| To whom correspondence should be addressed: Kenji Irie, Department of Molecular Cell Biology, Graduate School of Comprehensive Human Sciences, University of Tsukuba, 1-1-1 Tennoudai, Tsukuba 305-8575, Japan. Tel: +81–29–853–3066, Fax: +81–29–853–3066 E-mail: kirie@md.tsukuba.ac.jp Abbreviations: CDS, coding sequence; eIF4G, translation initiation factor 4G; GBD, Gal4p DNA binding domain; hnRNP K, heterogeneous nuclear ribonucleoprotein K; KH, K homology; KHD1, KH-domain protein 1; mRNA, messenger RNA; nts, nucleotides; P-body, processing body; poly(A), polyadenosine; PCBP, poly(C)-binding protein; rRNA, ribosomal RNA; SDS-PAGE, SDS-polyacrylamide gel electrophoresis; SP, signal peptide; TAP, tandem-affinity purification; TBS, Tris-buffered saline; TM, transmembrane domain. |
Regulation of gene expression is not only achieved at the transcriptional level but also at the post-transcriptional level. In the nucleus, the primary transcript is processed by the addition of the 5'-cap structure, the removal of introns and the addition of a polyadenosine (poly(A)) tail. Upon export to the cytoplasm, the mature mRNA is subject to quality control and occasionally localized to particular subcellular compartments for translation. Finally, mRNAs are degraded in processing bodies (P-bodies), a process that must also be tightly controlled. The integration of all these regulatory steps ensures that a given mRNA produces the proper proteins at the right time and place. RNA-binding proteins play important roles in post-transcriptional regulation of gene expression (Glisovic et al., 2008; Halbeisen et al., 2008; Hogan et al., 2008; Keene, 2007; Siomi and Dreyfuss, 1997). Eukaryotic cells encode a large number of RNA-binding proteins, each of which has unique RNA-binding activity and protein-protein interaction characteristics. A yeast RNA-binding protein, KH-domain protein 1 (Khd1p), contains three K homology (KH) RNA-binding domains, and is most related to mammalian heterogeneous nuclear ribonucleoprotein K (hnRNP K) and poly(C)-binding proteins (PCBP1–4) (Bomsztyk et al., 2004; Makeyev and Liebhaber, 2002). Khd1p is required for efficient localization of ASH1 mRNA to the bud-tip in dividing yeast by possibly acting as a repressor of translation during mRNA transport (Irie et al., 2002; Paquin et al., 2007). ASH1 encodes a transcriptional repressor for HO endonuclease and thus prevents mating type switching in daughter cells (Bobola et al., 1996; Sil and Herskowitz, 1996). Khd1p/Hek2p is also involved in the regulation of the telomeric position effect and telomere length (Denisenko and Bomsztyk, 2002, 2008). More recently, Khd1p appears to regulate asymmetric expression of FLO11 to determine daughter cell fate during filamentous growth (Wolf et al., 2010). We have previously identified target mRNAs for Khd1p on a genome-wide level and found that Khd1p associates with hundreds of mRNAs comprising almost 20% of the yeast’s transcriptome (Hasegawa et al., 2008). A significant fraction of the potential Khd1p mRNA targets encode proteins localized to the cell periphery such as the cell wall and plasma membrane, and also nuclear proteins involved in transcriptional regulation.
The initiation of translation is triggered by the assembly of translation initiation factors at the 5'-cap of messages, which in turn recruit the small ribosomal subunit. Poly(A)-binding protein Pab1p is an essential, highly conserved protein that binds with high affinity to the poly(A) tail at the 3'-end of mRNAs (Kahvejian et al., 2001; Preiss and Hentze, 1998; Tarun and Sachs, 1995). Pab1p is a multifunctional protein and appears to be an important mediator of the multiple roles of the poly(A) tail in mRNA biogenesis, stability, and translation. The coordinated recruitment of factors at the poly(A) tail and the 5'-cap structure are required for efficient translation. Translation initiation factor 4G (eIF4G) associates with Pab1p and eIF4E, which is the cap binding protein, constructing an Pab1p-eIF4G-eIF4E initiation complex that enhances translation. Paquin et al. (2007) have demonstrated that Khd1p represses translation of ASH1 mRNA by inhibiting Pab1p-eIF4G-eIF4E complex formation. Khd1p binds to the coding sequence (CDS) of ASH1 mRNA and interacts with the C-terminal domain of eIF4G1 and thereby prevents the recruitment of ribosomes to the mRNA during transport to the bud-tip. At the bud-tip, Khd1p dissociates from ASH1 mRNA through phosphorylation mediated by the casein kinase Yck1p and consequently translational repression is released. Besides ASH1, at least 23 additional mRNAs were also known to be transported to the bud-tip of daughter cells, and some of these bud-localized mRNAs including MTL1 and MID2 mRNAs are potential Khd1p targets (Andoh et al., 2006; Hasegawa et al., 2008; Shepard et al., 2003; Takizawa et al., 2000).
While Khd1p negatively regulates expression of ASH1 mRNA by translational repression, Khd1p positively regulates expression of MTL1, possibly by stabilizing the mRNA (Hasegawa et al., 2008). MTL1 encodes a transmembrane protein, which is very similar to Mid2p. Mid2p and Mtl1p are thought to regulate cell integrity as cell membrane sensors for the Pkc1p-Mpk1p pathway (Lommel et al., 2004; Vilella et al., 2005). Although Khd1p binds both MTL1 and MID2 mRNAs, only MTL1 expression is regulated by Khd1p (Hasagawa et al., 2008). To further investigate how Khd1p regulates the stability of MTL1 mRNA, we searched for cis-acting elements in MTL1 mRNA and trans-acting factors that control the stability of MTL1 mRNAs. Our analyses revealed that MTL1 mRNA, but not MID2 mRNA, has a cis-acting element involved in destabilization by the decapping enzyme Dcp1/2p and the 5'-3' exonuclease Xrn1p and that Khd1p stabilizes MTL1 mRNA by binding to this element and possibly preventing Dcp1/2p and the Xrn1p exonuclease to degrade the mRNA.
Escherichia coli DH5α was used for DNA manipulations. Strains used in this study are described in Table I. Tandem-affinity purification (TAP)-tagged Mtl1p strain (BY4741 back ground; MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was obtained from Open Biosystems (Ghaemmaghami et al., 2003). Standard procedures were followed for yeast manipulations (Kaiser et al., 1994). The media used in this study included rich medium, synthetic complete medium (SC), and synthetic minimal medium (SD) (Kaiser et al., 1994). SC media lacking amino acids or other nutrients (e.g. SC-Ura corresponds to SC lacking uracil) were used to select transformants. SR and SG were identical to SC except that it contained raffinose and galactose, respectively, instead of 2% glucose. Recombinant DNA procedures were carried out as described (Sambrook et al., 1989).
Plasmids used in this study are described in Table II. Plasmid YCplac33-MTL1-TAP is YCplac33 carrying MTL1-TAP. Plasmids YCplac33-MTL1 (Δ106–1032)-TAP, YCplac33-MTL1 (Δ532–1032)-TAP, YCplac33-MTL1 (Δ331–531)-TAP, YCplac33-MTL1 (Δ532–723)-TAP, YCplac33-MTL1 (Δ724–1032)-TAP, and YCplac33-MTL1 (Δ724–792)-TAP were used to map cis-acting elements controlling MTL1 mRNA stability. Plasmid YCplac33-MID2-TAP is YCplac33 carrying MID2-TAP. Plasmid YCplac33-MTL1/MID2-TAP is YCplac33 carrying MID2 (1–75)-MTL1 (475–1083)-MID2 (673–1128)-TAP, in which the region of nucleotides (nts) 76–672 of MID2 coding sequence was replaced with nts 475–1083 of MTL1 coding sequence. YCplac33- MID2/MTL1-TAP is YCplac33 carrying MID2 (1–672)-MTL1 (1084–1653)-TAP, in which the region of nts 673–1128 of MID2 coding sequence encoding transmembrane and cytoplasmic domains of Mid2p was replaced with nts 1084–1653 of MTL1 coding sequence encoding transmembrane and cytoplasmic domains of Mtl1p. Plasmid pBG4D expresses HA-tagged-Gal4p DNA binding domain (GBD) from ADH promoter. Plasmid pBG4D-MTL1 (532–1032) expresses HA-tagged-Gal4p DNA binding domain fused with Mtl1 (532–1032). Plasmid pBG4D-SL-MTL1 (532–1032) is a derivative of plasmid pBG4D-MTL1 (532–1032) containing a short stem-loop sequence, GAGCAGGAGACTGCTC, before ATG codon.
Deletions of KHD1, CCR4, POP2, SKI2, SKI3, SKI7, SKI8, and XRN1 were constructed by PCR-based gene-deletion method (Baudin et al., 1993, Schneider et al., 1996, Sakumoto et al., 1999, Tadauchi et al., 2001). Primer sets were designed such that 46 bases at the 5' end of the primers were complementary to those at the corresponding region of the target gene, and 20 bases at their 3' end were complementary to the pUC19 sequence outside the polylinker region in the plasmid pCgLEU2 containing the C. glabrata LEU2 gene as selectable marker. Primer sets for PCR were designed to delete the ORF completely. The PCR products were transformed into the wild-type strain and selected for Leu+. The GALp-DCP1 and GALp-DCP2 strains were prepared by the method of Longtine et al. (1998) using pFA6a-kanMX6-GAL1p-3HA.
Extracts were prepared by the method of Kushnirov (2000). Extracts were subjected to SDS-PAGE on 8% acrylamide gels followed by electroblotting onto an Immobilonmembrane (MILLIPORE). To detect the TAP-tagged proteins, the blots were blocked for 30 min at room temperature with TBS-M buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 5% nonfat dry milk) and further incubated with 1:4000 diluted peroxidase-anti-peroxidase soluble complex (PAP; Sigma) in TBS-M buffer overnight at 4°C. After three final washes with TBS buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl), blots were developed with the enhanced chemiluminescence detection kit (Millipore). To detect HA-tagged proteins, the membrane was incubated with anti-HA antibody (Santa Cruz Biotechnology Inc.: 1:2000) and then with HRP-labeled secondary antibody (Calbiochem: 1:4000). To control for equal loading of the lanes, the blots were probed with anti-Mcm2 antibody (Santa Cruz Biotechnology Inc.: 1:1000) and peroxidase-conjugated secondary antibody (Calbiochem: 1:3000).
Total RNA were prepared from cells using Isogen reagent (Nippon Gene) and RNeasy mini kit (Qiagen). RNA samples were separated by 1% denatured agarose gel electrophoresis and transferred to nylon membrane. RNA was then hybridized using digoxigenin (DIG)-labeled antisense probe. The primer sets, TN264 (GGTCGACGGATCCCCGGGTTA) and TN266 (TCACTGATGATTCGCGTCTAC), and i1008 (ATGAAGCTACTGTCTTCTATCGA) and i1009 (CGATACAGTCAACTGTCTTTGAC), were used to detect transcript containing TAP and GBD, respectively. After washing and blocking, the membrane was incubated with alkaline phosphatase-conjugated anti-DIG antibody, and the signal was detected by enhanced chemiluminescence.
Cells were grown to an OD600 of 0.5 in the appropriate medium and prepared for microscopy. For observation, cells were washed twice with SC supplemented with amino acids and glucose and resuspended in the same medium. For glucose depletion conditions, cells were washed in SC supplemented with appropriate amino acids without glucose and resuspended in the same medium for 10 min before being collected. Cells were examined by phase contrast microscopy using a ×100 magnification and NA 1.4 lens (Carl Zeiss). Images were captured with a cooled charged-coupled device (Carl Zeiss) and digital images displayed with Adobe Photoshop (Adobe Systems Inc.).
We have previously shown that Mtl1p and MTL1 mRNA levels are increased in Khd1p-overexpressing cells and decreased in khd1Δ mutant cells (Hasegawa et al., 2008). MTL1 mRNA has at least two Khd1p-binding regions, nts 331–533 and 534–1031 (numbering starts at the first nucleotide of the start codon), within the coding region of MTL1 mRNA (Hasagawa et al., 2008). We then examined whether these two regions were involved in the Khd1p-mediated control of MTL1 mRNA stability (Fig. 1A). For this purpose, we used MTL1-TAP gene, in which the TAP-tag followed by the ADH 3'UTR was fused to the 3' end of MTL1 open reading frame on a plasmid. We introduced MTL1-TAP into wild-type and khd1Δ mutant cells and compared the Mtl1-TAP protein levels. As observed previously (Hasegawa et al., 2008), in khd1Δ mutant cells, the Mtl1-TAPp level derived from full-length MTL1 was reduced compared to wild-type cells (Fig. 1B, lanes 1 and 2). Likewise, also the MTL1 mRNA was reduced in khd1Δ mutant cells (Fig. 1C, lanes 1 and 2). Surprisingly, an in-frame internal deletion construct, MTL1 (Δ106–1032)-TAP, in which the two Khd1p-binding regions are missing, expressed the Mtl1 (Δ106–1032)-TAP protein and mRNA at similar levels in wild-type and khd1Δ mutant cells (Fig. 1B and 1C, lanes 3 and 4). We further created other deletion constructs, MTL1 (Δ532–1032) and MTL1 (Δ331–531), which more specifically lack the Khd1p-binding region, and examined the Mtl1-TAP protein and mRNA levels. As seen before with the MTL1 Δ106–1032 construct, the protein and mRNA levels derived from the MTL1 (Δ532–1032)-TAP construct were similar in wild-type and khd1Δ mutant cells (Fig. 1B and 1C, lanes 7 and 8). However, the protein and mRNA levels derived from the MTL1 (Δ331–531)-TAP construct were reduced in khd1Δ mutant cells compared to wild-type cells, similarly to the full-length MTL1-TAP construct (Fig. 1B and 1C, lanes 5 and 6). These results suggest that the MTL1 (532–1032) region is required for the destabilization of MTL1 mRNA.
 View Details | Fig. 1. Deletion of the coding region 532–1032 of MTL1 mRNA restored the decreased Mtl1p and MTL1 mRNA levels caused by khd1Δ mutation. (A) Schematic representation of the location of the two Khd1p-binding regions in MTL1 mRNA coding sequence and the three fragments deleted Khd1p-binding region in MTL1 mRNA. SP, signal peptide; TM, transmembrane domain. (B) Effect of deletion of the MTL1 coding region on Mtl1-TAP protein levels. (C) Effect of deletion of the MTL1 coding region on MTL1 mRNA levels. Each yeast cell harboring the indicated plasmid was grown in SC-Ura medium at 25°C, then incubated for 4 hours at 37°C, and harvested. Western blot analysis was performed to quantitate the level of Mtl1-TAP protein (upper panel) and Mcm2 protein (lower panel) as a quantity control. MTL1 transcripts were quantified by Northern blotting as described in Materials and Methods. rRNA was included as a quantity control. Lane 1, WT (MTL1-TAP Full-length); lane 2, khd1Δ (MTL1-TAP Full-length); lane 3, WT (MTL1Δ 106–1032-TAP); lane 4, khd1Δ (MTL1Δ 106–1032-TAP); lane 5, WT (MTL1Δ 331–532-TAP); lane 6, khd1Δ (MTL1Δ 331–532-TAP); lane 7, WT (MTL1Δ 532–1032-TAP); and lane 8, khd1Δ (MTL1Δ 532–1032-TAP). |
To further confirm that the MTL1 (532–1032) sequence is involved in the Khd1p-mediated stability control of mRNA, we examined whether addition of the MTL1 (532–1032) sequence into other mRNAs conferred instability of mRNA (Fig. 2). For this purpose, we first made chimeric constructs comprised of the MTL1 and MID2 genes. MID2 encodes a transmembrane protein that has a similar structure and function as Mtl1p (Fig. 2A; Lommel et al., 2004; Vilella et al., 2005). We have previously reported that Mid2p and MID2 mRNA levels are not affected by khd1Δ mutation (Fig. 2B and 2C, lanes 3 and 4), although Khd1p directly binds to the regions 140–319 and 387–559 of the coding sequence of MID2 mRNA (Hasegawa et al., 2008). We made a MTL1/MID2 chimeric construct, in which nts 76–672 of the MID2 CDS containing two Khd1p-binding regions was replaced with nts 475–1083 of MTL1 CDS containing the MTL1 (532–1032) sequence (Fig. 2A, MTL1/MID2 chimera). We also made another chimeric construct MID2/MTL1, in which nts 560-1128 of MID2 CDS coding for the transmembrane and cytoplasmic domains of Mid2p was replaced with nts 1084–1653 of MTL1 coding sequence, which likewise code for the transmembrane and cytoplasmic domains of Mtl1p (Fig. 2A, MID2/MTL1 chimera). We then examined protein and mRNA levels of MTL1/MID2 and MID2/MTL1 chimeric constructs in wild-type and khd1Δ mutant cells (Fig. 2B and 2C). Both protein and mRNA levels derived from the MTL1/MID2 construct containing the MTL1 (532–1032) sequence were decreased in khd1Δ mutant cells compared to those in wild-type cells (Fig 2B and 2C, lanes 5 and 6). In contrast, those derived from the MID2/MTL1 construct were not altered in khd1Δ mutant cells compared to wild-type cells (Fig. 2B and 2C, lanes 7 and 8). These results indicate that the MTL1 (532–1032) sequence is sufficient for the Khd1p-mediated control of mRNA stability.
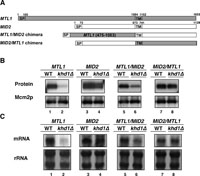 View Details | Fig. 2. MTL1 (532–1032) region is sufficient for destabilization in khd1Δ mutants. (A) Description of the two versions of chimeric constructs with MTL1 sequence and MID2 sequence. SP: signal peptide, TM: transmembrane domain. (B) Effect of MTL1 (532–1032) region on the TAP-tagged protein levels. (C) Effect of MTL1 (532–1032) region on the TAP-tagged mRNA levels. Each yeast cell harboring the indicated plasmid was grown in SC-Ura medium at 25°C, then incubated for 4 hours at 37°C, and harvested. Western blot analysis was performed to quantitate the level of TAP-tagged protein (upper panel) and Mcm2 protein (lower panel) as a quantity control. Transcripts were quantified by Northern blotting as described in Materials and Methods. rRNA was included as a quantity control. Lane 1, WT (MTL1-TAP); lane 2, khd1Δ (MTL1-TAP); lane 3, WT (MID2-TAP); lane 4, khd1Δ (MID2-TAP); lane 5, WT (MTL1/MID2-TAP); lane 6, khd1Δ (MTL1/MID2-TAP); lane 7, WT (MID2/MTL1-TAP); and lane 8, khd1Δ (MID2/MTL1-TAP). |
To further confirm that the MTL1 (532–1032) sequence is sufficient for the Khd1p-mediated control of mRNA stability, we constructed a fusion gene comprised of the GAL4 DNA binding domain (GBD) with a part of the MTL1 coding sequence (532–1032). GAL4 mRNA is not a target mRNA for Khd1p (Hasegawa et al., 2008). Both protein and mRNA levels derived from the MTL1 (532–1032)-GBD construct were decreased in khd1Δ mutant cells compared to that in wild-type cells, while those derived from the GBD vector were not altered in khd1Δ mutant cells compared to wild-type cells (Fig. 3A and 3B, lanes 1–4). These results confirm that the MTL1 (532–1032) sequence is sufficient for the Khd1p-mediated control of mRNA stability.
 View Details | Fig. 3. MTL1 (532–1032) region is sufficient for destabilization in khd1Δ mutants in GBD constructs. (A) Effect of MTL1 (532–1032) region on the GBD-fusion protein levels. (B) Effect of MTL1 (532–1032) region on the GBD mRNA levels. Each yeast cell harboring the indicated plasmid was grown in SC-Trp medium at 25°C, then incubated for 4 hours at 37°C, and harvested. Western blot analysis was performed to quantitate the level of GBD-fusion protein (upper panel) and Mcm2 protein (lower panel) as a quantity control. Transcripts were quantified by Northern blotting as described in Materials and Methods. rRNA was included as a quantity control. Lane 1, WT (GBD); lane 2, khd1Δ (GBD); lane 3, WT (MTL1-GBD); lane 4, khd1Δ (MTL1-GBD); lane 5, WT (Stem-loop-MTL1-GBD); and lane 6, khd1Δ (Stem-loop-MTL1-GBD). |
We next examined whether translation is involved in the instability of the MTL1 (532–1032) mRNA in khd1Δ mutant cells. We inserted a short stem-loop structure in the 5'UTR of the MTL1 (532–1032)-GBD construct in order to reduce its level of translation. The insertion of the stem-loop structure abolished the protein expression (Fig. 3A, lanes 5 and 6). However, the mRNA levels derived from the stem-loop-MTL1 (532–1032)-GBD construct were also decreased in khd1Δ mutant cells compared to that in wild-type cells, similarly to the MTL1 (532–1032)-GBD construct (Fig. 3B, lanes 5 and 6). Thus, the instability of the MTL1 (532–1032) mRNA in khd1Δ mutant cells does not involve a translation.
The MTL1 (532–1032) region contains seven CNN repeats (Fig. 4A), and this region translates into an amino acid sequence containing stretches of serine residues (Fig. 4A). To examine whether these CNN repeats in the MTL1 (532–1032) region were involved in the Khd1p-mediated control of MTL1 mRNA stability, we created shorter deletion constructs, MTL1 (Δ532–732), MTL1 (Δ724–1032), and MTL1 (Δ724–792) (Fig. 4A). We then examined the MTL1-TAP mRNA levels in wild-type and khd1Δ mutant cells (Fig. 4B). The mRNA level derived from the MTL1 (Δ523–723)-TAP construct lacking the first two CNN repeats was reduced in khd1Δ mutant cells compared to wild-type cells (Fig. 4B, lanes 3 and 4), similarly to the full-length MTL1-TAP construct (Fig. 4B, lanes 1 and 2). In contrast, the mRNA level derived from the MTL1 (Δ732–1032)-TAP construct lacking latter 5 CNN repeats was similar in wild-type and khd1Δ mutant cells (Fig. 4B, lanes 5 and 6), as seen with the MTL1 (Δ532–1032) construct (Fig. 1C). Thus, the latter 5 CNN repeats are more important for the Khd1p-mediated control of MTL1 mRNA stability. The mRNA level derived from the MTL1 (Δ724–792)-TAP construct lacking a longest CNN repeat (CNN23) was still partially reduced in khd1Δ mutant cells compared to wild-type cells (Fig. 4B, lanes 7 and 8), but its reduction was moderate than that seen with the full-length MTL1-TAP construct (Fig. 4B, lanes 1, 2, 7, and 8). Thus, the longest CNN repeat seems to be involved in both the destabilization of mRNA in khd1Δ cells and the stabilization of mRNA through the binding of Khd1p. The other four CNN repeats, CNN7, CNN6, CNN8, and CNN6, strengthen the effect of destabilization of mRNA in khd1Δ cells and stabilization by Khd1p.
 View Details | Fig. 4. Shorter deletion analyses of CNN repeats in the MTL1 (532–1032) region. (A) Schematic representation of the location of CNN repeats in the MTL1 (532–1032) region. (B) Effect of deletion of CNN repeats on MTL1 mRNA levels. Each yeast cell harboring the indicated plasmid was grown in SC-Ura medium at 25°C, then incubated for 4 hours at 37°C, and harvested. MTL1 transcripts were quantified by Northern blotting as described in Materials and Methods. rRNA was included as a quantity control. Lane 1, WT (MTL1-TAP Full length); lane 2, khd1Δ (MTL1-TAP Full length); lane 3, WT (MTL1 Δ532–723-TAP); lane 4, khd1Δ (MTL1 Δ532–723-TAP); lane 5, WT (MTL1 Δ724–1032-TAP); lane 6, khd1Δ (MTL1 Δ724–1032-TAP); lane 7, WT (MTL1 Δ724–792-TAP); and lane 8, khd1Δ (MTL1 Δ724–792-TAP). |
We next searched for trans-acting factors controlling the stability of MTL1 mRNA. For this purpose, we constructed mutant cells lacking genes known to be involved in mRNA decay and measured the MTL1 mRNA levels in these mutants. In budding yeast, it has been believed that most cytoplasmic mRNA decay is initiated by poly(A) tail shortening, which is catalyzed by Ccr4p-Notp deadenylase complex (Coller and Parker, 2004). After poly(A) tail shortening, these mRNAs are subject to either 5' to 3' or 3' to 5' decay. The former case depends on the 5'-3' exonuclease Xrn1p. The latter case depends on the 3'-exonuclease complex called exosome containing Ski protein complex. Therefore, we constructed single mutants of CCR4, CAF1/POP2, SKI2, SKI3, SKI7, SKI8, and XRN1 and double mutants of these genes and KHD1 in a strain harboring MTL1-TAP gene, and measured the MTL1-TAP mRNA levels (Fig. 5). As described above, the level of MTL1 mRNA in khd1Δ mutant was reduced compared with wild-type cells (Fig. 5A, lanes 1, 2, 5, and 6, WT vs khd1Δ). Mutations in CCR4 and CAF1/POP2 genes did not restore the decreased MTL1 mRNA levels caused by khd1Δ mutation (Fig. 5A, lanes 2, 4, 6, and 8, khd1Δ vs khd1Δ ccr4Δ khd1Δ vs khd1Δ caf1Δ). Similarly, mutations in SKI genes also did not restore the decreased MTL1 mRNA levels caused by khd1Δ mutation (Fig. 5B, lanes 3-10, khd1Δ vs khd1Δ skiΔ). In contrast, mutation in XRN1 restored the decreased MTL1 mRNA levels in khd1Δ mutants (Fig. 5C, lanes 2 and 4, khd1Δ vs khd1Δ xrn1Δ). These data demonstrate that destabilization of MTL1 mRNA in khd1Δ mutants is mediated by 5'to 3' decay, but not 3'to 5' decay.
 View Details | Fig. 5. Mutations in DCP1, DCP2 and XRN1 restored the decreased MTL1 mRNA levels caused by khd1Δ mutation. MTL1 transcripts were quantified by Northern blotting as described in Materials and Methods. rRNA was included as a quantity control. (A) Effect of the ccr4Δ and caf1Δ mutations on levels of MTL1 mRNA. Lane 1, WT (MTL1-TAP); lane 2, khd1Δ (MTL1-TAP); lane 3, ccr4Δ (MTL1-TAP); lane4, ccr4Δkhd1Δ (MTL1-TAP); lane 5, WT (MTL1-TAP); lane 6, khd1Δ (MTL1-TAP); lane7, caf1Δ (MTL1-TAP); and lane 8, caf1Δkhd1Δ (MTL1-TAP), (B) Effect of the ski2Δ, ski3Δ, ski7Δ, and ski8Δ mutations on levels of MTL1 mRNA. Lane 1, WT (MTL1-TAP); lane 2, khd1Δ (MTL1-TAP); lane 3, ski2Δ (MTL1-TAP); lane 4, ski2Δkhd1Δ (MTL1-TAP); lane 5, ski3Δ (MTL1-TAP); lane 6, ski3Δkhd1Δ (MTL1-TAP); lane 7, ski7Δ (MTL1-TAP); lane 8, ski7Δkhd1Δ (MTL1-TAP); lane 9, ski8Δ (MTL1-TAP); and lane 10, ski8Δkhd1Δ (MTL1-TAP), (C) Effect of the xrn1Δ mutation on levels of MTL1 mRNA. Lane 1, WT (MTL1-TAP); lane 2, khd1Δ (MTL1-TAP); lane 3, xrn1Δ (MTL1-TAP); and lane 4, xrn1Δkhd1Δ (MTL1-TAP), (D) Effect of the dcp1 and dcp2 mutations on levels of MTL1 mRNA. Lane 1, WT (MTL1-TAP); lane 2, khd1Δ (MTL1-TAP); lane 3, GAL-DCP1 khd1Δ (MTL1-TAP); and lane 4, GAL-DCP2 khd1Δ (MTL1-TAP). |
The observation that the destabilization of MTL1 mRNA in khd1Δ mutants is mediated by 5' to 3' decay raised the possibility that the decapping reaction may be involved in MTL1 mRNA destabilization in khd1Δ mutants. Decapping is catalyzed by the decapping enzymes Dcp1p and Dcp2p. However, cells lacking DCP1 or DCP2 gene were lethal in the strains we used. Therefore, to elucidate the involvement of DCP1 and DCP2 genes in MTL1 mRNA destabilization in khd1Δ mutants, we carried out the transcriptional shut-off analysis using the GAL1 promoter. The transcription of genes under the control of GAL1 promoter is activated in the presence of galactose, but, conversely, repressed in the presence of glucose. Therefore, the transcription of DCP1 and DCP2 genes under the control of GAL1 promoter can be abolished by nutrient substitution of galactose to glucose. In cells incubated for 5 hours after the shut-off of the transcription of the DCP genes, the MTL1 mRNA levels in khd1Δ mutants showed similar levels to wild-type cells (Fig. 5D, lanes 2–4, khd1Δ vs khd1Δ dcp). This data demonstrate that MTL1 mRNA destabilization in khd1Δ mutants also involved a decapping reaction. Taken together, these data revealed that the decapping enzyme Dcp1/2p and the 5' to 3' exonuclease Xrn1p are involved in MTL1 mRNA destabilization in khd1Δ mutants.
It has been reported that the decapping and decay of mRNA occurs in the cytoplasmic foci, termed P-bodies, which are aggregates of nontranslating mRNAs associated with translational repressors and the mRNA decapping machinery (Eulalio et al., 2007; Parker and Sheth, 2007). In budding yeast, the decapping enzyme Dcp1/2p and the 5'-3' exonuclease Xrn1p are known to localize to the P-bodies. During glucose deprivation, translation is repressed and untranslated mRNAs accumulate in P-bodies (Parker and Sheth, 2007). Polyadenylated mRNAs, Pab1p, eIF4E, and eIF4G as well as Dcp1/2p and Xrn1p also accumulate in the P bodies during glucose deprivation (Brengues and Parker, 2007). Since our results suggested that Dcp1/2p and Xrn1p are involved in MTL1 mRNA destabilization in khd1Δ mutants, we reasoned that Khd1p would localize to the P-bodies during glucose deprivation. Therefore, we constructed cells coexpressing GFP-tagged version of Khd1p and RFP-tagged version of Dcp1p and examined their localization before and after glucose deprivation. In glucose-containing medium, both Khd1p-GFP and Dcp1p-RFP diffusely localized in cytoplasm (data not shown). After glucose depletion, both Khd1p-GFP and Dcp1p-RFP displayed some large particles in cytoplasm and Khd1p-GFP particles overlapped with Dcp1p-RFP particles (Fig. 6). Since the Dcp1p particle is a marker for P-bodies, these results indicate that Khd1p accumulates in P-bodies during glucose deprivation. This observation supports the possibility that MTL1 mRNA is destabilized by the decapping enzyme and the 5'-3' exonuclease, and that Khd1p stabilizes MTL1 mRNA through binding to the region involved in destabilization.
 View Details | Fig. 6. Khd1p localizes to P-bodies Colocalization of Khd1p-GFP with Dcp1p-RFP. Wild-type cells coexpressing Khd1p-GFP (green) and Dcp1p-RFP (red) were grown in medium without glucose for 10 min. Yeast strains; WT (KHD1-GFP) [YCplac33-DCP1-mRFP]. Bars, 5 μm. |
We have previously shown that Khd1p preferentially associates with CNN repeats, repetitive patterns of cytosines at every third position, in the coding regions of target mRNAs (Hasegawa et al., 2008). This is in contrast to previously characterized KH domain proteins, such as hnRNP K and ZBP-1, that have been reported to bind to sequences in the 3'UTR of mRNAs (Berglund et al., 1997; Buckanovich and Darnell, 1997; Holcik and Liebhaber, 1997; Kanamori et al., 1998; Ostareck et al., 1997; Ross et al., 1997). Although Khd1p binds to coding sequences of target mRNAs including ASH1, MID2, and MTL1 mRNAs, the effects of its binding are different in the target mRNAs. While Khd1p negatively regulates gene expression of Ash1p by the translational repression, Khd1p positively regulates gene expression of Mtl1p by stabilization of the mRNA. Neither KHD1 overexpression nor khd1Δ mutation affects the expression of other target mRNAs including MID2, although Khd1p associates and colocalizes with them (Hasegawa et al., 2008). We have shown here that MTL1 mRNA has a cis-acting element that is required and sufficient to mediate the destabilization. While a deletion of the coding region 532–1032 of MTL1 mRNA restored the decreased Mtl1p and MTL1 mRNA levels caused by khd1Δ mutation, addition of the MTL1 (532–1032) sequence into MID2 and GAL4 mRNAs conferred instability of mRNA in khd1Δ mutants. The MTL1 (532–1032) region contains Khd1p-binding CNN repeats, and this region translates into an amino acid sequence containing stretches of serine residues. A shorter deletion of the coding region 724–792 of MTL1 mRNA containing a longest CNN repeat partially restored the decreased MTL1 mRNA levels caused by khd1Δ mutation. Thus, the longest CNN repeat seems to be involved in both the destabilization of mRNA in khd1Δ cells and the stabilization of mRNA through the binding of Khd1p. Additional four other CNN repeats strengthen the effect of destabilization of mRNA in khd1Δ cells and stabilization by Khd1p. Since both MTL1 and MID2 mRNAs are bound to Khd1p and encode similar serine-rich membrane proteins, it remains unclear why these two mRNAs are differently regulated by Khd1p. A stretch of Ser residues in the MTL1 (532–1032) region is longer than that in MID2 mRNA and deletion of longest CNN repeat (724–792) partially restored the decreased MTL1 mRNA level caused by khd1Δ mutation. Thus, the longer Ser stretch may cause the destabilization of the mRNA via translation. Since the non-translated mRNA derived from the stem-loop-MTL1 (532–1032)-GBD construct was still destabilized in khd1Δ mutant, a specific secondary structure within MTL1 mRNA may contribute to the destabilization of mRNA in a translation-independent manner.
Decapping is a key step in mRNA turnover because the removal of the 5'-cap structures by the decapping enzyme allows 5' to 3' exonucleolytic decay. Alternatively, the 5'-cap structure plays an important role in the initiation of translation that needs the accumulation of translation initiation factors in the 5'-cap structure. Thus, the protection of the 5'-cap structure is important for keeping the stability and the translational ability of mRNA. We found here that the destabilization of MTL1 mRNA in khd1Δ mutants involved a decapping enzyme Dcp1/2p and a 5' to 3' exonuclease Xrn1p, but not Ccr4p and Caf1p/Pop2p deadenylases and Ski protein complex functioning in 3' to 5' decay. This result suggests that the repression of the decapping reaction by the decapping enzyme is important for the MTL1 mRNA stabilization by Khd1p. We found that Khd1p colocalized with Dcp1p at P-bodies during glucose deprivation. Polyadenylated mRNA, Pab1p, eIF4E, and eIF4G are also known to accumulate in P-bodies during glucose deprivation (Brengues and Parker, 2007). Taken together, our results presented here suggest that Khd1p protects MTL1 mRNA from decapping and degradation at P-bodies.
In conclusion, our results suggest that MTL1 mRNA has a cis-acting element involved in destabilization by the decapping enzyme and the 5'-3' exonuclease, and Khd1p stabilizes MTL1 mRNA through binding to the region involved in destabilization.
We are grateful to Dr. Kunihiro Matsumoto (Nagoya University, Japan) and Dr. André P. Gerber (ETH Zurich, Switzerland) for critically reading this manuscript. K.I. is supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (2009–2010).
|