| To whom correspondence should be addressed: Hans Peter Bächinger, Research Department, Shriners Hospital for Children, 3101 SW Sam Jackson Park Road, Portland, OR 97239. E-mail: hpb@shcc.org Grant sponsor: Shriners Hospital for Children Research (HPB & HSS) |
Prolyl hydroxylation is known to be a critical post translational event during collagen biosynthesis and is catalyzed by multiple members of the iron-dependent dioxygenase superfamily. Collagen prolyl 4-hydroxylase catalyzes the formation of 4-hydroxyproline in -X-Pro-Gly- triplets in collagens and prolyl 3-hydroxylase 1 (P3H1) has been shown to catalyze the formation of 3-hydroxyproline in procollagens (Vranka et al., 2004). Interestingly, although 4(R)-hydroxyproline is clearly required for proper assembly and folding of the collagen triple helix, the importance of 3(S)-hydroxyproline is only just beginning to be elucidated. 3-hydroxyproline is found in almost all collagens in the sequence -Gly-3Hyp-4Hyp-Gly- (Fietzek et al., 1973, 1972; Rexrodt et al., 1975); however, the extent of 3-hydroxylation varies with the different types of collagens and occurs in the largest amounts in collagen types IV and V (Kefalides, 1972, 1973; Rhodes and Miller, 1978). Importantly, prolyl 3-hydroxylase 2 was recently shown to preferentially hydroxylate two synthetic peptides corresponding to sequences that are hydroxylated in type IV collagen (Tiainen et al., 2008).
The prolyl 3 hydroxylase family of proteins consists of 3 members, all of which share the conserved residues of the active site domain of the iron-dependent dioxygenases, including those of the prolyl 4-hydroxylases and lysyl hydroxylases. Additionally, P3H1 has been shown to specifically interact with cartilage associated protein (CRTAP) and cyclophilin B (Vranka et al., 2004). CRTAP is a protein of unknown function that shares homology with the amino terminal domain of the P3H family of proteins (Morello et al., 1999; Tonachini et al., 1999; Vranka et al., 2004). Multiple recent reports have shown that P3H1 deficiency in humans, as well as mutations in CRTAP, causes a recessive disorder resembling osteogenesis imperfecta (OI) (Barnes et al., 2006; Cabral et al., 2007; Morello et al., 2006; Willaert et al., 2008). More importantly, the 3-hydroxylation of key residues in types I and II collagens was reduced in the patients with OI indicating the importance of P3H1 and CRTAP in collagen stability, secretion, and ultimately in bone development.
The aim of the present work was to study the expression patterns of the three prolyl 3-hydroxylase genes in adult and fetal mouse tissues in order to determine in vivo sites expressing the prolyl 3-hydroxylase enzymes and to identify tissues most likely to be affected by their loss of function.
Mouse multiple tissue Northern blots containing 2 μg of poly A+ RNA per lane were purchased from BD Biosciences (Clontech). Hybridization was carried out at 60°C using express hybridization buffer (BD Biosciences). The membranes were washed 4×20 min in 2x sodium chloride-sodium citrate buffer (SSC) and 1% SDS at room temperature, then washed in 1x SSC and 0.1% SDS twice and autoradiographed. 3'UTR probes of approximately 600 nucleotides in length were PCR-amplified from mouse fibroblast cDNA for P3H1 and P3H2 probes with the following gene specific oligonucleotides: for the P3H1 probe 5'AGGCAGATGACCTGGTGAAG and 5'GCCTACATAGGCCCCAGGGTGG; for the P3H2 probe 5'ATTCAGAGCCCATTCACACC and 5'TTAATCCAGTGGTGGCTTCC. Internal sequences were selected for the P3H3 probe where the gene was most divergent from P3H1 and P3H2 and these amplified a 274 bp product by PCR. The P3H3 gene specific oligonucleotides selected were: 5'GACACTGGATACGGGTTCTG and 5'ATGTCCTCTCGTGGTCCCAG. The GAPDH oligonucleotide sequences selected amplified a 150 bp product by PCR and were as follows: 5'CACTGCCACCCAGAAGACTGT and 5'GGAAGGCCATGCCAGTGA. The PCR products were labeled using a kit for random priming (Roche Random Primed DNA Labeling Kit) and [α32P]dCTP.
Internal probes of approximately 600 nucleotides in length were also generated to P3H1 and P3H2 internal sequences for each gene. The following oligonucleotides were used to PCR-amplify the gene-specific products: 5'GCTGGCTTCCCACCCAAGTA and 5'GCAGCTCCTGGCACTCATCA for the P3H1 probe and 5'CCAAGCCACACCTGGAAAGC and 5'CCTGCCGTCCTCCATAGCTG for the P3H2 probe.
Total mouse RNA was purchased from BD Biosciences (Mouse Total RNA Master Panel). Each representative RNA (from adult mouse heart, brain, eye, lung, kidney, liver, uterus, testis, spleen, thymus, salivary gland, and total RNA from embryonic stages E7, E11, E15 and E17) was reverse transcribed using Superscript Reverse Transcriptase III (Invitrogen) according to the manufacturer’s instructions with Oligo(dT)12–18 primer (Invitrogen). Additional RNA was extracted from adult mouse bones, joint cartilages and skin using the TRIzol extraction method and reverse transcribed using random hexamers according to the manufacturer’s instructions (Invitrogen). Resulting cDNA was used as the template in real-time quantitative PCR experiments with the same P3H 1, 2, or 3 gene specific oligonucleotides described above, and the iQ SYBR Green Supermix according to the manufacturer’s instructions (Bio-Rad). Quantitative PCR data were measured and analyzed using the iQ5 Multicolor Real-Time PCR Detection System and software version 2.0 (Bio-Rad). The following gene specific oligonucleotides to 18srRNA were synthesized 5'CAGTAAGTGCGGGTCATAAGC and 5'AGGGCCTCACTAAACCATCC and amplified a 96 bp product, and additional PCR experiments were performed using the 18srRNA primers and the same GAPDH sequences as mentioned above as reference genes for data normalization. Each PCR reaction was 25 μl and apart from that used for the standard curves, the total input cDNA was identical for all reactions. Because SYBR Green binding is not sequence specific, careful validation of the primer pairs was undertaken to ensure that only the target gene sequence was amplified. To verify the specificity of each amplicon, a single gel band of the expected size was amplified for each primer pair when tested on a mouse fibroblast cDNA and melting curve analysis confirmed the presence of a single PCR product. Standard curves were generated for each amplified product and data obtained in all experiments were confirmed to correspond to a linear portion of the standard curve generated for each experiment. Reaction efficiencies determined from calibration curves for each set of primers were between 83–93%. Fold differences in target genes were normalized either to GAPDH expression or 18srRNA expression in each tissue where indicated (ΔΔCT method). Additionally gene specific data were quantified using the standard curves for each gene and normalized to total input RNA (ΔCT method). Data analysis was performed using the iQ5 Optical System Software version 2.0.
E10–E15 embryos were fixed overnight in 4% paraformaldehyde in 1x phosphate buffered saline (PBS) at 4°C, then dehydrated in methanol and stored at –20°C until use. Hybridization was carried out overnight at 70°C as described previously with the addition of the digioxigenin-labeled P3H1, P3H2, and P3H3 RNA probes at 0.5 μg/ml (Carpenter et al., 1993; Manley and Capecchi, 1995; Morgan et al., 2003). Alkaline phosphatase-conjugated anti-digoxigenin Fab fragments were used at 1:3000 (Roche) as described by manufacturer. Color reactions were carried out over time periods ranging from 2 hours to overnight for optimized signal. For the antisense probes the same oligonucleotides as described in the methods above were used to PCR-amplify 600 bp gene-specific 3'UTR products. These were subsequently cloned into the pCR4 cloning vector (Invitrogen) which contains RNA Polymerase T3 and T7 promoters. Antisense riboprobes were generated using the plasmids and digoxigenin UTP and T7 or T3 RNA polymerase at 37°C for 2 hours according to manufacturer’s instructions (Roche). For cryosection analysis, paraformaldehyde-fixed embryos were washed 30 min in 1x PBS. Cryoprotection of the tissues was achieved using a sequential series of 10–40% sucrose in 1x PBS. The embryos were oriented in OCT (Tissue-Tek) and frozen rapidly on dry ice. The embryos were sectioned to 16 μm using a Leitz Kryostat 1740, and mounted on Superfrost plus slides (Fisher) for analysis. Sections were rinsed for 10 min three times in 1x PBS to remove the OCT. They were then treated with 1 μg/ml proteinase K for 5 min at room temperature, washed in PBS with 0.1% Tween 20 (PBST) and fixed in 4% paraformaldehyde. Sections were washed in PBST and acetylated in acetic anhydride and 0.1 M triethanolamine for 15 min at room temperature. Slides were washed in PBST and then air dried followed by the addition of prewarmed hybridization solution and incubation at 65°C for 1 hour in humidified boxes. Antisense riboprobes were added to slides at 0.5 μg/ml and incubated overnight at 65°C. Sections were washed in 1x SSC/50% formamide for 30 min at 65°C and then treated with 20 μg/ml RNase A (Roche) for 30 min at 37°C. Slides were then washed in 2x SSC, and twice in 0.2x SSC each for 20 min at 65°C. Sections were then blocked in 20% heat-inactivated sheep serum (HISS) for 1 hour at room temperature and then incubated in a 1:2500 dilution of anti-digoxigenin-AP (Roche) in 5% HISS overnight at 4°C. Slides were washed four times in 0.1 M maleic acid, 0.15 M NaCl for 5 min each wash and then developed with 250 μg/ml NBT and 125 μg/ml BCIP in 0.1 M NaCl, 0.1 M Tris, pH 9.5, and 0.05 M MgCl2 overnight at 4°C.
Northern blot analysis was performed on various adult mouse tissues to determine transcript size using probes derived from the 3'UTR for all three P3H genes (Fig. 1A–C). Two transcripts of approximately 2.8 and 3.5 kb were detected for P3H1 in heart, liver, and kidney (Fig. 1A). The two different transcripts are thought to represent a long form and an alternatively spliced shorter form, both of which have previously been found to be associated with certain human tissues and transformed cells (Kaul et al., 2000). P3H1 expression was not detected in skeletal muscle by northern blot analysis with either the 3'UTR probe or with an internal probe (see Methods). When the blot was hybridized with a GAPDH probe (Fig. 1D) the presence of a single 1.3 kb transcript in all lanes indicated that RNA was present for all samples on the blot; however, GAPDH expression appeared to vary significantly from tissue to tissue (see Discussion.) Prolyl 3-hydroxylase 2 (P3H2) was detected approximately as a 3.7 kb transcript in kidney, heart, and liver, as well as a 2.0 kb transcript only found in liver (Fig. 1B). It is not clear whether the smaller transcript in liver represents a splice variant or a degradation product. Previous studies of human P3H2 expression reported an additional 6.5 kb transcript in skeletal muscle (Jarnum et al., 2004). Finally, prolyl 3-hydroxylase 3 (P3H3) was detected as a 3.0 kb transcript in heart, liver, and kidney (Fig. 1C). A 3.8 kb P3H3 transcript was also detected in the brain whereas neither P3H1 nor P3H2 was detected in this same tissue. Differences in transcript size may be due to alternatively processed transcripts and may result in tissue-specific differences of overall enzyme activity as the enzymatic activity resides in the carboxy-terminal domain of all three proteins. It is important to note here that although northern blots are a good method to determine correct transcript size, quantitative PCR is the primary method used in this work to assess actual quantitative differences in P3H expression levels across various tissue samples.
 View Details | Fig. 1. Northern blot analysis of RNA from adult mouse tissues. Hybridizations were performed using radiolabeled 3'UTR probes to detect prolyl 3-hydroxylase 1 (A) transcripts, prolyl 3-hydroxylase 2 (B) transcripts, and prolyl 3-hydroxylase 3 (C) transcripts. RNA from adult mouse tissues were loaded in the following lanes: 1. Heart, 2. Brain, 3. Liver, 4. Skeletal Muscle, 5. Kidney. P3H1 transcripts were detected at approximately 2.8 and 3.5 kb (A). P3H2 transcripts were detected at approximately 3.7 kb in most tissues with an additional 2.0 kb transcript in liver (B). P3H3 transcripts were detected approximately as a 3.0 kb transcript in heart, lung, liver and kidney, as well as a 3.8 kb transcript in brain (C). GAPDH was detected in all tissues as a 1.3 kb transcript and verifies the integrity of the RNA on the blots (D). |
Real-time quantitative PCR on reverse-transcribed RNA from a variety of adult mouse tissues, as well as from embryonic mice at developmental stages (E7–E17) was performed to quantitate the expression levels of P3H1, P3H2, and P3H3 genes (Table I and Table II). The most striking difference in gene expression was a tenfold higher expression of P3H2 in the adult kidney, a tissue rich in basement membrane type IV collagen which is a likely substrate of the P3H2 enzyme (Table II and Fig. 2A). P3H2 was also expressed at higher levels in the heart, spleen, and eye (Table II and Fig. 2A). Slight differences in expression levels of P3H2 between Fig. 2 and Table II are likely due to the normalization of expression levels to either 18srRNA or to total input RNA, respectively. Interestingly, collagen IV is an essential component of the basement membranes in kidney, spleen and the lens of the eye (Maatta et al., 2004; Pöschl et al., 2004).
 View Details | Fig. 2. Graphical representation of real-time quantitative PCR analysis of adult and embryonic mouse tissues. Data are represented as the fold change differences and are represented as the ratio of target gene expression to 18srRNA signal (A, B) per 100 ng of input RNA from each tissue. All experiments were performed in triplicate and mean values are represented on each graph. P3H1 gene expression is represented by the black boxes, P3H2 is in white and P3H3 is in gray. Graph A shows relative gene expression in adult tissues. Graph B shows relative P3H gene expression in embryonic mouse tissues. |

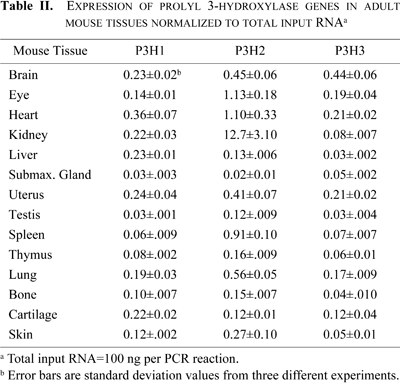
For P3H3 the highest levels of expression were detected in brain, heart, and uterus. P3H1 was expressed at low levels in heart, liver, and kidney and at slightly higher levels in cartilage relative to P3H2 or P3H3 (Table II and Fig. 2). All three genes were expressed at low levels in adult thymus, testis, submaxillary gland and bone (Fig. 2A, 2B and Table II).
Differences in the embryonic expression of P3H1, P3H2 and P3H3 were also detected. For P3H1, embryonic expression appeared highest at approximately equal levels at both E7 and E15 (Table I and Fig. 2B). P3H2 and P3H3 were also expressed but at the mid-embryonic stages E11 and E15 (Table I and Fig. 2B). These results demonstrate some overlap in the expression of the three genes as well as some distinct expression differences in specific tissues supporting the idea that the enzymes may be important in development and have some unique tissue specificities. Some small discrepancies in the expression levels of a gene are present in the data and are thought to be due to the method of normalization as well as the housekeeping gene used, as represented in Fig. 2 (fold expression relative to 18srRNA) versus in Table II (expression normalization to total input RNA). As mRNA expression of reference genes has been shown to differ among tissues and various cells the P3H gene levels were analyzed by the method of data normalization which relates the mRNA data to the total RNA content of the sample preparation subjected to the reverse transcription reaction, (Bustin, 2002; Bustin and Nolan, 2004; Sindelka et al., 2006). Very low levels of expression of any of the 3 prolyl 3-hydroxylase genes in the tissues presented in Table II are not thought to be significant.
Our previous characterization of P3H1 in the developing chick suggested that tissues containing fibrillar collagens such as cartilage, tendon, bone, skin, and aorta were likely targets of P3H1 enzymatic activity (Vranka et al., 2004). In this study embryonic mouse tissues were analyzed by whole mount and section in situ hybridization using riboprobes specific for P3H1, P3H2 and P3H3 to determine whether there was a similar pattern of P3H distribution. Common and unique sites of expression of P3H1, P3H2 and P3H3 were detected in the embryos throughout the developmental stages analyzed (E11.5–E13.5). Among the regions expressing all 3 prolyl 3-hydroxylase genes were the forelimbs and hindlimbs, and maxillary and mandibular components of the first embryonic arch at E11.5 (Fig. 3A–C), in the distal tip of the tail and in the vibrissae at E12.5 (Fig. 3D–F), and in the developing pinnae at E13.5 (Fig. 3G–I). P3H2 and P3H3 were also expressed in the developing vertebral bodies (Fig. 3E and 3F) and in the developing tail (Fig. 3G–I). P3H3 expression was also detected in the hair follicles of the skin at E13.5 (Fig. 3I).
 View Details | Fig. 3. Detection of prolyl 3-hydroxylase gene expression in whole mouse embryos by in situ hybridization. Antisense riboprobes were generated to a 600 bp fragment of the 3'UTR region of P3H1 (A, D, G), P3H2 (B, E, H), and P3H3 (C, F, I) and used to detect gene expression levels. P3H1 detected in the forelimb and hindlimb at E11.5 (A), E12.5 (D), and E13.5 (G). P3H2 detected in the forelimb and hindlimb of E11.5 embryos (B), E12.5 (E) and also in the distal tip of the tail (asterisks) (E), and limbs of the E13.5 embryo (H). P3H3 detected in the forelimb and hindlimb at stages E11.5 (C), E12.5 (F), and E13.5 (I) as well as in the distal tip of the tail (asterisks), vertebral bodies (arrows), vibrissae (v), and hair follicles in the skin (arrowheads) (I). fl=forelimb; hl=hindlimb, p=pinnae, t=tail. |
Finally to identify the cell types expressing the P3H genes in situ hybridization was performed on E12.5 cryosections (Fig. 4). P3H1 was expressed in prechondrogenic skeletal elements, such as the vertebral bodies (Fig. 4A) and cranial primordia (Fig. 4C). P3H1 was also strongly expressed in the aortic arch (Fig. 4B). In contrast, P3H2 was not expressed in precartilaginous structures (Fig. 4D) or in the aortic arch (Fig. 4E). P3H2 expression was observed in the cells forming part of the intervertebral discs (Fig. 4D) and in the lens of the eye (Fig. 4F), whereas P3H1 expression was not detected in the eye at E12.5 (Fig. 4C). P3H3 was also expressed in the eye in subpopulations of cells within the lens (Fig. 4I). Overall, P3H3 appeared to be more ubiquitously expressed throughout the E 12.5 mouse embryo (Fig. 4G–I) and showed some overlap with P3H1 expression in the vertebral bodies (Fig. 4A–C) and with P3H2 expression in the eye (Fig. 4D–F).
 View Details | Fig. 4. Detection of prolyl 3-hydroxylase gene expression in E12.5 mouse tissue cryosections by in situ hybridization. Antisense riboprobes made to P3H1 (A–C), P3H2 (D–F), and P3H3 (G–I) were used to detect expression levels in the cartilage condensations of the developing vertebrae (arrowheads) (A, D, G), the aortic arch (arrows) and Meckel’s cartilage (B, E, H), and the developing eye (C, F, I). P3H1 is detected in cartilage condensations in the vertebrae (A), whereas P3H2 localizes to intervertebral zone (D), and P3H3 is detected throughout the entire vertebral area (G). P3H1 is detected within the aortic arch (arrows) and in Meckel’s cartilage (B), whereas P3H2 (E) and P3H3 (H) are not localized to either tissue. P3H1 is detected in the developing mouse eye in cartilage primordium well below the retina (C), however P3H2 is localized to the lens of the eye (F) and P3H3 is detected in the retina, cornea, and within lens cell subpopulations (I). iv=intervertebral zone; lg=lung; mc=Meckel’s cartilage; aa=aortic arch; c=cornea; l=lens; r=retina; c.p.=cartilage primordium. |
A developmental role for P3H1 is most likely linked to that of the fibrillar collagens, particularly types I, II, and III which are abundantly expressed during development (Niederreither et al., 1995). Mutations in P3H1 have been shown to cause a recessive form of osteogenesis imperfecta demonstrating a crucial role for P3H1 in facilitating collagen stability and bone development (Cabral et al., 2007). Additionally, type II collagen is expressed in chondrogenic tissue in advance of chondrogenesis, as well as in some nonchondrogenic tissues of the developing mouse embryo including the heart, epidermis, inner ear, and nervous system (Cheah et al., 1991; Ng et al., 1993; Sakai et al., 2001) indicating the involvement of prolyl 3-hydroyxlase enzyme activity during development. Collagen IV has also been shown to be indispensable for the structural integrity of basement membranes at later stages of development and in adult tissues (Pöschl et al., 2004). A recent study demonstrated that P3H1 protein was present in the stromal elements of the rat kidney during development with its expression persisting into adulthood (Lauer et al., 2007). This data is in agreement with our gene expression data in which P3H1 is present in kidney but at limited levels. The highly elevated level of P3H2 expression in the adult kidney supports the idea that its enzymatic activity is essential to the development and formation of functional basement membranes. These results are supported by the recent work of Tiainen et al. showing P3H2 expression in fetal kidney (Tiainen et al., 2008). Additionally, a very recent study has demonstrated not only conserved notochord expression of all three prolyl 3-hydroxylase genes but also the co-expression of the three genes in areas of the mouse embryos at various developmental stages suggesting a functional importance for the development of specific regions (Capellini et al., 2008). More detailed studies on the expression of P3H3 in a wider variety of tissues, cell types, and ages will help elucidate its potential substrates and cellular activities.
In summary we conclude from the data presented here that the prolyl 3-hydroxylases have both unique and overlapping regions of expression in adult and embryonic tissues. This differential expression may reflect the utilization of these proteins in their abilities to act upon a unique set of substrates in discrete tissues. P3H1 appears to be coordinately expressed and to recognize fibrillar collagens such as types I, II, III, and V collagen in adult and developing tissues. P3H2 appears to be coordinately expressed with and therefore recognizes basement membrane collagens such as type IV collagen, which is known to contain up to 15 3-(S)-hydroxyproline residues in 1000. Other basement membrane associated collagens may be targets of this enzyme as well. The potential substrates of the third P3H family member are ambiguous, but may be collagens or other proteins predominantly expressed in brain or uterus and expressed in some developing mouse tissues. The data presented herein help to further the current understanding of the family of prolyl 3-hydroxylase genes as well as to suggest potentially novel individual enzyme substrates.
This study was supported in part by a Shriners Hospital Postdoctoral Research Fellowship (J. Vranka) and grants from Shriners Hospital for Children Research (H.P.B. & H.S.S.)
|