| To whom correspondence should be addressed: Takao Hijikata, Department of Anatomy and Cell Biology, Faculty of Pharmacy, Research Institute of Pharmaceutical Sciences, Musashino University, 1-1-20 Shinmachi Nishitokyo-shi, Tokyo 202-8585, Japan. Tel & Fax: +81–42–468–9290 E-mail: hijikata@musashino-u.ac.jp Abbreviations: CTX, cardiotoxin; CXMDJ, canine X-linked muscular dystrophy in Japan; DMD, Duchenne muscular dystrophy; mdx, X chromosome-linked muscular dystrophy; RT-PCR, reverse transcription polymerase chain reaction; TA, tibialis anterior. |
MicroRNAs (miRNAs) are small, ~22-nucleotide, non-coding RNA molecules that post-translationally regulate gene expression. Many miRNAs are expressed in a tissue-specific manner, and seem to contribute to tissue specification during differentiation (Mansfield et al., 2004; Hornstein et al., 2005; Lagos-Quintana et al., 2002; Chang et al., 2004; Fazi et al., 2005). In addition, they have been implicated in the pathogenesis of cancers and infectious diseases (for review, see Boyd, 2008; Iorio et al., 2005; Jopling et al., 2005).
The three muscle-specific miRNAs, miR-1, miR-133, and miR-206 have been shown to play important roles in the regulation of muscle development. miR-1 and miR-133 are expressed in cardiac and skeletal muscle and are transcriptionally regulated by the myogenic differentiation factors, such as MyoD, myogenin, Mef2, and serum response factor (SRF) (Rao et al., 2006; Chen et al., 2006; Kwon et al., 2005; Sokol and Ambros, 2005; Zhao et al., 2005). miR-1 promotes differentiation of cardiac and skeletal progenitors and their exit from the cell cycle in mammals (Zhao et al., 2005, 2007; Kwon et al., 2005), while miR-133 inhibits their differentiation and maintains them in a proliferative state (Chen et al., 2006). miR-206 is expressed only in skeletal muscles, and its expression appears to be induced by MyoD and myogenin during myogenesis and promotes muscle differentiation (Kim et al., 2006; Rosenberg et al., 2006). These muscle-specific miRNAs also seem to participate in muscle diseases, including cardiac hypertrophy, heart failure, cardiac arrhythmias, congenital heart disease, and muscular dystrophy (Carè et al., 2007; van Rooij et al., 2006, 2007; Yang et al., 2007; McCarthy et al., 2007; Eisenberg et al., 2007).
To gain insight into the pathophysiological roles of muscle-specific miRNAs in dystrophin-deficient muscular dystrophy, we analyzed the profiles of miR-1, miR-133a, and miR-206 expression in TA muscles of mdx mice and CXMDJ dogs by semiquantitaive RT-PCR and in situ hybridization. In addition, their temporal and spatial expression patterns were examined in C2C12 cells during myogenesis and in tibialis anterior (TA) muscles during a degeneration-regeneration process induced by cardiotoxin (CTX)-injury. Here, we demonstrated that miR-206 was highly expressed within muscle fibers newly formed from satellite cells during regeneration in CTX-injured and dystrophic mdx muscles. However, miR-206 was not abundantly expressed in CXMDJ TA muscles, which exhibited much more severe and progressive degeneration than those of mdx mice. The degree of miR-206 expression might depict the potential of muscle regeneration and maturation.
The use of animals was approved by the Animal Ethics Committee of Musashino University or the Ethics Committee for Treatment of Laboratory Animals at NCNP. Three-week-old to one-year-old male control (strain C57BL/10; B10) and mdx mice were anesthetized with diethyl ether and sacrificed by cervical dislocation to dissect out the TA muscles. For regeneration assay, injury was performed on TA muscles of 12-week-old C57BL/6 (B6) mice by injecting 100 μl of 10 μM cardiotoxin (CTX). Mice were sacrificed at 1, 2, 3, 4, 5, and 7 days, 2, 4, and 8 weeks post-injection to collect the TA muscles. Wild-type and dystrophic CXMDJ dogs were sacrificed by exsanguination under anesthesia with isoflurane to dissect out the TA muscles.
C2C12 myoblasts were cultured on the collagen-coated dishes in growth medium consisting of DMEM and 20% fetal calf serum. Differentiation into myotubes was induced by switching the culture medium to differentiation medium consisting of 5% horse serum and 10 μg/ml insulin in DMEM, followed by culture for 1, 3, or 6 days.
Muscle-specific miR-1, miR-133a, and miR-206 were quantified by real-time reverse transcription polymerase chain reaction (RT-PCR) using total RNA obtained from C2C12 cells and TA muscles as well as a TaqMan MicroRNA Assay kit (Applied Biosystems). Each miRNA expression was represented relative to the expression of small RNA U6 used as an internal control in RT-PCR. Expression data were given as means of relative expression values obtained from three samples in conjunction with standard deviation. Statistical comparisons were performed by t-test or Aspin-Welch-test.
In situ hybridization was performed using a locked nucleic acid (LNA) detection probe for mmu-mir-206 (Exiqon), which was labeled with digoxigenin (DIG) using a DIG oligonucleotide tailing kit (Roche). C2C12 cells fixed with 4% paraformaldehyde (PFA) in PBS were immersed in 100% methanol, rehydrated, treated with proteinase K, and then re-fixed with PFA, whereas cryosections prepared from TA muscles fixed with 4% PFA were treated with proteinase K, re-fixed with PFA, and then acetylated with acetylation buffer (0.1 M triethanolamine pH 8.0). After washing with PBS, both types of specimen were incubated with DIG-labeled miR-206 probes at 55°C overnight. After stringent wash at 55°C in 50% formamide, 2×SSC, 0.1% Tween-20, and at room temperature in 0.2×SSC and then PBS, they were incubated with blocking solution, followed by alkaline phosphatase-conjugated anti-DIG antibody (Roche). Hybridized probes were detected and visualized by color reaction with nitroblue tetrazolium (NBT) and 5-bromo 4-chloro-3-indolyl phosphate (BCIP).
Using RT-PCR Taqman assay, we analyzed the expression profiles of miR-1, miR-133a, and miR-206 in TA muscles from control B10 and dystrophic mdx mice at different ages. As shown in Fig. 1A, miR-206 was constantly expressed in mdx TA muscles throughout the ages examined from 3 weeks to 1 year, whereas its expression was reduced with age in control B10 muscles. Thus, a comparison between age-matched B10 and mdx mice indicated significantly increased expression of miR-206 in mdx TA muscles. On the other hand, there were no differences in the expression of miR-1 or miR-133a between B10 and mdx mice at any age examined except at 5 and 8 weeks old, when mdx TA muscles showed slightly reduced expression of miR-133a as compared to B10 TA muscles.
 View Details | Fig. 1. A, Expression levels of miR-1, miR-133a, and miR-206 in TA muscles of mdx and B10 mice at different ages. All expression levels were determined by real-time RT-PCR. Results are presented as mean relative expression±SD; n=3. *, P<0.05; **, P<0.01. B, Serial cross-sections from TA muscles of 8-week-old mdx mice. In situ hybridization using miR-206 probes on one section (right) and hematoxylin and eosin (H&E) staining on an adjacent section (left). In situ hybridization analyses showed intense signals for miR-206 probes in newly formed muscle fibers with centralized nuclei, or regenerating fibers. Bar, 50 μm. |
Next, to identify which types of cells abundantly express miR-206 within mdx TA muscle, in situ hybridization analysis was performed by using DIG-labeled miR-206 probes. Intense signals for miR-206 probes were observed in newly formed myotubes and immature muscle fibers with centralized nuclei, or regenerating muscle fibers (Fig. 1B). However, intact (pre-degenerated) muscle fibers and many small mononucleated cells, such as inflammatory infiltrates, fibroblasts, and proliferating satellite cells, appeared to lack miR-206 signals. These results clearly indicated that increased expression of miR-206 in mdx TA muscles was due to newly formed muscle fibers. For control experiments, the tissue sections were hybridized with LacZ probes or treated without miR-206 probes, thereby presenting no specific and positive signals.
To compare to the results obtained in mdx TA muscles, the expression levels of the three miRNAs were analyzed in TA muscles from wild-type or CXMDJ dogs. CXMDJ dogs exhibit a much more severe and more progressive dystrophic form than mdx mice and almost recapitulate dystrophinopathy phenotype DMD in human (Shimatsu et al., 2005). As shown in Fig. 2, CXMDJ TA muscles expressed smaller amounts of miR-1, miR-133a, and miR-206 than wild-type controls. These reduced levels of expression in CXMDJ TA muscles, as assessed by semiquantitaive RT-PCR from total RNA, may represent either a decrease in the number of cells expressing the three muscle-specific miRNAs or in the amount of miRNA per cell. The former is likely, since CXMDJ TA muscles contain many degenerated muscle fibers, inflammatory infiltrates, and fibroblasts (Fig. 3A), none of which express muscle-specific miRNAs. To assess the latter, i.e., whether CXMDJ muscle fibers, including those in the process of regenerating, express smaller amounts of miR-206 than muscle fibers of wild-type dogs or mdx mice, we calculated the relative levels of expression of miR-206 and miR-133 to miR-1, which was induced only in differentiated myotubes (Rao et al., 2006). As shown in Fig. 3B, the relative expression of miR-206 to miR-1 was elevated in CXMDJ TA muscles as compared to controls. However, CXMDJ muscles did not exhibit such a marked increase in relative expression of miR-206 as observed in mdx muscles (Fig. 3C). On the other hand, the expression of miR-133a relative to that of miR-1 showed a similar ratio of around 1 as compared between mice and dogs.
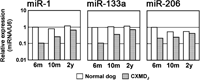 View Details | Fig. 2. Expression levels of miR-1, miR-133a, and miR-206 in TA muscles of CXMDJ and wild-type dogs at different ages (n=1 at each age). |
 View Details | Fig. 3. A, Representative sections of B10, mdx, normal dog, and CXMDJ TA muscles stained with hematoxylin and eosin. Note degenerating muscle fibers and inflammatory infiltrates in CXMDJ muscle, in contrast to well-regenerated muscle fibers with central nuclei in mdx muscle. Bar, 50 μm. B, Expression levels of miR-206 and miR-133a are represented relative to that of miR-1, based on data shown in Fig. 1A and 2. Relative expression levels of miR-206 were elevated in mdx and CXMDJ TA muscles as compared to respective control muscle. *, P<0.05; **, P<0.01. C, The relative expression of miR-206 or miR-133a to miR-1 in mdx and CXMDJ TA muscles was compared to and represented relative to that in control TA muscles. Highly increased expression of miR-206 was observed in mdx muscles, as compared to CXMDJ muscles . |
As shown above, dystrophic TA muscles contain many degenerating and regenerating muscle fibers as well as inflammatory infiltrates. These observations prompted us to assess how muscle degeneration and regeneration affect the expression of miR-1, miR-133a, and miR-206. The temporal and spatial expression patterns of these miRNAs were analyzed in C2C12 cells during muscle differentiation as an in vitro model of muscle regeneration, and in cardiotoxin (CTX)-injured TA muscles as an in vivo model of the degeneration-regeneration process. The degeneration-regeneration process induced by CTX-injury was well documented as follows: After CTX injection, satellite cell proliferation occurs within 2 days, myogenic differentiation is initiated within 3 days, new myotube formation is evident within 5 days, and muscle architecture is largely restored within 10 days (Hawke and Garry, 2001; see also Fig. 5B).
Consistent with previous studies (Kim et al., 2006; Rao et al., 2006), not only the expression of miR-206 but also those of miR-1 and miR-133a were markedly up-regulated during C2C12 differentiation (Fig. 4A). In situ hybridization analysis revealed that miR-206 was abundantly expressed in differentiated myotubes, in which intense signals for miR-206 were found in the sarcoplasm, especially in the perinuclear regions (Fig. 4B). Numerous mononucleated myoblasts, still found even in the differentiation medium, almost lacked miR-206 signals. For control experiments, hybridization with the omission of miR-206 probes or with the use of LacZ probes did not detect any specific and positive signals in C2C12 myoblasts or myotubes.
 View Details | Fig. 4. A, Expression levels of miR-1, miR-133a, and miR-206 during C2C12 differentiation. C2C12 cells cultured in the differentiation medium for 0, 1, 3, or 6 days were used to determine expression levels of the three miRNAs by real-time RT-PCR. Results are presented as mean relative expression±SD; n=3. *, P<0.05; **, P<0.01. B, In situ hybridization analyses showed intense miR-206 signals in a newly formed myotube with two nuclei in day 1 cultures (1d) and large myotubes in day 3 cultures (3d). Pictures on the right side show enlarged views of the same myotubes indicated by arrows in the pictures on the left. Bar, 50 μm. |
The CTX injection into TA muscles resulted in a decrease of ~100-fold in expression level of miR-206 on the first day post-injury and >100-fold decrease in those of miR-1 and miR-133a 3 days after CTX injection (Fig. 5A). The expression of miR-206 was induced on day 2 post-injury and increased markedly by 10-fold on day 5 post-injury. Its elevated expression level lasted until at least 4 week post-injury and was still significantly higher than the pre-operative level even 8 weeks after CTX injection. On the other hand, both miR-1 and miR-133a were similarly induced later on day 4 post-injury and their levels of expression increased gradually, returning close to the pre-operative levels by 4 weeks after CTX injection.
 View Details | Fig. 5. A, Temporal expression profiles of miR-1, miR-133a, and miR-206 during the degeneration-regeneration process induced by CTX-injury. Using real-time RT-PCR, expression levels were determined in CTX-injected TA muscles at 0 day to 8 weeks post-injury. Results are presented as mean relative expression±SD; n=3. B, In situ hybridization analyses show intense miR-206 signals in regenerating fibers with central nuclei, but no signals in intact muscle fibers and many small cells, possibly inflammatory infiltrates and proliferating satellite cells. Bar, 50 μm. |
In situ hybridization analysis using miR-206 probes showed that newly formed myotubes or immature muscle fibers with centralized nuclei were intensely labeled with miR-206 probes in CTX-injured TA muscles (Fig. 5B). These results, together with the quantitative results described above, clearly indicated that miR-206 was highly expressed in regenerating muscle fibers. This is consistent with the present findings in dystrophic TA muscles of mdx mice.
This study provided a detailed temporal analysis of muscle-specific miR-1, miR-133a, and miR-206 expression during the complete degeneration-regeneration process of mouse TA muscles injured by CTX. This quantitative analysis as well as the results of in situ hybridization analyses clearly indicated that miR-206 was highly expressed in myotubes newly formed from satellite cells. Similar increases in the expression of miR-206 in regenerating muscle fibers were found in mdx TA muscles, which have considerable regenerative capacity (Tanabe et al., 1986; Coulton, et al., 1988; Grounds and McGeachie et al., 1992; Itagaki et al., 1995). In contrast, CXMDJ TA muscles, which exhibit much more severe and more progressive degenerative alterations than those of mdx mice, expressed smaller amounts of miR-206 than controls.
miR-1, miR-133a, and miR-206 are transcribed during myogenesis and seem to be regulated by MyoD and myogenin (Rao et al., 2006; Rosenberg et al., 2006). miR-206 is transcribed independently of miR-1 and miR-133a, which are transcribed as a common pri-miRNA precursor from the miR-1/miR-133a locus, followed by alternative splicing to generate different primary transcripts (Rao et al., 2006; Liu et al., 2007). This is consistent with the present finding that miR-1 and miR-133a expression showed similar patterns in their induction and increase during CTX-induced regeneration. On the other hand, the onset of miR-206 expression temporally preceded those of miR-1 and miR-133a in CTX-induced regeneration. These observations suggested that miR-206 might be induced by MyoD, while miR-1 and miR-133a might be induced by myogenin, since the onset and temporal sequence of miR-206 and miR-1/miR-133a expression seem to coincide with those of MyoD and myogenin expression during myogenesis and CTX-induced regeneration (Megeney et al., 1996; Yun and Wold, 1996; Launay et al., 2001). After their induction by MyoD and myogenin, however, other factors must be involved in the expression of these miRNAs at later stages.
The early induction of miR-206 and its increased expression even after apparent recovery in CTX-induced regeneration emphasizes the suggestion that miR-206 may possess a variety of in vivo functions, such as myogenesis, synapse formation/elimination during reinnervation, maturation of muscle fibers, and maintenance of muscle integrity or contractility. Some functional roles of miR-206 during myogenesis, including myoblast fusion, have been proposed based on identification of its targets. miR-206 down-regulates DNA polymerase α, resulting in inhibition of DNA synthesis and withdrawal of myoblast proliferation, and thereby promoting muscle differentiation (Kim et al., 2006). During myoblast fusion, connexin43 (Cx43), a component of gap junction channels, was down-regulated by miR-206 (Anderson et al., 2006). During maturation of muscle fibers, utrophin is down-regulated and replaced at the sarcolemma by dystrophin. This suppression of utrophin has been shown to occur through miR-206 targeting to its 3’UTR (Rosenberg et al., 2006). In addition, a search using TargetScan (www.targetscan.org/) predicted 480 potential targets for miR-206, including brain-derived neurotrophic factor (BDNF), nerve growth factor receptor (NGFR), insulin-like growth factor 1 (IGF-1), and insulin-like growth factor binding protein 5 (IGFBP5). These proteins may be involved in synapse formation/elimination during reinnervation and muscle mass regulation during muscle maturation, although it has yet to be verified whether their levels of expression are eventually down-regulated by miR-206.
Increased expression of miR-206 in mdx TA muscle may reflect active and efficient regeneration, whereas its decreased expression in CXMDJ TA muscles may depict inactive and inefficient regeneration. In support of these suggestions, miR-206 introduction promotes C2C12 differentiation despite the presence of serum, whereas its inhibition by antisense oligonucleotide retards cell cycle withdrawal and differentiation (Kim et al., 2006). As the expression of miR-206 is induced by MyoD and myogenin (Rao et al., 2006; Rosenberg et al., 2006), its decreased expression in CXMDJ dystrophic muscles may suggest poor expression or instability of the myogenic factors. Their paucity and instability would affect satellite cell activation, myoblast differentiation into muscle fibers, and maturation of muscle fibers. MyoD mutant muscle was reported to be severely deficient in regenerative ability (Megeney et al., 1996), whereas myogenin-deficient mice showed failure of myotube formation from myoblasts (Hasty et al., 1993).
An additional point of interest is that the expression of miR-206 in control TA muscles is reduced with age, although newly formed muscle fibers show abundant expression. This fact implies that miR-206 functions in maintenance of muscle integrity and contractility may decline with age. To understand the multiple functions of miR-206 in not only myogenesis but also muscle maintenance, further studies, especially the identification of bona fide, biologically relevant targets for miR-206, will be required.
This work was supported in part by a Research Grant (17A-10, 20B-13) for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare, and by the High-Tech Research Center Project for Private Universities (MEXT. HAITEKU, 2004-2008).
|