| To whom correspondence should be addressed: Eisuke Mekada, Department of Cell Biology, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamadaoka, Suita, Osaka 565-0871, Japan. Tel: +81–6–6879–8286, Fax: +81–6–6879–8289 E-mail: emekada@biken.osaka-u.ac.jp Abbreviations: EGF, epidermal growth factor; HB-EGF, heparin-binding EGF-like growth factor; proHB-EGF, membrane-anchored form of HB-EGF; DT, diphtheria toxin; hHB-EGF, human HB-EGF; mHB-EGF, mouse HB-EGF; hzHB-EGF, humanized HB-EGF; tRA, all-trans retinoic acid. |
Tumor progression is a complex process that involves the interaction of cancer cells with the cells in the cancer-surrounding tissues (cancer stroma) including endothelial cells, fibroblasts, lymphocytes, and other cell types. The cancer stroma directly or indirectly influences cancer cell growth and metastatic potential (Bhowmick et al., 2004; Folkman and Shing, 1992; Kalluri and Zeisberg, 2006; Mueller and Fusenig, 2004; Tlsty and Coussens, 2006). Although much attention has recently been paid to the roles of the cancer stroma in tumor development and progression, the molecular mechanism underlying the crosstalk between cancer cells and stromal cells remains largely unknown.
The ErbB family of receptor tyrosine kinases and their ligands, the epidermal growth factor (EGF) family, play important roles in tumorigenesis (Normanno et al., 2003). Heparin-binding EGF-like growth factor (HB-EGF), a member of the EGF family, is synthesized as a membrane-anchored form (proHB-EGF) and its soluble form is subsequently released from the cell surface by ectodomain shedding (Goishi et al., 1995; Higashiyama et al., 1991). The soluble HB-EGF has potent mitogenic and chemoattractant activities for a number of cell types (Higashiyama et al., 1993). Increasing evidence has accumulated to indicate that HB-EGF plays pivotal roles in oncogenic transformation, tumor invasion and metastasis (Bos et al., 2009; Miyamoto et al., 2004; Miyamoto et al., 2006; Ongusaha et al., 2004), and that HB-EGF elaborated by cancer cells themselves is involved in tumorigenesis. However, the contribution of HB-EGF expressed in tumor stromal cells to tumor growth remains unclear, although HB-EGF is often expressed in fibroblasts and endothelial cells (Raab and Klagsbrun, 1997).
ProHB-EGF serves as the receptor for diphtheria toxin (DT) (Iwamoto et al., 1994). DT and CRM197, a non-toxic mutant form of DT, bind to the EGF-like domain of human HB-EGF (hHB-EGF), thereby inhibiting the binding of hHB-EGF to EGF receptor (Mitamura et al., 1995). Consequently, CRM197 is used as a specific inhibitor of hHB-EGF. Furthermore, it strongly inhibits tumor growth in mouse xenograft tumor models (Miyamoto et al., 2004). However, CRM197 does not bind to mouse HB-EGF (mHB-EGF) and cannot inhibit mHB-EGF derived from cancer stromal cells in mouse tumor models (Mitamura et al., 1995). In the present study, we generated knock-in mice in which the mHB-EGF gene was replaced with CRM197-inhibitable humanized HB-EGF (hzHB-EGF) and studied the role of HB-EGF produced by cancer stromal cells in tumor growth.
DT and CRM197 were prepared as described previously (Uchida et al., 1973).
All cell lines were maintained in DMEM supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin and 10% fetal bovine serum (ICN Biomedicals, Costa Mesa, CA, USA).
The constructions of plasmids encoding hHB-EGF cDNA, mHB-EGF cDNA and human/mouse HB-EGF chimeras inserted into the eukaryotic expression vector pRc/CMV (Invitrogen, Tokyo, Japan) were described previously (Mitamura et al., 1995). For the construction of hzHB-EGF, we used the H (106–186) chimera HB-EGF cDNA as the starting material for hzHB-EGF construction (Mitamura et al., 1995). This cDNA encodes mouse HB-EGF containing human sequence between Asp106 and Tyr186. The EGF-like domain locates between Cys108 and Pro149. Two amino acid residues in the sequence from Pro149 to Tyr186 differ between human and mouse HB-EGF. In order to restrict the substitution within the EGF-like domain, R153P and I162V mutations were introduced into the H (106–186) chimera HB-EGF cDNA inserted into the pRc/CMV vector by site-directed mutagenesis, resulting in an hzHB-EGF cDNA. A HindIII-MscI fragment of the hzHB-EGF cDNA inserted into the pRc/CMV vector was substituted with the corresponding fragment of the H (54–73) chimera HB-EGF cDNA inserted into the pRc/CMV vector, resulting in an H6-hzHB-EGF cDNA. Stable transfectants of LC cells expressing H6-mHB-EGF, hHB-EGF, H6-hzHB-EGF or hzHB-EGF were obtained by transfection with the corresponding plasmids using Lipofectamine 2000 (Invitrogen, Tokyo, Japan) according to the manufacturer’s instructions.
DT sensitivity was measured by inhibition of protein synthesis by DT as described previously (Umata et al., 1990). Binding of DT to cells was measured as described previously (Iwamoto et al., 1994).
L cells (5×105 cells) were transfected with hHB-EGF, hzHB-EGF, H6 epitope-tagged hzHB-EGF (H6-hzHB-EGF) or H6 epitope-tagged mHB-EGF (H6-mHB-EGF) and cultured in RPMI 1640 containing 10% fetal calf serum and 10 μg/ml of heparin for 72 h. The conditioned media were collected. The mitogenic activities of HB-EGF in the conditioned media with and without CRM197 were assayed by measuring their effects on the proliferation of DER cells as described previously (Takazaki et al., 2004).
All experimental use of animals complied with the Guidelines for Animal Care of Osaka University.
A 6.0-kb EcoRI-SacII fragment containing exon 1 of the HB-EGF gene and an 8.0-kb EcoRI-EcoRV fragment downstream of exon 3 were used as homology arms. A LoxP-flanked mHB-EGF cDNA with a poly(A) signal, a neo cassette driven by the phosphoglycerate kinase promoter and intron 5 of the mHB-EGF gene as a splicing donor (SD) were fused at exon 1. The hzHB-EGF cDNA linked to the GFP cDNA with a poly(A) signal via the internal ribosome entry site (IRES) was inserted downstream of intron 5 of the mHB-EGF gene (SD). The XhoI-linearized DNA of the targeting vector was electroporated into D3 embryonic stem (ES) cells. To obtain the HBhz-flox allele, individual clones were screened for homologous recombination by Southern blot analysis of HindIII-digested DNA and SpeI-digested DNA with 5' and 3' probes that corresponded to sequences flanking the targeting vector of the 5'- and 3'-arms, respectively. SpeI-digested DNA was also analyzed with a neo probe as an internal probe. Hybridization was carried out as described previously (Iwamoto et al., 2003).
The targeted ES clones were injected into C57BL/6J blastocysts, and the chimeric mice were bred with C57BL/6J female mice to obtain HBhz-flox mice. Homozygous HBhz-flox/hz-flox mice were obtained by interbreeding of HBhz-flox mice. Subsequently, homozygous HBhz-flox/hz-flox mice were bred with CAG-Cre transgenic mice (Sakai and Miyazaki, 1997) to generate HBhz/+ mice. Cre-mediated recombination was confirmed by PCR analysis using primer sets for Cre (fwd, 5'-AGGTTCGTTCACTCATGGA-3' and rev, 5'-TCGACCAGTTTAGTTACCC-3'), neo (fwd, 5'-ATGGGATCGGCCATTGAACA-3' and rev, 5'-GAAGAACTCGTCAAGAAGGC-3') and GFP (fwd, 5'-CAAGCAGATCCTGAAGAACA-3' and rev, 5'-GAACATCTCCTCGATCAGGT-3') as well as a wild-type allele-specific primer set (wt) (fwd, 5'-CATGGGGTTGTGACTCTCCT-3' and rev, 5'-AGCCTGCACACACAAAAGTG-3'). Finally, HBhz/+ heterozygous mice were intercrossed to generate HBhz/hz mice. Elimination of mouse HB-EGF and alternative expressions of hzHB-EGF and GFP were confirmed by RT-PCR analysis using an hHB-EGF-specific primer set (fwd, 5'-ATGTGAAGGAGCTCCGGG-3' and rev, 5'-TCAGTGGGAGCTAGCCAC-3'), an mHB-EGF-specific primer set (fwd, 5'-ACCTGCAGGAGTTCCGTA-3' and rev, 5'-TCAGTGGGAGCTAGCCAC-3'), a primer set based on the consensus sequence of hHB-EGF and mHB-EGF (fwd, 5'-ATGAAGCTGCTGCCGTCGGT-3' and rev, 5'-TCAGTGGGAGCTAGCCACGC-3'), a primer set for GFP and a primer set for G3PDH (fwd, 5'-ACCACAGTCCATGCCATCAC-3' and rev, 5'-TCCACCACCCTGTTGCTGTA-3').
cDNAs were prepared by a reverse transcriptase, ReverTra Dash (Toyobo, Osaka, Japan) from total RNA extracted using ISOGEN (Nippon Gene, Tokyo, Japan) from all-trans retinoic acid (tRA)-treated mouse skins, and PCR was performed by using the primer sets as described above. (Yamazaki et al., 2003).
HBdel/+;nu/nu mice were obtained by crossing HBdel/+ mice with nude (nu/nu) mice. A total volume of 0.1 ml containing 5×106 cells suspended in serum-free DMEM was subcutaneously injected into the HBdel/+;nu/nu mice at 5 weeks of age. At 4 weeks after the tumor cell injection, the formed tumors were excised, fixed with 4% paraformaldehyde and embedded in OCT compound (Sakura Fine-Tek, Tokyo, Japan). Frozen sections (8 mm) were stained with 5-bromo-4-chloro-3-indolyl β-D-galactoside (X-Gal) and an anti-CD31 antibody (clone MEC13.3; BD PharMingen, Franklin Lakes, NJ, USA) or anti-α-smooth muscle actin (SMA) antibody (clone 1A4; Sigma, Tokyo, Japan). Other mouse tissues, including the hearts, were fixed by perfusion of 4% paraformaldehyde, dehydrated and embedded in paraffin. Paraffin sections (4 mm) were stained with hematoxylin/eosin or subjected to Azan-Mallory staining.
A total volume of 0.1 ml containing 5×106 cells suspended in serum-free DMEM was subcutaneously injected into nu/nu mice or HBhz/hz; nu/nu mice at 5 weeks of age. At several time points after the cell injection, the sizes of the tumors were measured using a caliper. The tumor volumes were calculated as described previously (Bissery et al., 1991). CRM197 was dissolved in 1 ml of PBS containing 8% sucrose at 2.88 mg/ml and then further diluted to 8 μg/ml with saline. CRM197 at a dose of 0.2 mg/kg was injected intraperitoneally into the tumor-bearing mice every day.
The statistical significance was determined by Student’s t test as implemented by Excel 2007 (Microsoft Corp., Redmond, WA, USA).
Cancer stroma expresses a number of growth factors, cytokines and chemokines to enhance cancer cell proliferation and metastasis. However, the expression of HB-EGF in cancer stroma has not yet been reported. We examined whether HB-EGF was expressed in cancer-associated stromal cells upon tumor formation. To this end, we utilized an HB-EGFdel/+ lacZ reporter allele (Iwamoto et al., 2003). In HB-EGFdel/+ mice, lacZ is expressed under the HB-EGF promoter and localized in the nucleus because of an additional nuclear localizing signal, thereby enabling us to detect HB-EGF expression by lacZ staining. To facilitate the implantation of cancer cells into mice, HBdel/+;nu/nu mice were used for this study. Tumors were generated by subcutaneously injecting human or mouse cancer cell lines into the HBdel/+; nu/nu mice. We tested the following cell lines for tumor formation: RMG-1, a human ovarian cancer cell line; HT29, a human colon cancer cell line; MMT, a mouse breast cancer cell line; and LLC, a mouse lung cancer cell line. At 1 month after implantation, the tumors were histologically analyzed for the expression of host-derived HB-EGF by lacZ staining. Although lacZ-positive cells were scarcely observed in the corresponding normal tissues, lacZ-positive cells were detected in the stromal region of the tumors formed when RMG-1, HT29 or MMT cells were injected (Fig. 1), indicating that these cells induced HB-EGF expression in the tumor-surrounding tissues upon tumorigenesis. Immunohistochemistry for CD31, a marker of vascular endothelial cells, and SMA, a marker of cancer-associated fibroblasts (CAFs), revealed that vascular endothelial cells and/or CAFs were contained in the cell types expressing HB-EGF in the cancer stroma (Fig. 1). When LLC cells were injected, the tumor was successfully formed. However, the tumor stroma did not induce any lac-Z positive cells (Fig. 1).
 View Details | Fig. 1. HB-EGF is expressed in the cancer stroma. RMG-1, HT29, MMT and LLC cells were subcutaneously injected into HBdel/+;nu/nu mice. Tumor sections were stained for lacZ and also stained with an anti-CD31 antibody or anti-SMA antibody, followed by counter-staining with nuclear fast red. Blue spots indicate the nuclei of lacZ-positive HB-EGF-expressing cells. CD31 and SMA are stained in brown and gray, respectively. Insets show magnified views of lacZ and CD31 double-positive cells or lacZ and SMA double-positive cells. |
CRM197 is a specific inhibitor of hHB-EGF, but does not inhibit mHB-EGF expressed in cancer-associated stromal cells in mouse cancer models. To examine whether HB-EGF expressed in stromal cells is involved in cancer progression, we generated knock-in mice expressing CRM197-inhibitable hzHB-EGF. To this end, we first constructed hzHB-EGF by swapping the EGF-like domain of mHB-EGF with the corresponding region of hHB-EGF (Fig. 2A). There are 39 and 10 amino acid substitutions within the whole molecule and the EGF-like domain, respectively, between hHB-EGF and mHB-EGF. DT and CRM197 bind to the EGF-like domain and only the EGF-like domain is sufficient for the binding (Mitamura et al., 1995; Mitamura et al., 1997). Therefore, to create hzHB-EGF (i.e. DT-sensitive and CRM197-inhibitable), we constructed a mouse/human chimeric HB-EGF in which the EGF-like domain of mHB-EGF was swapped with the corresponding region of hHB-EGF. For recognition with anti-HB-EGF antibody H6, H6-mHB-EGF and H6-hzHB-EGF, in which the H6 region of mHB-EGF was swapped with the corresponding region of hHB-EGF, were also constructed (Fig. 2A). Each HB-EGF construct was transfected into mouse LC cells and the DT sensitivities were measured by inhibition of protein synthesis (Mitamura et al., 1995). Cells transfected with hzHB-EGF, H6-hzHB-EGF or hHB-EGF became equally DT-sensitive, whereas those transfected with H6-mHB-EGF did not (Fig. 2B). DT-binding assays revealed that the Ka value of hzHB-EGF for DT was 3.9×108 M–1, and similar to a previously reported value for hHB-EGF (3.6×108 M–1) (Mitamura et al., 1995). More importantly, hzHB-EGF exhibited similar degrees of mitogenic activity to hHB-EGF and mHB-EGF, and CRM197 inhibited the mitogenic activity of hzHB-EGF similarly to that of hHB-EGF (Fig. 2C).
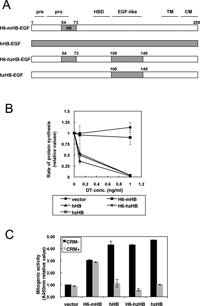 View Details | Fig. 2. Generation and characterization of hzHB-EGF knock-in mice. (A) Structures of hzHB-EGF and the related constructs. pre, signal sequence; pro, pro-domain; HBD, heparin-binding domain; TM, transmembrane domain; CM, cytosolic domain; H6, region from amino acids 54–73 of hHB-EGF recognized by the anti-H6 antibody. (B) DT sensitivity of hzHB-EGF-expressing mouse cells. hzHB-EGF, hHB-EGF, H6-hzHB-EGF and H6-mHB-EGF were separately transfected into mouse LC cells, and the DT sensitivities of the transfected cells were determined by measuring their rates of cellular protein synthesis as described in the supplementary data online. The cells expressing hzHB-EGF and H6-hzHB-EGF are sensitive to DT, similar to the cells expressing hHB-EGF. (C) Mitogenic activity of hzHB-EGF and inhibition by CRM197. Conditioned media prepared from LC cells stably expressing H6-mHB-EGF, hHB-EGF, H6-hzHB-EGF or hzHB-EGF were tested for their mitogenic activities in the DER cell system with or without 10 μg/ml of CRM197 as described in the supplementary data online. Bars, SE. |
Using the hzHB-EGF construct, hzHB-EGF knock-in (HBhz) mice were generated by a gene replacement strategy that replaced the mHB-EGF gene with hzHB-EGF (Fig. 3). The targeting vector contained the mHB-EGF cDNA flanked by loxP sites linked to the hzHB-EGF cDNA (Fig. 3). This vector was introduced into mouse ES cells to generate chimeric mice carrying the HBhz-flox allele. Homologous recombination in ES cells was confirmed by Southern blot analysis (Fig. 4A). Chimeric mice were bred with C57BL/6J mice to produce heterozygous mice (HBhz-flox/+). Homozygous (HBhz-flox/hz-flox) mice were identified by PCR analysis (data not shown). To generate mice expressing the hzHB-EGF gene systemically, HBhz-flox/+ mice were crossed with CAG-Cre transgenic mice. Cre-mediated recombination was detected by PCR analysis (Fig. 4B). To confirm the expression of hzHB-EGF in HBhz/hz mice, RT-PCR analysis was performed (Fig. 4C). To detect the expression of HB-EGF in this assay more easily, the mouse skins were treated with tRA (Yamazaki et al., 2003). After Cre-mediated recombination, the expression of mHB-EGF was eliminated and alternative expressions of hzHB-EGF and GFP were detected. DT sensitivity of HBhz/hz mice was also confirmed (Fig. 5). No overt abnormalities, including lethality and infertility, were observed in the HBhz/hz mice, at least within 10 months after birth.
 View Details | Fig. 3. Gene-targeting strategy for the generation of hzHB-EGF knock-in mice. A LoxP-flanked mHB-EGF cDNA with the neomycin-resistance gene (neo) and intron 5 of the mHB-EGF gene as a splicing donor site (SD) was fused to the first exon of the mHB-EGF gene. The hzHB-EGF cDNA linked to the GFP cDNA via the internal ribosome entry site (IRES) was inserted downstream of the mHB-EGF cDNA. The targeting vector also contained the DT A-fragment (DT-A) gene. Cre-mediated recombination resulted in the deletion of the mHB-EGF cDNA and the neo cassette. Instead, hzHB-EGF and the GFP gene were expressed. Exon sequences and the loxP site are shown as gray boxes and triangles, respectively. Probes for a 5'-arm and 3'-arm are shown as black boxes. Primers for p1 and p2 are shown as arrowheads. H, HindIII; V, EcoRV; SI, SpeI; X, XhoI; E, EcoRI; S, SacII. |
 View Details | Fig. 4. Characterization of hzHB-EGF knock-in mice. (A) Southern blot analysis of homologous recombination in ES cells. The genomic DNA was digested with HindIII for hybridization with the 5' probe or SpeI for hybridization with the 3' probe. The 5' probe yields a 19-kb fragment from the wild-type allele and a 14-kb fragment from the hz-flox allele, while the 3' probe yields a 32-kb fragment from the wild-type allele and an 18-kb fragment from the hz-flox allele. SpeI-digested DNA was also analyzed with an internal probe corresponding to a sequence of the neo cassette. As a result, a 14-kb fragment was detected from the hz-flox allele, but no fragment was detected from the wild-type allele. (B) PCR analysis of genomic DNA from adult mouse tails to confirm Cre-mediated recombination. PCR analysis was performed using primer sets for wild-type allele-specific amplification (p1 and p2), GFP cDNA amplification and neo cassette amplification. To generate mice expressing hzHB-EGF, HBhz-flox/+ mice were crossed with CAG-Cre transgenic mice. In the presence of Cre recombinase, the band corresponding the neo gene disappeared. (C) RT-PCR analysis of the expression of hzHB-EGF. Skins from wild-type (+/+) and HBhz/hz mice (hz/hz) were treated with tRA for 4 days. Total RNA extracts from these skins were analyzed by RT-PCR using the indicated primer sets as follows: hHB-EGF, for hHB-EGF-specific amplification; mHB-EGF, for mHB-EGF-specific amplification; HB-EGF, for amplification of both human and mouse HB-EGF; GFP, for amplification of GFP; G3PDH, for amplification of G3PDH (loading control). In HBhz/hz mice, the expression of mHB-EGF is eliminated and hzHB-EGF and GFP are expressed instead. |
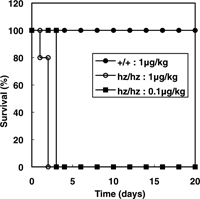 View Details | Fig. 5. DT sensitivity of hzHB-EGF knock-in mice. Survival curves of wild-type (+/+) and HBhz/hz (hz/hz) mice intraperitoneally injected with DT at various doses. All mice were injected with DT at 9–10 weeks of age (n=5). |
It should be noted that HBhz-flox/hz-flox mice unexpectedly showed a leaky expression of hzHB-EGF as well as mHB-EGF and were therefore sensitive to DT (data not shown). Consequently, the generated HBhz-flox/hz-flox mice are not appropriate for conditional expression of hzHB-EGF in a tissue-specific manner.
We used the generated HBhz/hz mice to examine whether host-derived stromal HB-EGF contributes to tumor growth. For this purpose, the following xenograft and allograft tumor models were evaluated. For the xenograft model, the human colon cancer cell line HT-29 cells were used. In this model, CRM197 should neutralize HT-29-derived hHB-EGF but not stroma-derived mHB-EGF when HT-29 cells are injected into nu/nu mice, whereas it should neutralize both HT-29-derived hHB-EGF and stroma-derived hzHB-EGF when HT-29 cells are injected into HBhz/hz;nu/nu mice. HT-29 cells express HB-EGF but the expression level is much lower than those in various other cell lines, including ovarian cancer cell lines (Yotsumoto et al., 2008). Consequently, we expected that the contribution of stromal HB-EGF to tumor growth would be observed more prominently using HT-29 cells than using other cell lines expressing high levels of HB-EGF. From 1 week after a subcutaneous injection of HT-29 cells, nu/nu mice and HBhz/hz;nu/nu mice were treated with CRM197 at 0.2 mg/kg every day. CRM197 significantly suppressed the tumor growth in HBhz/hz;nu/nu mice but did not have this effect in nu/nu mice when the tumor growths were compared between the mice with and without CRM197 treatment (Fig. 6A). These results suggested that HB-EGF derived from the host stroma predominantly contributed to the tumor growth of HT-29 cells, rather than the HB-EGF derived from the HT-29 cells themselves.
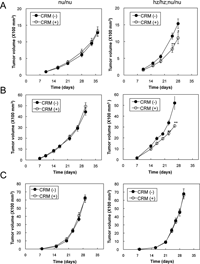 View Details | Fig. 6. Suppression of tumor growth by CRM197 administration. (A-C) HT29 (A), MTT (B) or LLC (C) cells were subcutaneously injected into nude mice (nu/nu) or HBhz/hz;nu/nu mice (hz/hz;nu/nu). From 1 week after the cell injection, 0.2 mg/kg of CRM197 or control saline was injected intraperitoneally every day. The tumor volumes were measured as described in Materials and Methods. Bars: SE (n=8). *p<0.05; **p<0.02. |
To further elucidate whether the host stroma-derived HB-EGF contributed to the tumor growth, we used allograft models in which mouse carcinoma cell line MMT and LLC cells were injected subcutaneously into nu/nu mice and HBhz/hz;nu/nu mice, followed by administration of CRM197. In these models, CRM197 should not neutralize either cancer cell-derived mHB-EGF or stroma-derived mHB-EGF when the cells are injected into nu/nu mice, whereas it should only neutralize stroma-derived hzHB-EGF when the cells are injected into HBhz/hz;nu/nu mice. While CRM197 did not inhibit the tumor growth of MMT cells in nu/nu mice, it significantly suppressed the tumor growth of MMT cells in HBhz/hz;nu/nu mice (Fig. 6B). MMT cells induced HB-EGF expression in the cancer-associated stroma, whereas LLC cells did not (Fig. 1). Consistent with the loss of HB-EGF expression in the stromal region, tumors derived from LLC cells were not suppressed by CRM197 in HBhz/hz;nu/nu mice, similar to the case for nu/nu mice (Fig. 6C). These results indicate that HB-EGF expressed in the cancer stroma plays a significant role in tumor growth, either directly or indirectly. Although further studies are required to clarify the role of stromal HB-EGF, we can nevertheless conclude that HB-EGF expressed in the cancer stroma could represent a potential molecular target for future cancer therapeutic strategies.
The generated HBhz/hz mice will be useful for pharmacological studies of therapeutic agents targeting hHB-EGF. The clinical development of CRM197 as an anticancer drug is in progress. We have shown in the present study that HB-EGF in the tumor stroma also contributes to tumor growth. Therefore, the knock-in mice would be more appropriate for evaluating the tumor-suppressive effects of CRM197 than conventional xenograft models using nude mice. The knock-in mice can also be used for evaluating the side effects of CRM197. CRM197 possesses a subtle toxicity (less than 10–6 of the toxicity of DT) (Kageyama et al., 2007). Single-dose toxicology tests showed that administration of CRM197 at high doses (greater than 10 mg/kg) resulted in death (Fig. 7A), while repeated-dose tests revealed that daily administration of CRM197 at 1 mg/kg to HBhz/hz mice caused body weight loss (Fig. 7B) and fibrosis of the cardiac muscle (Fig. 7C), although no overt abnormalities were observed in other tissues including the liver, kidney and brain (data not shown). Therefore, use of HBhz/hz mice enables evaluations of the efficacy and toxicity of CRM197, and possibly other therapeutic agents targeting HB-EGF, such as antibody-based drugs, in the same mice.
 View Details | Fig. 7. Toxicology of CRM197 evaluated using hzHB-EGF knock-in mice. (A) Survival curve for a single-dose study. The indicated doses of CRM197 were intraperitoneally injected into wild-type (+/+) and HBhz/hz (hz/hz) mice and their survival times were measured (n=5). (B) Effects of repeated doses of CRM197 on the body weights of mice. CRM197 at 0.2 or 1 mg/kg was intraperitoneally injected into wild-type (+/+) and HBhz/hz (hz/hz) mice every day for 28 days. The average body weights at 0 and 28 days are shown as ratios to the values of untreated mice. Bars indicate the mean±SE (n=4). ***p<0.005. (C) Histology of HBhz/hz mice treated with repeated doses of CRM197. CRM197 at 0.2 or 1 mg/kg was intraperitoneally injected into wild-type (+/+) and HBhz/hz (hz/hz) mice every day for 28 days. The hearts were then removed and sections were subjected to Azan-Mallory staining. Repeated doses of CRM197 at 1 mg/kg caused fibrosis of the cardiac muscle. |
HB-EGF is widely involved in physiological and pathological processes in the body. Although HB-EGF-null mice are available, these null mice show severe phenotypes in the heart and other tissues, and most of the mice die in the neonatal stage. HBhz/hz mice will be useful for future studies examining the roles of HB-EGF in the above-mentioned processes in combination with CRM197 or anti-HB-EGF antibodies.
We wish to thank M. Hamaoka, A. Kawai and Y. Esaki for technical assistance. The present study was supported in part by Grant-in-Aid 16207014 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to E. M.).
|