| To whom correspondence should be addressed: Kiyotoshi Sekiguchi, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan. Tel: +81–6–6879–8618, Fax: +81–6–6879–8619 E-mail: sekiguch@protein.osaka-u.ac.jp Abbreviations: 5'-RACE, rapid amplification of 5' cDNA ends; BSA, bovine serum albumin; CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; CeRME-8, C. elegans RME-8; CI-MPR, cation-independent mannose 6-phosphate receptor; DMEM, Dulbecco’s modified Eagle’s medium; DmRME-8, Drosophila melanogaster RME-8; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; GFP, green fluorescent protein; mAb, monoclonal antibody; PBS, phosphate-buffered saline; RME, receptor-mediated endocytosis; TfR, transferrin receptor; TGN, trans-Golgi network. |
All eukaryotic cells take up extracellular molecules by a process known as endocytosis via an elaborate intracellular membrane system. Endocytosis plays roles in many physiological processes, including uptake of extracellular nutrients, regulation of cell-surface receptor expression and maintenance of cell polarity, and is crucial for the construction and maintenance of multicellular systems (Mellman, 1996; Mukherjee et al., 1997; Sorkin and Von Zastrow, 2002). In clathrin-dependent receptor-mediated endocytosis, which represents the best-characterized endocytic pathway, ligands and receptors become internalized via clathrin-coated pits and vesicles (CCPs and CCVs, respectively) (Brodsky et al., 2001; Conner and Schmid, 2003), and are then transported to early endosomes for sorting to their appropriate destinations. Molecules destined for enzymatic degradation are delivered to lysosomes via late endosomes, while recycling receptors, such as the transferrin receptor (TfR), are returned to the plasma membrane via recycling endosomes (Mukherjee et al., 1997). In addition, certain molecules are targeted to other compartments, such as the trans-Golgi network (TGN). These membrane trafficking events are controlled by many proteins including adaptor molecules, Rab GTPases and SNAREs (van Vliet et al., 2003).
The protein designated RME (receptor-mediated endocytosis)-8 was first identified in Caenorhabditis elegans during a genetic screen of mutants showing defective endocytosis of yolk proteins (Fares and Grant, 2002; Zhang et al., 2001). This ubiquitously expressed protein has been implicated not only in fluid-phase and receptor-mediated endocytosis, but also in the survival and development of C. elegans. RME-8 is a large protein comprised of more than 2000 amino acid residues, but has no known domains other than a single DnaJ-domain. The DnaJ-domain has been shown to stimulate the ATPase activity of Hsp70 family proteins (Bukau and Horwich, 1998; Wall et al., 1994), and DnaJ-domain-containing proteins are therefore considered to function in protein folding as well as the association and dissociation of protein complexes. Indeed, the DnaJ-domain-containing protein auxilin interacts with Hsc70 and uncoats CCVs (Holstein et al., 1996; Ungewickell et al., 1995). In Drosophila, the DnaJ-domain of RME-8 was reported to bind to Hsc70-4 in an ADP (but not ATP)-dependent manner (Chang et al., 2004), although the binding of mammalian RME-8 to Hsc70 is dependent on ATP (Girard et al., 2005). The reasons for the different nucleotide requirements between Drosophila and mammalian RME-8 proteins remain unknown. RME-8 contains four conserved repeats of ~90 amino acids, designated IWN repeats, which are characterized by seven invariant residues, including isoleucine, tryptophan and asparagine residues. The functions of these repeats are also currently unknown.
RME-8 orthologues have been found in multicellular organisms from plants to humans. Arabidopsis RME-8 was identified by analysis of gravitropism defective 2 mutants showing reduced gravitropic responses in their shoots and hypocotyls (Silady et al., 2004). Drosophila RME-8 (DmRME-8) was identified in a screen for mutants showing enhancement of the rough eye phenotype induced by the expression of dominant-negative dynamin and implicated in clathrin-dependent endocytosis (Chang et al., 2004). Rat RME-8 was identified in a proteomic analysis of CCVs, and its orthologue in simian COS-7 cells was reported to be involved in the traffic between the TGN and the endosomal system (Girard et al., 2005). A cDNA encoding human RME-8 (hRME-8) was predicted using the human genome database (Girard et al., 2005) and has been cloned as KIAA0678, although the putative protein encoded by KIAA0678 does not start with a methionine. Despite accumulating evidence for the involvement of RME-8 in endocytosis, the connections between its functions and subcellular localizations remain to be defined. For example, RME-8 mutants of worms and flies showed marked defects in early endocytic pathways, such as those from the plasma membrane to early endosomes, while the RME-8 proteins in these organisms are primarily localized to multivesicular bodies and late endosomes, which are supposed to be involved in later endocytic pathways (Chang et al., 2004; Zhang et al., 2001).
In the present study, we cloned a full-length cDNA encoding hRME-8 and examined the biochemical properties and intracellular localizations of hRME-8 protein. Our results show that hRME-8 is tightly membrane-associated via its N-terminal region and juxtaposed with several markers for early endosomes. It is also localized in the membrane of vacuoles induced by expression of dominant-active Rab5, implying its role in membrane trafficking through early endosomes. We also attempted to identify the trafficking pathway involving hRME-8 by overexpressing hRME-8 truncation mutants.
The coding regions of hRME-8 were predicted by GENSCAN (http://genes.mit.edu/GENSCAN.html), and confirmed by sequencing of RT-PCR products amplified from A549 human lung adenocarcinoma cells with the following pairs of primers: sense primer, 5'-GAGCCTTTAGAATTCGAGCAA-3' (nucleotide positions 631–651) and antisense primer, 5'-AAAGCTAGCTCTGTAGCCGCA-3' (nucleotide positions 4325–4344); sense primer, 5'-GAGCTAGCTTTCCATACTGTC-3' (nucleotide positions 4270–4290) and antisense primer, 5'-ACACGTGAGTCTGATAGCCAAGGTC-3' (nucleotide positions 6712–6729). The 5'-untranslated region and remaining coding sequence of hRME-8 were obtained by rapid amplification of 5' cDNA ends (5'-RACE). The primers used in the first round of PCR were a 3'-primer (5'-CGAATTCTAAAGGCTCTTTCC-3'; nucleotide positions 626–646) and an abridged anchor primer (Invitrogen, Carlsbad, CA). The primers used in the second round of PCR were a nested 3'-primer (5'-CTCTTCTCTTTGCTCTGACGC-3'; nucleotide positions 547–567) and an abridged universal amplification primer (Invitrogen). After sequence verification, error-free cDNA fragments were ligated in tandem to construct a cDNA encompassing the whole open reading frame. The hRME-8 cDNA was inserted into the EcoRV site of pEAK10 (Edge Biosystems, Gaithersburg, MD) or KpnI/SmaI site of pEGFP-C1 (wild-type). Expression vectors for ΔC425 (nucleotides 1–5454), ΔC633 (nucleotides 1–4830), ΔC1165 (nucleotides 1–3234), ΔC1790 (nucleotides 1–1359), ΔN453 (nucleotides 1360–6729) and ΔN1083 (nucleotides 3250–6729) were obtained by digestion at unique restriction enzyme sites in the wild-type plasmid, followed by T4 polymerase treatment and self-ligation.
A cDNA encoding Hrs tagged with a myc epitope at its N-terminal end was a generous gift from Dr. Harald Stenmark (The Norwegian Radium Hospital, Oslo, Norway). cDNAs encoding mouse Rab family proteins (Rab4a, Rab5a, Rab7 and Rab11a) were amplified by RT-PCR as described previously (Kuroda et al., 2002). cDNAs encoding dominant-active forms of Rab4a (Q72L), Rab5a (Q79L), Rab7 (Q67L) and Rab11a (Q70L) were constructed by PCR using mutagenic oligonucleotides as previously described (Fukuda et al., 1995). The sequences of the mutagenic oligonucleotides used are available from the authors on request. The mutant Rab cDNAs were subcloned into the pEGFP-C1 vector at appropriate restriction enzyme sites and verified by DNA sequencing.
For the production of an anti-hRME-8 antibody, a cDNA encoding amino acids 1965–2243 of hRME-8 was amplified by PCR and cloned into the SalI/NotI sites of a pGEX vector (Amersham Biosciences, Piscataway, NJ). The PCR primers used were: sense primer, 5'-AGTCGACGAAGATTTTGCTGTGGTG-3' and antisense primer, 5'-AGCGGCCGCTATTTCAAGTCTGATAG-3'. The GST fusion protein was expressed in Escherichia coli and isolated as inclusion bodies, followed by cleavage with thrombin. The recombinant protein was separated by preparative SDS-PAGE, electroeluted from the gel, dialyzed against phosphate-buffered saline (PBS) and used to immunize rabbits. A polyclonal antibody against hRME-8 was prepared by affinity purification using the recombinant protein coupled to CNBr-activated Sepharose-4B (Amersham Biosciences). Mouse monoclonal antibodies (mAbs) against EEA1, caveolin-1 and GM130 were purchased from BD Transduction Laboratories (Lexington, KY). The production of a mAb against human TfR (N-2) was described previously (Yoshimori et al., 1988). A mAb against human TfR (H68.4) was obtained from Zymed Laboratories Inc. (South San Francisco, CA). A goat polyclonal antibody against integrin α3 and a mAb against myc were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mAb against human LAMP1 and rabbit serum against rat cathepsin D were kindly provided by Dr. Yukio Nishimura (Kyushu University, Fukuoka, Japan). Rabbit serum against rat aldolase and a mAb against LBPA were generous gifts from Dr. Eiki Kominami (Juntendo University, Tokyo, Japan) and Dr. Toshihide Kobayashi (RIKEN, Japan), respectively. Mouse mAbs against GFP and mono/poly-ubiquitin (FK2) were obtained from MBL (Nagoya, Japan). A mAb against human LAMP2 (H4B4) developed by Dr. Thomas August was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa (Iowa City, IA). A mAb against KDEL and a sheep polyclonal antibody against TGN46 were purchased from StressGen Biotechnologies (Victoria, BC, Canada) and Serotec Ltd. (Oxford, UK), respectively. Alexa 405-, 488- or 546-conjugated goat anti-mouse, goat anti-rabbit and donkey anti-sheep secondary antibodies were obtained from Invitrogen, as were human transferrin conjugated with Texas Red, biotinylated epidermal growth factor (EGF) complexed to Texas Red streptavidin (Texas Red-EGF) and Alexa 546 carboxylic acid succinimidyl ester. Human holo-transferrin was obtained from Sigma (St. Louis, MO). A fluorescently-labeled protein (Alexa 546-transferrin) was prepared according to the manufacturer’s instructions (Invitrogen).
HeLa (human cervical carcinoma), HT1080 (human fibrosarcoma), TYK-nu (human ovarian undifferentiated carcinoma), 293T (human embryonic kidney), A549 and COS-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. NB-1 (human neuroblastoma) and K562 (human erythroleukemia) cells were grown in RPMI 1640 containing 10% fetal calf serum.
For transfection, 293T cells were seeded on 6-well plates or 6-cm dishes at 1 day prior to transfection with an hRME-8 expression vector using FuGENE6 (Roche, Indianapolis, IN) or Lipofectamine 2000 (Invitrogen). At 36 h after transfection, the cells were used in experiments as described below. In the case of transfection with myc-tagged Hrs, GFP-Rab mutants or hRME-8 deletion mutants, A549, HeLa and COS-1 cells were seeded on coverslips in 24-well plates or 35-mm μ-Dishes (Nippon Genetics) at 1 day before transfection. The cells were fixed at 24 h after transfection and immunostained as described below. For immunoblot analyses, cells were lysed in Tris-buffered saline containing 1% Triton X-100, 1 mM PMSF, 5 μg/ml aprotinin and 5 μg/ml leupeptin, and centrifuged at 15,000 g for 10 min at 4°C. The proteins were resolved by SDS-PAGE, transferred to PVDF membranes and probed with antibodies using an ECL detection system.
Fractionation of HeLa and A549 cells was performed as described previously (Kihara et al., 2001). Briefly, harvested cells were lysed in cold homogenization buffer (20 mM HEPES pH 7.5, 0.25 M sucrose, 1 mM EDTA) containing 1 mM PMSF, 5 μg/ml aprotinin and 5 μg/ml leupeptin by extrusion through a polycarbonate filter with 8-μm pores, and the filter effluent was centrifuged at 500 g for 5 min at 4°C to remove cell debris and nucleic acids. The supernatant (S1) was centrifuged at 10,000 g for 10 min at 4°C to generate a pellet (P2) and a supernatant (S2). The S2 supernatant was centrifuged at 100,000 g for 1 h at 4°C to generate a pellet (P3) and a supernatant (S3). Each pellet was resuspended in homogenization buffer, and its volume was adjusted to be equal to that of the supernatant. For solubilization experiments, the P3 fraction was diluted with an equal volume of homogenization buffer containing either 2% Triton X-100, 4% n-octyl-β-D-glucoside, 2% SDS, 1.2 M NaCl, 8 M urea or 0.2 M Na2CO3, and solubilized for 15 min at room temperature. The solubilized materials were centrifuged at 100,000 g for 1 h at 4°C, and the resulting supernatants were subjected to acetone precipitation. The pellets were resuspended in an equal volume of SDS-PAGE sample buffer.
For sucrose-gradient centrifugation, the P3 fraction (1 ml) was adjusted to 1.5 M sucrose with 4.5 ml of 1.78 M sucrose in 20 mM HEPES (pH 7.5) containing 1 mM EDTA, and then overlaid with 22.8 ml of 1.2 M sucrose and 7.5 ml of 0.5 M sucrose in the same 20 mM HEPES buffer. The gradient was centrifuged at 140,000 g for 16 h at 4°C, and fractions were collected from the top of the gradient. Each fraction was diluted by 3-fold in homogenization buffer, and centrifuged at 100,000 g for 1 h at 4°C. The pellets were dissolved in SDS-PAGE sample buffer. For lipid raft isolation, cells on 15-cm dishes were lysed in 2.5 ml of buffer consisting of 0.2% Triton X-100, 20 mM Tris-HCl pH 7.4, 5 mM EDTA, 0.15 M NaCl and 1 mM PMSF, and passed through a 24-gauge needle five times. The lysate was mixed with an equal volume of 100% sucrose solution, sequentially overlaid with 21 ml of 35% sucrose in Buffer A (10 mM Tris-HCl pH 7.4, 2.5 mM EDTA, 75 mM NaCl) and 9 ml of 5% sucrose in Buffer A, and centrifuged at 140,000 g for 20 h at 4°C. Fractions were collected as described above. The proteins in the fractions were precipitated with 10% trichloroacetic acid, washed with cold acetone and dissolved in SDS-PAGE sample buffer for immunoblot analyses.
293T cells transfected with GFP-tagged wild-type hRME-8 or its deletion mutants were lysed as described above, and centrifuged at 500 g for 5 min at 4°C. The supernatants (total fractions, T) were centrifuged at 100,000 g for 1 h at 4°C to generate pellets (membrane fractions, M) and supernatants (cytosolic fractions, C). Each membrane fraction was resuspended in homogenization buffer, and its volume was adjusted to be equal to that of the corresponding cytosolic fraction.
Cells cultured on coverslips and μ-Dishes were fixed with 3% paraformaldehyde in PBS for 15 min at room temperature, or treated with a pre-permeabilization solution (80 mM PIPES pH 6.8, 50 μg/ml digitonin, 5 mM EGTA, 1 mM MgCl2) before fixation for 5 min at 4°C. After fixation, the cells were permeabilized with 50 μg/ml digitonin in PBS for 5 min, and quenched with 50 mM NH4Cl in PBS for 15 min. The cells were blocked with 1% bovine serum albumin (BSA) in PBS for 1 h, and then incubated with a primary antibody diluted in the blocking solution for 1 h. The following primary antibodies and dilutions were used: anti-hRME-8 antibody, 1:800; anti-LAMP2 antibody, 1:300; anti-TGN46 antibody, 1:400; anti-EEA1 antibody, anti-LBPA antibody, anti-GM130 antibody, anti-KDEL antibody, anti-myc antibody, anti-TfR antibody and anti-mono/poly-ubiquitin antibody, 1:100. After three washes with PBS, the cells were incubated with an appropriate secondary antibody diluted in blocking solution for 1 h, and washed three times with PBS. The stained cells were mounted and observed using an LSM5 Pascal confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
To label early and recycling endosomes, cells were incubated with serum-free DMEM containing 0.1% BSA (0.1% BSA/DMEM) for 30 min, and then with 30 μg/ml Texas Red-conjugated transferrin in 0.1% BSA/DMEM for 30 min at 37°C. For EGF internalization, cells were incubated with 0.1% BSA/DMEM for 1 h at 37°C and then with 3.3 μg/ml Texas Red-EGF in 0.1% BSA/DMEM for 1 h at 4°C. The cells were washed and incubated at 37°C for the indicated periods of time. For transfection with wild-type hRME-8 and its deletion mutants, COS-1 cells were preincubated with serum-free DMEM as described above, and pulsed with 20 μg/ml Alexa 546-transferrin or 3.3 μg/ml Texas Red-EGF in 0.1% BSA/DMEM at 37°C for 10 min, followed by washing and chasing at 37°C for the indicated times. After these treatments, the cells were washed, fixed and stained for hRME-8 or EEA1 as described above.
A549 cells were fixed in 4% paraformaldehyde-4% sucrose in 0.1 M phosphate buffer (pH 7.4) for 15 min at room temperature. The procedures used for immunogold labeling of thin cryosections were described previously (Waguri et al., 1999).
To explore the functions of hRME-8, we first cloned a full-length cDNA encoding hRME-8 by RT-PCR and 5'-RACE. RT-PCR was performed based on the sequence predicted by GENSCAN, and the putative exon 1 that was not amplified by RT-PCR was obtained by 5'-RACE. Comparison of the cloned cDNA sequence with the human genome sequence revealed that the hRME-8 gene is located at chromosome 3q22.1 and consists of 56 exons (Fig. 1A). The predicted open reading frame of the hRME-8 gene encodes a protein of 2243 amino acid residues (Fig. 1B). Blast searches revealed that “DnaJ homologue subfamily C member 13 (DNAJC13)” is identical to hRME-8. The full-length human RME-8 contains a DnaJ-domain and four IWN repeats (Fig. 1B), similar to other RME-8 orthologues, and shows 43% homology to C. elegans RME-8 (CeRME-8) and 96% homology to rat putative RME-8 (accession number XP_343463) predicted by automated computational analysis.
 View Details | Fig. 1. Schematic diagram of the hRME-8 locus and immunodetection of hRME-8. (A) Structure of the hRME-8 locus. Exons are indicated by vertical lines and filled rectangles. (B) Domain structure of hRME-8. The DnaJ-domain and four IWN repeats are indicated as a black box and white numbered boxes, respectively. The asterisk indicates the C-terminal region used as an antigenic fragment for antibody production. (C) Immunoblot analysis of hRME-8 in various human cell lines. Equivalent amounts of cell extracts were subjected to SDS-PAGE and subsequent immunoblotting with an anti-hRME-8 polyclonal antibody. The cells examined were A549 (lung adenocarcinoma), HeLa (cervical carcinoma), HT1080 (fibrosarcoma), NB-1 (neuroblastoma), K562 (erythroleukemia), TYK-nu (ovarian carcinoma), 293T (embryonic kidney-derived epithelial) and 293T cells transfected with hRME-8. The position of a molecular size marker is shown on the left. |
RME-8 is ubiquitously expressed throughout the body. Indeed, transcripts encoding KIAA0678 were detected in all human tissues examined (Ishikawa et al., 1998). To detect hRME-8 expression at the protein level, we produced an antibody against the C-terminal 279 amino acid residues (see Fig. 1B) and examined the expression levels of hRME-8 protein in various human cell lines. Immunoblot analyses using the affinity-purified antibody identified an ~220-kDa protein in all the cell lines examined, including those of epithelial and mesenchymal origins (Fig. 1C). Transfection of the hRME-8 cDNA into 293T cells produced the same 220-kDa band, confirming the authenticity of the hRME-8 cDNA and specificity of the anti-hRME-8 antibody.
CeRME-8 in coelomocytes and simian RME-8 in COS-7 cells are localized to vesicular structures, while DmRME-8 is predominantly detected in the cytosol (Chang et al., 2004; Girard et al., 2005; Zhang et al., 2001). To address this apparent discrepancy and examine whether hRME-8 is a cytosolic or membrane-associated protein, HeLa cells were homogenized and fractionated by consecutive centrifugations (Fig. 2A), and each fraction was subjected to immunoblot analysis for the presence of hRME-8 protein. hRME-8 was detected in the mitochondrial/lysosomal (P2) and microsomal (P3) fractions, but not in the cytosolic (S3) fraction, similar to the case for TfR and integrin α3, which are single-span membrane proteins (Fig. 2B). LAMP1 and cathepsin D (lysosomal markers) were mostly detected in the P2 fraction, while aldolase (a soluble cytosolic protein) was present in the S3 fraction, verifying the fractionation procedure. Similar results were obtained for A549 cell homogenates (data not shown).
 View Details | Fig. 2. Subcellular localization of hRME-8. (A) Schematic diagram of the subcellular fractionation by differential centrifugation. After lysis by extrusion through a polycarbonate filter, cell lysates were subjected to differential centrifugation to fractionate subcellular organelles and the cytosol. The pellets recovered by centrifugation at 10,000 g (P2) and 100,000 g (P3) are enriched with the indicated organelles. The 100,000 g supernatant (S3) is enriched with cytosolic proteins. (B) HeLa cell lysates were subjected to fractionation as described in (A), and each fraction was analyzed by immunoblotting with antibodies against hRME-8, TfR (early/recycling endosomal membrane protein), integrin α3 (plasma membrane protein), LAMP1 (late endosomal/lysosomal membrane protein), cathepsin D (lysosomal protein) and aldolase (cytosolic protein). (C) Aliquots of the P3 fraction from A549 cells were adjusted to final concentrations of 1% Triton X-100 (Triton), 2% n-octyl-β-D-glucoside (OG), 1% SDS, 0.6 M NaCl, 4 M urea or 0.1 M Na2CO3, and solubilized for 15 min at room temperature. All samples were separated into pellet (P) and supernatant (S) fractions by ultracentrifugation, followed by immunoblotting with antibodies against hRME-8, TfR and EEA1. (D) The P3 fraction from A549 cells was subfractionated by sucrose gradient ultracentrifugation. Each fraction was then diluted and centrifuged, and the resulting pellet was analyzed by immunoblotting as described for (C). (E) A549 cells were extracted with 0.2% Triton X-100 at 4°C and fractionated by sucrose gradient ultracentrifugation. A total of 12 fractions were collected, precipitated with 10% trichloroacetic acid and subjected to immunoblotting with antibodies against hRME-8, TfR and caveolin-1 (a lipid raft marker). |
Since hRME-8 was found in the membrane-associated fractions, we next examined whether hRME-8 is an integral or peripheral membrane protein by treating the microsomal fraction with detergents, high salt concentrations or denaturants. Immunoblot analyses revealed that hRME-8 was not solubilized in 0.6 M NaCl or 4 M urea, while the majority of microsomal hRME-8 was solubilized in 0.1 M Na2CO3 (Fig. 2C), indicating that hRME-8 is a peripheral membrane protein and not a typical integral membrane protein. EEA1 was solubilized in all reagents tested, i.e., 0.6 M NaCl, 4 M urea and 0.1 M Na2CO3. The distinctive behavior of hRME-8 after treatment with high salt concentrations and a protein denaturant indicates that hRME-8 and EEA1 bind to the membrane via different mechanisms, and that hRME-8 is more tightly associated with the membrane than EEA1. The behavior of hRME-8 against detergent treatment also differed from those of EEA1 and TfR. EEA1 and TfR were solubilized in Triton X-100 and n-octyl-β-D-glucoside, whereas hRME-8 was resistant to these detergents. These distinctions were not due to protein aggregation, since hRME-8 was recovered in the buoyant fraction, together with TfR, when the microsomal fraction was further fractionated by a float-up method using a sucrose gradient (Fig. 2D). hRME-8 was also not a typical raft-associated protein, since it was recovered in the bottom fraction, together with TfR, when the Triton X-100 lysate was fractionated by a float-up method (Fig. 2E), in which caveolin-1, a raft marker, was found in the buoyant fraction.
 View Details | Fig. 3. Effects of N-terminal and C-terminal truncations on the membrane association of hRME-8. (A) Schematic structures of wild-type hRME-8 and its deletion mutants with an N-terminal GFP tag. ΔC425, mutant lacking residues 1819–2243 including IWN4; ΔC633, mutant lacking residues 1611–2243 including IWN3 and IWN4; ΔC1165, mutant lacking residues 1079–2243 including IWN2 through IWN4 and the DnaJ-domain; ΔC1790, mutant lacking residues 454–2243 including IWN1 through IWN4 and the DnaJ-domain; ΔN453, mutant lacking residues 1–453; ΔN1083, mutant lacking residues 1–1083 including IWN1. (B) 293T cells were transiently transfected with GFP-tagged hRME-8 or its deletion mutants, homogenized (T) at 36 h after transfection and fractionated into membrane (M) and cytosolic (C) fractions by centrifugation at 100,000 g. The fractions were analyzed by immunoblotting with antibodies against GFP and TfR. The positions of molecular size markers are shown on the left. |
Given the tight association of hRME-8 with the membrane fraction, we next sought to localize the region of hRME-8 that interacts with membranes by expressing a series of deletion mutants with a green fluorescent protein (GFP) tag at their N-termini (Fig. 3A). After expression in 293T cells, these mutant proteins were fractionated into the membrane and cytosolic fractions upon homogenization of the transfected cells and subsequent centrifugation at 100,000 g. Immunoblot analysis using an anti-GFP antibody showed that not only wild-type hRME-8 but also deletion mutants lacking the C-terminal region, e.g., ΔC425, ΔC633 and ΔC1165, were predominantly associated with the membrane fraction, as was the case with TfR (Fig. 3B). The deletion mutant lacking three-quarters of the C-terminal region, ΔC1790, was also partially associated with the membrane, indicating that the N-terminal region spanning amino acid residues 1–453 is primarily involved in the membrane association of hRME-8. In support of this conclusion, the association of hRME-8 with the membrane was abolished by deletion of the N-terminal 453 amino acid residues.
CeRME-8 is localized to the membrane of large vesicles in coelomocytes, while DmRME-8 and RME-8 in COS-7 cells are partially colocalized not only with early endosomes but also with late endosomes, as evaluated by immunofluorescence imaging (Girard et al., 2005). To address the possibility that hRME-8 is associated with multiple endocytic compartments, we examined whether hRME-8 colocalizes with a panel of well-characterized organelle markers. In A549 cells, hRME-8 was juxtaposed or partially colocalized with EEA1 and transferrin, markers for early endosomes and early/recycling endosomes, respectively (Fig. 4A). Since DmRME-8 partially overlaps with Hrs, which resides in early endosomes (Pfeffer, 2003), we transfected A549 cells with myc-tagged Hrs and examined whether hRME-8 colocalized with Hrs. hRME-8 was tightly juxtaposed with myc-tagged Hrs, as was the case for EEA1. In contrast, hRME-8 did not colocalize with LBPA and GFP-Rab7 (late endosomal markers; Fig. 4A and Supplementary Fig. 1A) or LAMP2 (a late endosomal/lysosomal marker; Fig. 4A). These results indicate that hRME-8 differs from DmRME-8 and RME-8 in COS-7 cells regarding its subcellular localization, with the latter being partially localized in late endosomes (Chang et al., 2004; Girard et al., 2005). hRME-8 was not localized to either the cis-Golgi or endoplasmic reticulum, based on its distinct localization from those of GM130 and KDEL-containing proteins, respectively.
 View Details | Fig. 4. hRME-8 is mainly localized to early endosomes. (A) A549 cells were fixed and double-stained with antibodies against hRME-8 and the following organelle markers: EEA1 for early endosomes; LBPA for late endosomes; LAMP2 for late endosomes and lysosomes; GM130 for the Golgi apparatus; and the KDEL sequence for the endoplasmic reticulum. Cells were visualized by immunofluorescence confocal microscopy. For Texas Red-transferrin (Tf, an early and recycling endosomal marker) uptake assays, A549 cells were incubated with 30 μg/ml Texas Red-Tf at 37°C for 30 min, washed, fixed and stained with an anti-hRME-8 antibody for confocal microscopy. In addition, A549 cells were transiently transfected with myc-tagged Hrs (an early endosomal marker), fixed at 24 h after transfection and stained with anti-hRME-8 and anti-myc antibodies for confocal microscopy. The bar represents 20 μm. (B) A549 cells were incubated with 3.3 μg/ml Texas Red-EGF at 4°C for 1 h, washed and incubated at 37°C to allow internalization of the bound ligands. The cells were fixed at the indicated times, followed by staining with an anti-hRME-8 antibody for confocal microscopy. The insets show higher magnification images of the boxed areas. The bar represents 20 μm. (C) Thin cryosections of A549 cells were double-labeled with antibodies against hRME-8 (arrows) and TfR (arrowheads), followed by detection of the bound antibodies using secondary antibodies conjugated with 5 and 10 nm colloidal gold particles, respectively. The asterisk in the left panel shows a multivesicular body. PM, plasma membrane. The bars represent 0.5 μm. |
EGF, a marker for the endocytic pathway, binds to its receptor, and is then internalized and transported to lysosomes via early endosomes. To examine whether hRME-8 colocalizes with EGF, A549 cells were incubated with Texas Red-conjugated EGF for 1 h at 4°C and then chased for the indicated times at 37°C (Fig. 4B). The internalized EGF partially colocalized with hRME-8 after 15 min of chase, but ceased to overlap after 1 h of chase. EGF was degraded after 2 h of chase (data not shown). These results, together with the colocalization of hRME-8 with early endosomal markers, demonstrate that hRME-8 is primarily localized to early endocytic compartments.
To investigate the localization of RME-8 in more detail, we performed an immunoelectron microscopic analysis using antibodies against hRME-8 and TfR. As shown in Fig. 4C, hRME-8 (arrows) and TfR (arrowheads) were localized to the same endosomes and vesicle-like structures. Although TfR was also detected in vesicular structures beneath the plasma membrane, presumably CCPs, hRME-8 was not localized to these structures. Furthermore, hRME-8 was not localized to typical multivesicular bodies (asterisk), which correspond to late endosomal compartments (21 multivesicular bodies in 25 cell sections were examined). These results again support the conclusion that hRME-8 is predominantly distributed to early endosomes and endosome-like structures, and not to degradative organelles, such as late endosomes.
Rab GTPases are well-known to control the vesicular trafficking of endocytosis and exocytosis (Stenmark and Olkkonen, 2001; Zerial and McBride, 2001). In humans, more than 60 different Rab proteins have been identified (Pereira-Leal and Seabra, 2000), of which Rab4, 5, 7 and 11 are the major Rab proteins involved in endocytic transport (Stenmark and Olkkonen, 2001; Zerial and McBride, 2001). Rab4 and Rab11 are involved in the recycling pathways, while Rab5 and Rab7 function in the early and late endocytic pathways, respectively. Exogenous expression of dominant-active Rab4a (Rab4aQ72L) has been shown to induce an abnormal early endosomal compartment (McCaffrey et al., 2001), while dominant-active Rab11a (Rab11aQ70L) concentrates in the pericentriolar region and inhibits the recycling of transferrin (Ren et al., 1998; Ullrich et al., 1996). Overexpression of dominant-active Rab5a (Rab5aQ79L) has been shown to promote the fusion of endosomes and induce large vacuoles that contain endosomal markers such as transferrin and EEA1 (Stenmark et al., 1994), while expression of dominant-active Rab7 (Rab7Q67L) increases the aggregation and fusion of late endocytic structures in the cytoplasm or perinuclear region (Bucci et al., 2000; Meresse et al., 1995; Vitelli et al., 1997). Since hRME-8 is predominantly localized in early endosomes, we examined whether expression of dominant-active mutants of Rab4a, Rab5a, Rab7 and Rab11a could alter the localization of hRME-8. The localizations of the GFP-Rab mutants and hRME-8 in A549 cells were examined by immunofluorescence microscopy. hRME-8 was partially juxtaposed with Rab4aQ72 at unidentified membrane structures (possibly peripheral early endosomes), but no generalized or substantial colocalization was observed (Fig. 5A). hRME-8 was not detected on endosomes showing positive staining for Rab7Q67L and Rab11aQ70L (Figs. 5C, D). However, it was not only juxtaposed with large vacuoles but also distributed non-uniformly on the surface of enlarged endosomes showing positive staining for Rab5aQ79L (Fig. 5B). Similar results were obtained with HeLa cells (Supplementary Fig. 1B). hRME-8 was also found to juxtapose with wild-type Rab5a, another marker for early endosomes (Fig. 5E). We further examined whether the distribution of hRME-8 was influenced by the expression of dominant-active forms of other Rabs, including Rab1 through Rab10. With the exception of Rab5aQ79L, transfection of these Rab mutants did not alter the hRME-8 distribution within A549 cells (A.F., data not shown), supporting the conclusion that hRME-8 is involved in the membrane trafficking pathway through early endosomes, which is primarily regulated by Rab5.
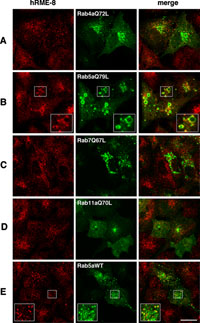 View Details | Fig. 5. Effects of dominant-active Rab proteins on the subcellular localization of hRME-8. A549 cells were transiently transfected with GFP-tagged Rab mutant proteins and wild-type Rab5a as indicated in the middle column, fixed at 24 h after transfection and stained for hRME-8. The cells were visualized by confocal microscopy. The insets show higher magnification images of the boxed areas. The bar represents 20 μm. |
To address the role of hRME-8 in membrane trafficking through early endosomes, we first attempted knockdown of endogenous hRME-8 expression in A549 cells by RNAi. Immunoblot and immunofluorescence analyses revealed that the expression of hRME-8 was significantly suppressed by >95% in siRNA-transfected cells, compared with control cells (Supplementary Fig. 2A). The silencing efficiency was comparable to that achieved by Girard et al. (Girard et al., 2005). RME-8-depleted cells showed no significant alterations in the subcellular localizations of EEA1 and clathrin heavy chain (Supplementary Fig. 2B) or LAMP2 (data not shown), compared to cells transfected with a control siRNA. Neither the EGF receptor (EGFR)/TfR expression levels nor the endocytic fate of their ligands were altered in hRME-8 knockdown cells (Supplementary Figs. 2A, B). We further examined the kinetics of the internalization, recycling and degradation of 125I-labeled EGF in hRME-8-depleted cells. There were no differences in the kinetics of 125I-EGF internalization and degradation between control and hRME-8-depleted cells, whereas the rate of 125I-EGF recycling was slightly (~10%, P<0.001), but reproducibly, increased in hRME-8-depleted cells (Supplementary Fig. 3A–C). Similarly, no significant differences in the internalization and recycling of 125I-Tf were observed between control and hRME-8-depleted cells (Supplementary Fig. 3D, E).
Since hRME-8 knockdown appeared to have no significant impact on endocytosis, we examined the effects of overexpression of hRME-8 and its truncated mutants on membrane trafficking through early endosomes. We used COS-1 cells due to their high levels of expression of exogenous proteins. When COS-1 cells were transfected with GFP-tagged hRME-8 or its deletion mutants, wild-type hRME-8 was predominantly localized to perinuclear regions, while the mutant lacking the N-terminal 453 amino acid residues (i.e., ΔN453) was diffusely distributed in the cytoplasm, consistent with its inability to associate with the membrane (Fig. 6A). Transfection of C-terminally-truncated mutants lacking the C-terminal half (ΔC1165) and C-terminal 425 amino acid residues including the IWN4 repeat (ΔC425) resulted in the formation of large puncta and vacuoles (Fig. 6A). In ΔC425-transfected cells, pronounced swollen vacuoles were observed throughout the cytoplasm, whereas large puncta were often detected in ΔC1165-transfected cells.
 View Details | Fig. 6. Effects of exogenous ΔC425 expression on receptor-mediated endocytosis. (A) COS-1 cells were transfected with GFP-tagged hRME-8 or its deletion mutants and then treated with a pre-permeabilization solution before fixation (wild-type) or fixed at 24 h after transfection for confocal microscopy. (B) COS-1 cells were transiently transfected with ΔC425, fixed and stained with antibodies against organelle markers as indicated in the left margin. (C) COS-1 cells were transfected with ΔC425 and then incubated with 3.3 μg/ml Texas Red-EGF or 20 μg/ml Alexa 546-transferrin (Tf) at 37°C for 10 min. For EGF uptake experiments, the cells were further incubated in EGF-free medium for 20 min. All cells were fixed for confocal microscopy. Arrowheads indicate cells transfected with ΔC425. The bars represent 20 μm (A, C) and 10 μm (B). |
To explore the mechanisms underlying the large swollen vacuole formation in ΔC425-transfected cells, we examined the distributions of various organelle markers. EEA1 was non-uniformly distributed along the ΔC425-induced large vacuoles, and exhibited significant colocalization with ΔC425. LAMP2 was partially distributed along the ΔC425-positive large vacuoles, but was also localized in small puncta interspersed between the large swollen vacuoles, where it did not colocalize with ΔC425. TfR, which is mainly localized in recycling endosomes in COS-1 cells, was also partially detectable in some ΔC425-positive large vacuoles, and the staining for TfR was less intense in transfected cells than in untransfected cells. In contrast to endosomal/lysosomal markers, the TGN marker TGN46 was not localized in the large vacuoles induced by ΔC425 overexpression, indicating that ΔC425 overexpression selectively interferes with endosomal compartmentalization. We further investigated whether expression of ΔC425 affects the internalization of EGF and transferrin. EGF was internalized and distributed as punctate structures in untransfected cells, whereas internalization of EGF was barely detectable in cells expressing ΔC425 (Fig. 6C). Transferrin uptake was also inhibited by the overexpression of ΔC425 (Fig. 6C), indicating that the early trafficking pathway was significantly compromised.
Next, we examined ΔC1165-transfected cells, in which large puncta instead of swollen vacuoles (ΔC425) were formed (Fig. 6A). Immunofluorescence analyses of various endocytic markers demonstrated that EEA1 colocalized with ΔC1165-positive vacuoles, while LAMP2, TfR and TGN46 did not (Fig. 7). Thus, overexpression of ΔC1165 causes the formation of enlarged early endosomes, on which ΔC1165 and EEA1 colocalize. Interestingly, mono- and poly-ubiquitinated proteins accumulated in ΔC1165-positive vacuoles (Fig. 7), suggesting that the degradation pathway bound for lysosomes, which is often associated with ubiquitinated proteins, may be halted at these enlarged early endosomes. To explore this possibility, we examined the uptake and early-to-late endosome transport of EGF in ΔC1165-transfected cells. In cells transfected with wild-type hRME-8, internalized EGF was partially colocalized with EEA1 and hRME-8 after 20 min of chase, and became well separated from these proteins after 1 h of chase (Fig. 8A). In contrast, in ΔC1165-transfected cells, EGF remained associated with enlarged endosomes showing positive staining for ΔC1165 and EEA1, even after 1.5 h of chase. Since EGF eventually became separated from the ΔC1165-positive vacuoles after 3 h of chase (Fig. 8B), the trafficking from early endosomes to lysosomes was significantly delayed, but not completely blocked, in ΔC1165-transfected cells. We further examined the recycling pathway through early endosomes using transferrin. After 1 h of chase, internalized transferrin was barely detected in untransfected cells due to recycling back to the plasma membrane (arrowheads), while transferrin mainly lingered on endosomes positive for ΔC1165 in transfected cells (Fig. 8C). Taken together, these results demonstrate that trafficking through early endosomes to either late endosomes (degradation pathway) or recycling endosomes (recycling pathway) was compromised by ΔC1165 expression.
 View Details | Fig. 7. Overexpression of the C-terminally-truncated mutant ΔC1165 induces the formation of large early endosomes. COS-1 cells were transiently transfected with ΔC1165, fixed at 24 h after transfection and stained with antibodies against organelle markers and mono/poly-ubiquitin (Ub) for confocal microscopy. The bar represents 20 μm. |
 View Details | Fig. 8. Transport of EGF and transferrin from early endosomes is delayed in cells expressing ΔC1165. COS-1 cells transiently transfected with wild-type hRME-8 (A) or ΔC1165 (B) for 24 h were incubated with Texas Red-EGF at 37°C for 10 min, washed and incubated at 37°C. The cells were treated with a pre-permeabilization solution before fixation (wild-type) or fixed at the indicated times, followed by staining with an anti-EEA1 antibody for confocal microscopy. The bar represents 20 μm. (C) COS-1 cells were transfected with ΔC1165 and incubated with Alexa 546-transferrin (Tf) at 37°C as described for Figure 6C. After washing, the cells were incubated for the indicated times and fixed for confocal microscopy. Arrowheads indicate untransfected cells. The bar represents 20 μm. |
In the present study, we cloned a full-length cDNA encoding hRME-8, determined its subcellular localization by cell fractionation and explored its involvement in endocytic membrane trafficking by transfection of dominant-active Rab mutants and hRME-8 deletion mutants. Our data reveal that hRME-8 is membrane-associated via its N-terminal region and predominantly localized in early endosomes and endosome-like vesicles, and that its subcellular localization is altered when a dominant-active mutant of Rab5a is expressed. Moreover, we noticed that overexpression of hRME-8 mutants lacking the C-terminal region induced the formation of large vacuoles and retarded EGF and transferrin transport from early endosomes, suggesting hRME-8 is involved in early endosomal trafficking pathways.
Biochemical analyses combined with cell fractionation indicated that hRME-8 is a membrane-associated protein, although DmRME-8 is mainly present in the cytosol. Since hRME-8 was solubilized by treatment with Na2CO3, it was assumed to be a peripheral protein and not a typical integral membrane protein. hRME-8 was not solubilized with high salt buffer or in the presence of denaturants, in marked contrast to EEA1, which was solubilized in both conditions. These results agree with previous observations on rat RME-8 (Girard et al., 2005), and indicate that hRME-8 is a peripheral protein tightly associated with the membrane. hRME-8 was also recovered in the Triton X-100-insoluble fraction, but not in the caveolin-rich lipid raft fraction. These data indicate that hRME-8 associates with and functions in Triton X-100-insoluble membrane domains that are distinct from the lipid raft. Therefore, it seems likely that hRME-8 not only binds to the membrane but also forms a complex with hitherto unknown Triton X-100-insoluble proteins or the cytoskeleton.
Rab proteins (e.g., Rab4 and Rab5) and Rab effectors, such as EEA1, are not intermixed but clustered in defined membrane domains on early endosomes and large endosomes induced by the expression of dominant-active Rab5a (McBride et al., 1999; Roberts et al., 1999; Zerial and McBride, 2001). Since hRME-8 was not evenly distributed on the surface of enlarged endosomes induced by dominant-active Rab5a (Fig. 5B), hRME-8 may also be localized in restricted membrane domains on early endosomes. Early endosomes have been shown to contain at least three functionally distinct domains enriched in Rab5, Rab4 and Hrs, respectively (Fig. 9). Rab5 regulates homotypic and heterotypic fusion of early endosomes together with EEA1 (Rubino et al., 2000), an effector of Rab5 that binds to phosphatidylinositol-3-phosphate on early endosomes via its FYVE domain (Simonsen et al., 1998). Rab4 is involved in the sorting and recycling of transferrin in early endosomes (McCaffrey et al., 2001; van der Sluijs et al., 1992). Hrs also interacts with phosphatidylinositol-3-phosphate, similar to EEA1, and functions in early-to-late endosome trafficking of ubiquitinated signaling receptors (Pfeffer, 2003; Raiborg et al., 2001). Since endogenous hRME-8 partially colocalized with Rab5a, Rab4aQ72L, EEA1 and Hrs (Figs. 4, 5), it seems likely that hRME-8 resides in a hitherto undefined subdomain of early endosomes that is distinct from those of rab5a, EEA1, Hrs and Rab4.
 View Details | Fig. 9. Schematic view of the subcellular localizations and functions of hRME-8. hRME-8 is localized in a part of the early endosomes and partially colocalizes with either EEA1 or Hrs. EEA1 plays a role in early endosome fusion, while Hrs is involved in the trafficking of EGF and its receptor from early to late endosomes. Rab4 functions in the recycling of transferrin and its receptor from early endosomes to recycling endosomes or the cell surface. hRME-8 is presumably located in a membrane domain of early endosomes that is distinct from those of EEA1, Hrs and Rab4, and may function in the sorting of these ligands and receptors from the Rab5/EEA1 domain to either the Hrs domain or Rab4 domain. |
Our conclusion that hRME-8 is predominantly localized in early endosomes is apparently contradictory to a report by Girard et al. (Girard et al., 2005), which demonstrated that RME-8 in COS-7 cells colocalized well with the cation-independent mannose 6-phosphate receptor (CI-MPR), indicative of its localization in late endosomes. However, our attempts to demonstrate the colocalization of hRME-8 with late endosomal and lysosomal markers (LBPA, Rab7 and LAMP2) were never successful. Furthermore, no significant association of hRME-8 with multivesicular bodies was observed by immunoelectron microscopy in A549 cells, further arguing against the localization of hRME-8 to late endosomal compartments. Although the reasons for these apparent discrepancies are not clear, CI-MPR may not be an authentic marker for late endosomes in COS-7 cells, since it was reported to reside primarily at the TGN in simian COS-1 cells (Kuronita et al., 2002).
Despite the localization of hRME-8 to early endosomes, hRME-8 knockdown by siRNA did not appear to have any significant effects on the distributions and membrane trafficking of endocytic markers, since neither apparent changes in the distributions of EEA1 and clathrin heavy chain nor significant differences in the kinetics of EGF and transferrin were detected in hRME-8-depleted A549 cells (Supplementary Figs. 2 and 3). These findings disagree with the previous observations by Girard et al. (Girard et al., 2005) that EGF internalization was inhibited by ~50% in RME-8-depleted COS-7 cells with a concomitant reduction in EGFR expression and altered localization of clathrin heavy chain. Although the reasons for these discrepancies remain unclear, depletion of hRME-8 may be compensated by other functionally redundant proteins in the A549 cells used in the present study. The expression levels of such proteins may be low in COS-7 cells. Alternatively, since the expression level of EGFR in COS-7 cells was higher than in A549 cells (Supplementary Fig. 2A), this difference may account for, at least in part, the different phenotypes between COS-7 and A549 cells upon RME-8 knockdown. Nonetheless, the phenotypes of RME-8-depleted COS-7 cells were not robust, compared with those of invertebrate RME-8 mutants that exhibit an almost complete block of endocytosis and are lethal (Chang et al., 2004; Zhang et al., 2001). Similarly, a loss-of-function mutation of Arabidopsis RME-8 (designated GRV2: gravitropism defective 2) did not affect cell viability, and the endocytic fate of FM1-43 in the mutant cells was not compromised (Silady et al., 2004). Likewise, depletion of RME-8 may be compensated in A549 and COS-7 cells by an alternate pathway that only operates in higher animals and plants.
Although hRME-8 knockdown had no apparent effects, the localization of hRME-8 still suggests its intimate involvement in the trafficking through early endocytic compartments. We sought to obtain clues addressing this issue by resorting to overexpression of truncated mutant hRME-8 proteins, in the hope that this approach may titrate out downstream factors through which other putative redundant proteins could function. Overexpression of C-terminally-truncated mutants (ΔC1165 and ΔC425) resulted in drastic alterations in the morphology of early endocytic compartments, such as the formation of puncta and vacuoles, and in the kinetics of endocytic ligands, such as EGF and transferrin, whereas overexpression of an N-terminally-truncated mutant (ΔN453) did not (data not shown). The phenotypes induced by overexpression of these C-terminally-truncated mutants indicate compromised trafficking pathways mainly through early endocytic compartments. The observation that ubiquitinated proteins accumulated in large endosomes in ΔC1165-overexpressing cells could be interpreted as compromised degradation trafficking from early endosomes to late endosomes. The more severe phenotype seen in ΔC425-overexpressing cells could be explained by inhibition of endocytic recycling of receptors from early endosomes to the cell surface, thereby leading to compromised receptor-mediated endocytosis from the plasma membrane. Given the existence of the DnaJ-domain in ΔC425, but not in ΔC1165, the region encompassing amino acid residues 1079 through 1818, which contains the DnaJ-domain, may interact with factors such as Hsp70 family proteins to facilitate targeting of the mutant protein to a proper subdomain on early endosomes, thereby more efficiently blocking the recycling of receptors from early endosomes through competitive inhibition of endogenous hRME-8.
Taken together, we propose a model for how hRME-8 functions in early endocytic pathways (Fig. 9). hRME-8 localizes to a subdomain of early endosomes that is distinct from those of EEA1, Hrs and Rab4, and functions in the sorting of EGF and transferrin from the Rab5/EEA1 domain to either the Hrs domain (for EGF) or the Rab4 domain (for transferrin). Further studies on the identification of putative proteins that bind to different regions of hRME-8 would yield insights into how hRME-8 functions in membrane trafficking through early endosomes.
We are grateful to Dr. Harald Stenmark for providing the myc-tagged Hrs cDNA and to Drs. Eiki Kominami, Yukio Nishimura and Toshihide Kobayashi for providing antibodies. We thank Yumi Yoshimura for performing the protein sequence analyses. We are also grateful to Dr. Atsuki Nara (Nagahama Institute of Bio-Science and Technology, Shiga, Japan), Hironobu Fujiwara and Tasuku Tsukamoto for their helpful comments and technical assistance. This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan. T.T. was supported by the Core Research for Evolutional Science and Technology (CREST), Grants-in-aid for Scientific Research (18050019), and Senri Life Science Foundation Grants.
|