| To whom correspondence should be addressed: Yoshihiro H. Inoue, Drosophila Genetic Resource Center, Kyoto Institute of Technology, Saga Ippongi-cho, Ukyo-ku, Kyoto 616-8354, Japan. Tel: +81–75–873–2653, Fax: +81–75–861–0881 E-mail: yhinoue@kit.ac.jp Abbreviations: GFP, green fluorescent protein; GSCs, germline stem cells; ILPs, insulin-like peptides. |
The study of spermatogenesis in Drosophila can aid in understanding the regulatory mechanisms underlying cell proliferation and growth during development. In young adult Drosophila males, 5 to 8 germline stem cells (GSCs) are usually observed at the tip of the testis. To maintain their multi-potential stem cell characteristics, GSCs receive signals from the adjoining hub cells. Both a ligand encoded by the unpaired gene and the JAK-STAT signaling cascade are involved in this signal transfer (Kiger et al., 2001; Tulina and Matunis, 2001). The proximal cell of the 2 daughter cells derived from asymmetric division of a stem cell exclusively receives the unpaired signal and becomes a self-renewed GSC. The distal daughter cell leaves the niche and differentiates as a spermatogonium, which then undergoes cell division 4 times to produce a 16-spermatocyte cyst. The 16 spermatocytes synchronously enter a growth phase during which they increase remarkably in volume by up to 25-fold. Upon completion of this stage of cell growth, the cell division essentially becomes meiotic.
The extracellular signal and the signaling cascade that maintain GSC numbers have been partially identified (Fuller and Spradling, 2007); however, the mechanism that induces stem cell division has yet to be reported. Identification of the growth factor and its signaling cascade that stimulate male stem cell division would help in understanding the regulatory mechanisms that maintain GSC numbers and produce germline cells from stem cells.
Insulin is known to play an essential role in glucose uptake in Drosophila cells (Ceddia et al., 2003), and 7 genes encoding insulin-like peptides (ILPs) have been identified in the Drosophila genome. These peptides are synthesized in clusters of medial neurosecretory cells in the Drosophila brain (Rulifson et al., 2002). An ILP receptor (insulin receptor, InR) and its downstream signaling cascade are well conserved in Drosophila (Fernandez et al., 1995; Bohni et al., 1999). It has been reported that InR and its signaling cascade can stimulate both cell proliferation and growth in cultured Drosophila cells as well as in larval imaginal cells (Chen et al., 1996; Brogiolo et al., 2001). In addition, hypomorphic InR mutant females exhibit loss of fertility, and the number of cysts produced from female GSCs was decreased in InR mutants. Furthermore, cell growth within cysts was also inhibited in the ovaries of mutants (LaFever and Drummond-Barbosa, 2005); however, cyst morphology and cell numbers within these cysts were not affected. These results suggest that insulin signaling is required for female GSC division and growth within the egg chambers. Another study on ovarian GSCs in Drosophila demonstrated that insulin signaling stimulated stem cell cycle progression, particularly at the G2 phase (Hsu et al., 2008). Furthermore, oogenesis in Drosophila is dependent on environmental nutrient conditions (Fuller and Spradling, 2007). Therefore, it is reasonable to assume that both cell number and growth in egg chambers are directly regulated by hormonal control via ILPs. In Drosophila spermatogenesis, it is still unclear if nutrient conditions affect sperm numbers. Moreover, the ligands and receptors that maintain GSC numbers are different in females and males (Fuller and Spradling, 2007). Hence, we sought to examine whether insulin signaling was involved in GSC division in males.
In order to synthesize the proteins required for oocyte development, ovarian cyst nurse cells activate protein expression and transport the proteins to a single oocyte. In spermatogenesis, on the other hand, all 16-cyst cells undergo marked cell growth before meiotic division. Hence, we also examined whether insulin signaling extrinsically induces spermatocyte growth or whether such growth is autonomously programmed within the cell.
In this study, we show that insulin signaling plays an important role in spermatogenesis by stimulating GSC proliferation and spermatocyte growth. We also present evidence suggesting that insulin signaling is required for cell cycle progression at the G2/M phase in male GSCs. These results indicate that insulin signaling plays an essential role in both oogenesis and spermatogenesis in Drosophila.
The following Drosophila stocks were used: (1) trans-heterozygotes for lethal alleles of InR, InRE19, InR339, and InR05545 (Fernandez et al., 1995; Brogiolo et al., 2001), which induce loss of fertility in both females and males without significant reduction in viability, and (2) hemizygotes for a chico mutation, a Drosophila gene encoding an ortholog of mammalian IRS-1 that results in reduced fertility in females and males (Bohni et al., 1999). To induce expression of the pericentrin/AKAP450 C-terminus protein (PACT) with a green fluorescent protein (GFP) tag in InRE19/InR339 mutants, we produced a recombined chromosome carrying P{UAS-GFP-PACT} (Martinez-Campos et al., 2004). Subsequently, InRE19/InR339 males and control heterozygous males carrying both P{hsp-Gal4} and P{UAS-GFP-PACT} were heat shocked at 37°C for 1 h within 24 h of eclosion. Their germline cells were dissected from the testes and fixed (Inoue et al., 2004) at 12, 18, and 24 h after heat shock induction. To construct males lacking brain ILP-producing cells, P{ilp2-Gal4} and P{UAS-reaper} were used as previously described (LaFever and Drummond-Barbosa, 2005).
Male germline cysts were dissected from young adults within 24 h of eclosion and fixed using the method of Inoue et al. (2004), unless otherwise indicated. GSCs in adult testes were identified as germline cells attached to the hub cells that had been visualized by anti-Fasciclin III immunostaining (Kiger et al., 2001). Alternatively, based on mitochondria abundance (Ichihara et al., 2007), hub cells were visualized by staining with Mitofluor 594 (Molecular Probes, Eugene, OR, USA). We regarded larger Vasa-positive cells that bound on the hub cells as male GSCs. The developmental stages of primary spermatocytes were determined as described in Cenci et al. (1994). Mitofluor 594 staining was used to identify stage S2b spermatocytes based on the apolar distribution of the nucleus and the presence of mitochondrial aggregates (Cenci et al., 1994; Ichihara et al., 2007).
Primary antibodies against Fasciclin III (Developmental Studies Hybridoma Bank (DSHB), Iowa, USA), cyclin E (Richardson et al., 1995), cyclin B (Whitfield et al., 1990) phosphorylated histone H3 (Upstate Biotechnology, Lake Placid, NY, USA), GFP (MBL, Japan), β-galactosidase (DSHB), and Vasa (rat antibody from A. Nakamura) were used for immunostaining experiments. A primary antibody against phosphorylated Drosophila Akt at Ser505 (Antibody #4054, Cell Signaling Technology, Beverly, MA, USA) was utilized for immunodetection of D-Akt activation. This antibody detects specific phosphorylation in cultured cells after treatment with insulin, as described in a protocol provided by the company (Dionne et al., 2006). The localization of primary antibodies was detected by Alexa 488- or 594-conjugated anti-IgG antibody (Molecular Probes). All preparations were observed with an ECLIPSE E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a cooled charge-coupled device camera (C4742-95, Hamamatsu Photonics Inc., Hamamatsu, Japan). The collected digital images were processed and merged in pseudocolor using Adobe Photoshop version 7 (Adobe Systems Inc., San Jose, CA, USA).
To examine the effects of insulin signaling mutations, we measured the size of stage 6 spermatocytes (Cenci et al., 1994) from intact cysts among testis cell preparations. The greatest diameter of 5 cells from each of these intact cysts was recorded, and the average calculated.
Some combinations of lethal InR mutations produced viable but sterile male and female adults. For example, the male trans-heterozygotes, InRE19/InR339 and InRE19/InR05545, exhibited almost complete loss of fertility without a significant reduction in viability due to intragenic complementation. Testis dissection revealed reduced numbers of germline cells or sperm in sterile InR mutants. To understand the primary defect that produced this male sterility, we examined whether GSC abundance was reduced in sterile mutants. In young adult males, an average of 6.5 GSCs were present in wild-type testes within 24 h of eclosion (Fig. 1A) and comparable GSC numbers were observed in InRE19/InR339 and InRE19/InR05545 mutant males (Fig. 1B, Table I). As the males aged, the numbers of GSCs gradually decreased in wild-type and mutants, with the largest decrease in InRE19/InR339 mutant males (Table I). Fourteen days after eclosion, InRE19/InR339 males had an average of 4.2 GSCs, i.e., 70% of the average number in newly eclosed males (Fig. 1D), while wild-type males maintained 90% of the GSC numbers (Fig. 1C). Thus, although the male GSC numbers in mutants did not significantly change at eclosion, the mutants themselves exhibited accelerated reductions in GSC numbers with age, thereby suggesting that stem cell renewal was affected.
 View Details | Fig. 1. Male GSCs and germline cysts derived from GSCs at the tip of the testis from sterile InR mutant males in Drosophila. (A–D) GSCs (arrowheads) can be identified as the larger cells stained with anti-Vasa antibody (green) and attached to the hub cells that were differentially immunostained with anti-Fasciclin III antibody (red). GSCs were maintained in the proliferation center of the testis in wild-type (A) or InRE19/InR339 (B) males within 24 h of eclosion, and also in wild-type males 14 days after eclosion (C). (D) A proliferation center containing decreased stem cells from InRE19/InR339 males 14 days after eclosion. (E, F) A view of germline cells at the tip of the testis stained with MitoFluor 594 (red) from a wild-type (E) or InRE19/InR339 (F) male within 24 h of eclosion. Spermatocyte cysts at stage S2b showing characteristic mitochondria aggregates are encircled by dotted lines. No S2b cysts were detected in this mutant testis containing less numbers of germline cells. The hub cells are indicated by an asterisk. Scale bar: 10 μm. |

We expected GSC division activities, such as stem cell renewal and gonialblast production, to be reflected in the numbers of cysts. Hence, after eclosion, we compared the numbers of stage S2b spermatocyte cysts in mutant and wild-type males. Stage S2b spermatocyte cysts were easily recognized because of their characteristic nuclear arrangement and mitochondrial aggregates (Fig. 1E) (Cenci et al., 1994; Ichihara et al., 2007). We found marked reduction in spermatocyte cyst numbers in InR or chico mutant testes even in newly eclosed males (Fig. 1F) and marked differences between wild-type and mutant males 7 days after eclosion (Table II). By 14 days after eclosion, stage S2b spermatocyte cysts were no longer detected in InRE19/InR339 or chico hemizygotes. These observations suggested that insulin signaling is involved in inducing GSC division to produce cysts.
We investigated whether inhibition of ILP production exerts the same effects on stem cell division. In order to remove insulin-producing cells from Drosophila brains, we induced ectopic expression of a cell death gene, reaper, in brain cells under the control of the ilp2 gene enhancer using the Gal4/UAS system (Rulifson et al., 2002). In flies executed by the ectopic expression of reaper (Chen et al., 1996), the phenotypes observed were identical to those in InR mutants, including a smaller body size. Young mutant males also exhibited a reduction in germline cyst numbers, as seen in InR and chico mutants (Table III). These observations confirmed that insulin signaling is involved in inducing GSC division in male Drosophila.
Further evidence for the activation of the insulin signaling cascade in male germline cells was obtained by immunostaining with an antibody (Antibody #4054, Cell Signaling Technology) that exclusively recognizes a phosphorylated form of D-Akt activated downstream of InR (Fig. 2A–D). Distinct antibody immunofluorescence was observed in wild-type GSCs, proliferating spermatogonia (Fig. 2A), and growing spermatocytes (Fig. 2C), while only a background level of immunofluorescence was observed in testis cells from InR mutants (Fig. 2B, D).
 View Details | Fig. 2. Accumulation of the activated form of Akt working as a signaling factor downstream of InR in testis cells. Immunofluorescence of a proliferation center containing GSCs and spermatogonia (A, B) or a growing spermatocyte cyst at stage 4 (C, D) with anti-phospho Akt antibody (green) from wild-type (A, C) or InR mutant (B, D) males. The hub cells indicated by an asterisk are red after immunostaining with anti-Fasciclin III antibody. Immunofluorescence signals for anti-phospho Akt staining are shown as black and white images in panels A' and B'. Note that only a background level of immunofluorescence was observed in proliferation center cells (B) and spermatocytes (D) from the InRE19/InR339 mutants. Scale bar: 10 μm. |
The above evidence suggested that insulin signaling was involved in inducing male GSC division. To estimate the activity of cell cycle progression of InR mutant stem cells, we examined the number of times stem cells proceeded through the S phase within a certain period. A centriolar protein, pericentrin, integrated into centrioles at the S phase along with the transient expression of C-terminal pericentrin with a GFP tag (GFP-PACT) by heat shock results in the production of cells containing GFP-labeled centrioles (Martinez-Campos et al., 2004) (Fig. 3A). After GFP-PACT induction, GFP fluorescence can be detected in the daughter centrosomes if the cells pass through the next cell cycle to the S phase (Fig. 3B). Therefore, from the number of labeled centrosomes, one can estimate the number of cells that pass through the S phase within a certain period (Yamashita et al., 2007). In control males, 25% of the stem cells (n=83) contained 2 GFP-labeled centrosomes 12 h after GFP-PACT induction (Fig. 3C), indicating that one fourth of the stem cells had passed through a single S phase in those 12 h. Twenty-four hours after GFP-PACT induction in control males, the proportion of stem cells with 2 labeled centrosomes decreased to 7% of the total GSCs (n=88). Further, in control males, stem cells containing single GFP-labeled centrosomes, which correspond to cells that passed through the S phase twice, were observed in 8% of the total GSCs 12 h after GFP-PACT induction; this proportion increased to more than 30% at 24 h. On the other hand, in InR mutants, the number of stem cells that passed through the S phase once was 5% of the total GSCs (n=56) 12 h after induction. Subsequently, the proportion decreased and no stem cells were observed at 18 h after induction. The GSCs that passed through the S phase twice were less than 6% of the total GSCs in InR mutants. Thus, cell cycle progression in GSCs was significantly compromised in InR mutant males, confirming that insulin signaling is required for cell cycle progression in male Drosophila GSCs.
 View Details | Fig. 3. Labeled centrosomes in GSCs in which expression of GFP-PACT (a centriolar protein with a GFP tag) was transiently induced by heat shock. GFP-PACT (green) was incorporated into centrosomes (arrows) at the S phase. (A) Stem cells in which both centrosomes were labeled with GFP correspond to cells that had passed through the S phase once in the presence of GFP-PACT. (B) The cells containing single labeled centrosomes underwent a second division cycle after the completion of GFP-PACT supply. The hub cells (asterisk) were immunostained with anti-Fasciclin III antibody and are shown in red (A) or yellow (B). For detection of labeled centrosomes 18 h after induction, we carried out sequential immunostaining with anti-GFP antibody following immunodetection of the hub cells. Scale bar: 10 μm. (C) Alteration in the number of GSCs containing labeled centrosomes after heat shock induction of GFP-PACT from control (closed square, closed diamond) or InRE19 (closed triangle, ×) males. GSCs in which both centrosomes (or only a distal centrosome) were labeled with GFP correspond to those undergoing their first (or second) cell cycle after heat shock induction. Scale bar: 10 μm. |
We next investigated the cell cycle phase in which insulin signaling promotes cell cycle progression in GSCs. For this purpose, we examined the proportion of GSCs exhibiting accumulations of the cell cycle markers cyclin E, cyclin B, or phosphorylated histone H3 in males within 24 h of eclosion (Fig. 4). GSCs that accumulate cyclin B and phosphorylated histone H3 correspond to those at G2/M and M phases, respectively. GSCs containing higher amounts of cyclin E correspond to those at the G1 phase (Boyle et al., 2007).
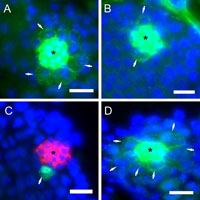 View Details | Fig. 4. Immunofluorescence of GSCs around the hub cells at the tip of the testis from wild-type (A–C) or InRE19/InR339 mutant (D) males using antibodies against the cell cycle markers cyclin E (A), cyclin B (B, D), or phosphorylated histone H3 (C). The hub cells (asterisk) were stained with anti-Fasciclin III antibody and are shown in green (A, B, D) or red (C). Scale bar: 10 μm. |
In wild-type males, an average of 6.5 GSCs was attached to hub cells (Table I). Among the GSCs in the proliferation center of wild-type testes, there were on an average 2.9 cyclin E-positive cells (n=16 testes), 1.6 cyclin B-positive cells (n=18), and 0.8 cells containing phosphorylated histone H3 (n=20) (Fig. 4A–C, Table IV), although some variations existed between individual flies. On the other hand, the average number of cells classified into each cell cycle stage changed in InRE19/InR339 males (Table IV). In particular, the average number of G2 phase cells in mutant males was more than twice that in wild-type males; however, this number was decreased at the other phases (Fig. 4D). These data suggest that GSC cycle progression at the G2/M phase was less efficient in InR mutant males than in wild-type males.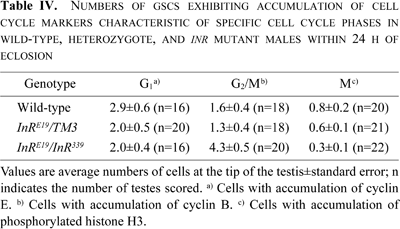
Drosophila spermatocytes increase in size by up to 25-fold before the initiation of the first meiotic division (Fuller and Spradling, 2007). To determine whether insulin signaling is required for the greatest cell growth among Drosophila diploid cells, we examined spermatocyte sizes in cysts at the S6 stage when cell growth is complete (Cenci et al., 1994) in both wild-type and insulin signaling mutants. In contrast with the average diameter of wild-type S6 cells (31.7 μm), that of InRE19/InR339 S6 spermatocytes was 21.7 μm, which was 68% that of wild-type spermatocytes (Table V). The average diameter of S6 spermatocytes of hemizygous chico mutant males was 25.4 μm, which was 80% that of wild-type males. These results suggest that insulin signaling plays a role in the remarkable cell growth of spermatocytes before entry into meiosis. We also detected the accumulation of activated Akt in growing (S4) spermatocytes, the immunofluorescence signal of which was shown to be dependent on insulin signaling (Fig. 2C, D).
Our results show that the inhibition of insulin production or insulin signaling results in a lower abundance of germline cells in Drosophila testes. The results of insulin signaling mutants suggest that insulin signaling is required for cell division in male GSCs to produce new germline cysts and for GSCs to self-renew. In addition, our results suggest that insulin signaling is required for cell cycle progression of GSCs at the G2/M phase. Thus, insulin signaling plays an essential role in Drosophila spermatogenesis and oogenesis. In addition, spermatocyte growth prior to meiosis was compromised in insulin signaling mutants, suggesting that insulin signaling has an extrinsic role in inducing the remarkable growth seen in spermatocytes.
In order to determine whether the efficiency of stem cell division was affected in InR mutants, we examined the number of cysts produced and the progression of stem cells through the cell division cycle. Based on the numbers of GFP-labeled centrosomes, we could estimate the frequency of the GSCs to undergo S phase. This is a convenient method to assess cell division of a specific cell type in living multi-cellular organisms. Results showed that a lack of insulin signaling decreased cell division efficiency.
The results showing that insulin signaling in Drosophila is required for stimulation of cell division in male GSCs suggests that insulin signaling affects sperm production. It has been reported that the production of ILPs is regulated by nutrient conditions in Drosophila (Ikeya et al., 2002) and that cell division of GSCs in Drosophila ovaries is under ILP control (LaFever and Drummond-Barbosa, 2005). Such evidence suggests that nutrient conditions may influence sperm abundance via hormonal control of ILPs and our results support this suggestion. In theory, a feedback system in which nutrient conditions affect germ cell division and growth would reduce Drosophila’s expense to produce excess progenies and allow Drosophila to survive under varying environmental conditions.
It has been shown that ILPs from neurosecretory cells operate directly upon the niche including GSCs in ovaries; however, these peptides are also produced by follicle cells (LaFever and Drummond-Barbosa, 2005). Here, we observed reduced cell division in GSCs in males without neurosecretory cells. As other peptide-producing cells (besides brain cells) have not been identified in male Drosophila, it is likely that male GSC maintenance may be under the direct control of ILPs from brain cells.
As shown in Fig. 2A, a similar level or somewhat more dominant accumulation of the activated Akt was also observed in hub cells rather than in surrounding GSCs or spermatogonia. As another interpretation of our genetic data and the immunostaining data, it might be possible that the insulin signaling mutations would influence GCSs via hub cells. Recently, a recent report showed that insulin signaling controls GSC maintenance via the niche in Drosophila females (Hsu and Drummond-Barbosa, 2009). We then checked whether a proliferation of the hub cells would be compromised in the InR mutants. We scored an average of 9 to 10 hub cells in newly eclosed males or two weeks old males after eclosion from both wild-type and the InR mutants. Although we have not obtained evidence to support the possibility that the ILPs activate InR in the hub cells and that it might influence GSC maintenance and/or division, it is still an interesting hypothesis to be examined in future.
The Drosophila protein Unpaired, produced by the hub cells, functions as a ligand to maintain male GSC numbers and plays a role in the repression of GSC division (Wu et al., 2007). In this study, we suggest that insulin signaling is required for inducing stem cell division. However, it has been shown that over-expressing ILP-2 using hsp70-ilp2 failed to produce extra GSCs or germline cysts in males (Ueishi and Inoue, unpublished). In oogenesis, Decapentaplegic (Dpp), a member of the Bone Morphogenetic Protein (BMP) ligand family in Drosophila, is independently used as a ligand to maintain GSC abundance and promote GSC division (Xie and Spradling, 1998). Different ligands and signaling cascades are used for renewal or maintenance of stem cells in males and females (Fuller and Spradling, 2007), but common insulin-like protein growth factors appear to function in both sexes. In order to maintain a certain number of stem cells and produce a regular number of germ cells, a balance between the maintenance and renewal signals is required. The regulatory mechanism that integrates these 2 signaling pathways has yet to be determined.
We also found that insulin signaling promoted cell cycle progression in male GSCs at the G2/M phase, similar to that observed in ovaries (Hsu et al., 2008). It has also been reported that insulin signaling influences cell cycle progression in cultured Drosophila cells at both the G1/S and G2/M phases; however, it was concluded that insulin functions as a negative factor rather than as a promoter of cell cycle progression at the G2/M phase (Wu et al., 2007). We speculate that insulin signaling affects cell cycle progression differently among cell types and/or developmental stages.
Insulin signaling was also found to be required for the remarkable spermatocyte growth in Drosophila, which occurs prior to meiosis. Primary spermatocytes in mammals also show considerable cell growth before meiosis (Sugiyama et al., 2001). The regulatory mechanism that controls such rapid growth has not yet been characterized, but here we have shown that spermatocyte growth can be regulated extrinsically by ILPs. Thus, such peptides regulate the two essential stages of division and growth in spermatogenesis.
Complete inhibition of spermatocyte growth was not observed in sterile InR mutant males in this study. This may be a consequence of some amount of residual insulin signaling activity in hypomorphic mutants. Alternatively, this suggests the involvement of other pathways that may be activated parallel to the insulin signaling pathway. It is known that the target of rapamycin (TOR) pathway is required for protein synthesis and cell growth (Zhang et al., 2000). Based on its involvement in synthesis and growth, the TOR pathway may also be activated during spermatocyte growth parallel to the insulin signaling pathway. Studies to examine the involvement of the TOR pathway in spermatocyte growth are underway.
Although insulin signaling is required for induction of GSC division and growth of germline cells within germline cysts in both Drosophila females and males, such influences of insulin signaling in mammals have not been reported. Nevertheless, we cannot exclude the possibility that a less severe form of diabetes in mammals is insufficient to detect the influence of insulin on mammalian spermatogenesis as in Drosophila chico mutants. It would be therefore interesting to perform careful characterizations of the phenotypes of testis-specific knockouts or knockdown of InR in other mammals such as mouse.
We wish to thank Daniela Drummond-Barbosa, Yasuyoshi Nishida, Yukiko Yamashita and the Kyoto Drosophila Genetic Resource Center for providing fly stocks. We also wish to acknowledge Helena Richardson, David Glover and Akira Nakamura for their generous gifts of antibodies. This work was supported by Grants-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Culture, Sports, Science and Technology of Japan to Y.H.I.
|