were mutated (Fig. 2, G–G”). Furthermore, when GFP-Tsg101(M95A) and wild type HA-Tom1L1 were coexpressed, the latter failed to colocalized on punctate structures positive for the Tsg101 mutant (Fig. 2, H–H”). These observations are in good agreement with the data of pull-down experiments (Fig. 1), and indicate that both the PTAP and PSAP sequences contribute to the Tom1L1 interaction with the Tsg101-UEV domain in the cell.
The above data indicate that the Tsg101-Tom1L1 interaction takes place in the cell. To further support the intracellular interaction, we exploited a most recent finding that the ESCRT machinery components, including Tsg101, transiently localize to the midbody during cytokinesis (Carlton and Martin-Serrano, 2007; Morita et al., 2007). As expected, when coexpressed with GFP-Tsg101, mCherry-tagged Tom1L1 was colocalized with Tsg101 to the midbody (indicated by white arrows) as well as on punctate endosomal structures in cells undergoing cytokinesis (Fig. 3, A–A”’). The identity of the Tsg101-positive dot structure on the intercellular bridge as the midbody was confirmed by the complete overlap of the GFP-Tsg101 structure with the structure positive for MKLP1 (mitotic kinesin-like protein 1) (Fig. 3, D–D”’ and E–E”’), which is a component of the centralspindlin complex and is known to associate with the midbody matrix during cytokinesis (Matuliene and Kuriyama, 2004; reviewed in Glotzer, 2005). In cells with GFP-Tsg101 at the midbody during cytokinesis, 77% of the cells (33 out of 43 cells counted) had mCherry-Tom1L1 (Fig. 3, A–A”’). In considerable contrast, the Tom1L1 mutant of both the PTAP and PSAP sequences (ATAA+ ASAA) was no longer found at the midbody or endosomes (Fig. 3, B–B”’); no cells out of randomly selected 20 cells with GFP-Tsg101 at the midbody had overlapping mCherry-Tsg101(ATAA+ASAA). Furthermore, when coexpressed with Tsg101(M95A), wild type Tom1L1 did not localize to the midbody or endosomes (Fig. 3C’), even though the Tsg101 mutant retained its ability to associate with the midbody (Fig. 3, E–E”’) as reported previously (Carlton and Martin-Serrano, 2007). Thus, through the UEV-P(T/S)AP interaction, Tsg101 is able to recruit Tom1L1 not only to endosomes but also to the midbody.
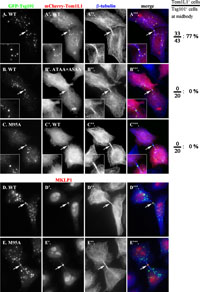 View Details | Fig. 3. Tsg101-dependent localization of Tom1L1 onto the midbody during cytokinesis. (A–C) HeLa cells were co-transfected with an expression vector for either GFP-tagged wild type Tsg101 (A and B) or its M95A mutant (C) and that for mCherry-tagged wild type Tom1L1 (A’ and C’) or its ATAA+ASAA mutant (B’) and stained for β-tubulin (A”–C”). (D and E) HeLa cells were transfected with an expression vector for GFP-tagged wild type Tsg101 (D) or its M95A mutant (E) were doubly stained for MKLP1 (D’ and E’) and β-tubulin (D” and E”). Images of cells during cytokinesis were taken by focusing on the midbody region. Merged images are shown in A”’–E”’. Arrows indicate the midbody positions. On the right side of the panels, percentages of GFP-Tsg101-positive midbodies with mCherry-Tom1L1 signals are indicated. |
Assembly and budding of HIV-1 and other enveloped viruses are directed by their viral Gag protein, which is a major structural component of the viruses. A conserved PTAP sequence within the C-terminal p6 region of Gag is required for efficient HIV-1 egress from most cell types by forming a docking site for Tsg101. The viruses thus highjack the ESCRT machinery for their egress from the cells (Martin-Serrano, 2007; Morita and Sundquist, 2004; Slagsvold et al., 2006).
HIV-1 Gag was previously reported to colocalize with Tsg101 on endosomes (Eastman et al., 2005) (also see Fig. 4, A–A”). On the other hand, our data presented so far have suggested that Tom1L1 interacts with Tsg101 through the PTAP and PSAP sequences to associate with endosomal structures (Fig. 2). We therefore performed immunofluorescence analyses to examine whether Tom1L1 and the HIV-1 Gag protein share the same ESCRT machinery. When coexpressed with GFP-Tsg101 in HeLa cells, Myc-tagged wild type Gag (Fig. 4, A–A”) but not Gag(AAAA) (Fig. 4, B–B”), in which the PTAP sequence was mutated to AAAA, was found to be recruited onto the Tsg101-positive endosomal structures. When HA-Tom1L1 was coexpressed with Gag-Myc, Tom1L1 remained cytosolic and did not show significant colocalization with the Gag protein (Fig. 4, C–C).
 View Details | Fig. 4. HIV-1 Gag competes with Tom1L1 in the interaction with Tsg101. HeLa cells were co-transfected with GFP-Tsg101 (A and B) and either Gag-Myc (A’) or Gag(AAAA)-Myc (B’), or with Gag-Myc (C) and HA-Tom1L1 (C’), or triply transfected with GFP-Tsg101 (D and E), HA-Tom1L1 (D’ and E’) and either Gag-Myc (D”) or Gag(AAAA)-Myc (E”). The cells were singly stained with anti-Myc antibody (A’ and B’), or doubly stained with antibodies to Myc (C, D” and E”) and HA (C’, D’ and E’). Merged images are shown in A”–C”, D”’ and E”’. (F) Lysates of control HeLa cells (lane 1) or HeLa cells triply transfected with GFP-Tsg101, HA-Tom1L1 and either Gag-Myc (lane 2) or Gag(AAAA)-Myc (lane 3) were subjected to immunoblot analysis using antibody to GFP (upper panel), HA (middle panel) and Myc (lower panel). |
Next, we simultaneously expressed GFP-Tsg101, Gag-Myc and HA-Tom1L1 in HeLa cells. As can be seen in Fig. 4, D–D”’, GFP-Tsg101 and Gag-Myc were colocalized on endosomal structures, whereas Tom1L1 remained predominantly cytosolic. On the other hand, when Gag(AAAA)-Myc was coexpressed with GFP-Tsg101 and HA-Tom1L1, the latter two were colocalized on endosomes, which did not contain Gag(AAAA)-Myc (Fig. 4, E–E”’). The redistribution of HA-Tom1L1 from GFP-Tsg101-positive endosomes was specific to the expression of wild-type Gag-Myc and did not result from extreme overexpression of the Gag protein, since the expression levels of Gag-Myc and Gag(AAAA)-Myc were comparable (Fig. 4F). These observations together indicate that the HIV-1 Gag protein is superior to Tom1L1 in terms of interaction with Tsg101.
 View Details | Fig. 4F. |
In the present study, we have characterized the interaction between Tom1L1 and Tsg101. By GST pull-down assays, we have shown that the interaction is mediated by the PTAP sequence in the GAT domain and the PSAP sequence in the C-terminal region of Tom1L1 and the UEV domain of Tsg101 (Fig. 1). In addition to Tom1L1, Tsg101 is known to interact with a variety of proteins containing P(T/S)AP sequences, such as Hrs and the HIV-1 Gag protein. We have then shown that Tom1L1 is recruited onto Tsg101-positive endosomal structures through the P(T/S)AP-UEV interactions. Components of the ESCRT machinery in the MVB pathway have been reported to be transiently recruited from the cytosol onto endosomal membranes, where they function sequentially in the sorting of ubiquitinated cargo proteins (Piper and Katzmann, 2007; Slagsvold et al., 2006; Williams and Urbé, 2007). Taken together, it is possible that Tom1L1 is recruited from the cytosol to endosomes via Tsg101 and regulates sorting of ubiquitinated proteins into the MVB pathway in concert with Tsg101 and other ESCRT components (Piper and Katzmann, 2007).
We have shown that both the PTAP sequence in the GAT domain and the PSAP sequence in the C-terminal region are able to interact with the Tsg101-UEV domain, whereas Puertollano previously reported that the VHS+GAT domain, but not the C-terminal region, of Tom1L1 could interact with Tsg101 (Puertollano, 2005). Although we do not know the exact reason for the discrepancy, it might be due to the experimental systems used: Puertollano revealed the interaction solely by using the yeast two-hybrid system, while we have demonstrated the dual interaction through the PTAP and PSAP sequences in vitro (Fig. 1) and at the cellular level (Fig. 2).
Like Tom1 and GGAs, Tom1L1 interacts with ubiquitin through the GAT domain (Bonifacino, 2004; Katoh et al., 2004; Nakayama and Wakatsuki, 2003; Shiba et al., 2004; Yamakami et al., 2003) and, like Tom1, with clathrin through the C-terminal region (Colilin et al., 2007; Katoh et al., 2006; Katoh et al., 2004; Yamakami et al., 2003). In the MVB pathway, ubiquitin functions as a sorting signal for degradation; ubiquitinated cargo proteins, such as the EGF receptor, are recognized by Hrs and sorted into the MVB by sequentially interacting with ESCRT complexes, including Tsg101-containing ESCRT-I (Piper and Katzmann, 2007; Slagsvold et al., 2006; Williams and Urbé, 2007). On the other hand, flat clathrin coats have been suggested to play a role in retention of ubiquitinated cargos on the limiting membrane of the MVB (Raiborg and Stenmark, 2002). We have shown here that Tom1L1 is recruited onto Tsg101-positive endosomes, suggesting that Tom1L1 plays a role in sorting of ubiquitinated proteins for degradation together with Tsg101 and clathrin. In this context, it is interesting to note that Tom1L1 is Tyr-phosphorylated by Src family kinases downstream of some growth factor receptors and is able to negatively regulate mitogenic signaling induced by the growth factors (Colilin et al., 2007; Franco et al., 2006; Hinsby et al., 2003; Li et al., 2005). It is therefore likely that Tom1L1 functions as a negative regulator of growth factor signaling at multiple levels.
Accumulating lines of evidence showed that the ESCRT machinery is required for egress of HIV-1 particles from the cell. The egress process is topologically equivalent to the formation of lumenal vesicles of the MVB (Martin-Serrano, 2007; Morita and Sundquist, 2004; Slagsvold et al., 2006) (see Supplementary Fig. S1). HIV-1 Gag has a PTAP sequence in its p6 late domain, and is thought to mimic the Tsg101-recruiting function of Hrs, thereby hijacking the machinery of the MVB formation for egress from the cell. In the present study, we have shown that HIV-1 Gag competes with Tom1L1 in terms of the interaction with Tsg101 (Fig. 4). In the presence of overexpressed HIV-1 Gag, Tom1L1 remains cytosolic, suggesting its inferiority to Gag in Tsg101 binding. These observations are compatible with the general strategy of viruses to proliferate in host cells; namely, viral proteins must overcome endogenous proteins to make exclusive use of the host cell machinery.
Most recently, the ESCRT components, including Tsg101, have been found to be transiently associated with the midbody and to be essential for cytokinesis (Carlton and Martin-Serrano, 2007; Morita et al., 2007; Spitzer et al., 2006). Since the final abscission stage of cytokinesis to give rise to two daughter cells is topologically equivalent to the final fission events of the MVB lumenal vesicle formation and the viral egress (see Supplementary Fig. S1), these results suggest that essentially the same mechanism underlies the regulation of the three distinct cellular processes; the MVB formation, viral egress and cytokinesis (Supplementary Fig. S1). In line with this notion, we observed that Tom1L1 is able to transiently associate with the midbody in a Tsg101-dependent manner (Fig. 3). Overall, Tom1L1 has a potential to regulate all the three processes involving the ESCRT machinery. To understand how Tom1L1 is implicated in the regulation of these processes, experiments are underway in our laboratory.
We would like to thank Drs. Nobuyuki Tanaka of the Miyagi Cancer Center Research Institute and Roger Tsien of UCSD for providing materials. This study was supported in part by grants in aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Japan Society for Promotion of Science, the Protein 3000 Project, and the Targeted Proteins Research Program. R. I. was supported as a research assistant by the 21st Century COE Program “Knowledge Information Infrastructure for Genome Science”, and S. T. and Y. K. were supported as research fellows of the Japan Society for Promotion of Science.
|