| To whom correspondence should be addressed: Mitsunori Fukuda, Laboratory of Membrane Trafficking Mechanisms, Department of Developmental Biology and Neurosciences, Graduate School of Life Sciences, Tohoku University, Aobayama, Aoba-ku, Sendai, Miyagi 980-8578, Japan. Tel: +81–22–795–7731, Fax: +81–22–795–7733 E-mail: nori@m.tohoku.ac.jp Abbreviations: CA, constitutive active; CC, coiled-coil; CN, constitutive negative; DENN, differentially expressed in normal and neoplastic; EGFP, enhanced green fluorescent protein; FYVE, Fab1, YOTB/ZK632.12, Vac1, and EEA1; GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; GFP, green fluorescent protein; GST, glutathione S-transferase; HRP, horseradish peroxidase; mStr, monomeric Strawberry; NGF, nerve growth factor; RBD35, Rab-binding domain specific for Rab35; RFP, red fluorescent protein; RUFY, RUN and FYVE domain-containing; RUN, RPIP8, UNC-14, and NESCA; RUSC, RUN and SH3 domain-containing; TBC, Tre-2/Bub2/Cdc16. |
The small GTPases of the Ras superfamily play a crucial role in a variety of cellular events, including cell proliferation, cell morphological change, intracellular membrane trafficking, and nucleocytoplasmic transport (reviewed in Takai et al., 2001). In general, these small GTPases function as a molecular switch by cycling between two nucleotide (GTP/GDP)-bound states, and the GTP-bound active form promotes specific cellular events through interaction with a specific effector molecule. The Rab small GTPases constitute the largest family of members of the Ras superfamily, and they are implicated in the control of intracellular membrane trafficking (reviewed in Stenmark, 2009; Zerial and McBride, 2001). Approximately 60 Rab members have been reported in humans and mice (Bock et al., 2001; Itoh et al., 2006; Pereira-Leal and Seabra, 2001), and each member is thought to regulate a specific type or specific step of membrane traffic. During the past decade, many more Rab effector molecules have been identified in an effort to improve our understanding the molecular mechanism of Rab-mediated membrane trafficking (Fukuda, 2008; Grosshans et al., 2006; Schwartz et al., 2007). Furthermore, systematic screening for novel Rab effectors by using 60 different mammalian Rab isoforms as bait has recently been performed, and several candidate Rab effector domains, e.g., a coiled-coil (CC) domain, an ankyrin repeat (ANKR) domain, and a phosphotyrosine binding (PTB) domain, have been identified (Fukuda et al., 2008; Kanno et al., 2010). However, we do not think that all Rab effector domains have been identified, because the public databases contain several uncharacterized protein motifs that have been implicated in the control of Rab-mediated membrane trafficking.
The RUN domain is one such protein motif (Callebaut et al., 2001) and was originally identified in RPIP8 (Rap2-interacting protein 8)/RUNDC3A (Janoueix-Lerosey et al., 1998), UNC-14 (Ogura et al., 1997), and NESCA (new molecule containing SH3 at the carboxy-terminus)/RUSC1 (Matsuda et al., 2000). Since RUN domains are present in several proteins that have been linked to the function of small GTPases, e.g., Rap and Rab, they have been suggested to be involved in Ras-like GTPase signaling in animals (Callebaut et al., 2001). As examples, RUNDC3A/RPIP8 and RUFY3 (RUN and FYVE domain-containing 3)/RPIPX/SINGAR1 (referred to as RUFY3 below) interact with Rap2 through their N-terminal domain containing the RUN domain (Janoueix-Lerosey et al., 1998; Kukimoto-Niino et al., 2006). Rab6IP1 (Rab6-interacting protein 1)/DENND5A, a DENN (differentially expressed in normal and neoplastic) domain-containing protein implicated in Rab regulation (Levivier et al., 2001; Yoshimura et al., 2010), interacts with Rab6A through the first RUN domain (Recacha et al., 2009), and IPORIN (interacting protein of rab1)/RUSC2 (RUN and SH3 domain-containing 2) interacts with Rab1 through the RUN domain (Bayer et al., 2005). In addition, several RUN domain-containing proteins (simply referred to as RUN proteins below) include a TBC (Tre-2/Bub2/Cdc16) domain, which is a putative Rab-GAP (GTPase-activating protein) domain (Bernards, 2003; Fukuda, 2011; Yang et al., 2007), and RUTBC3/RABGAP5/DJ1042K10.2/CIP85 interacts with Rab5 isoforms through its TBC domain (Haas et al., 2005; Itoh et al., 2006). Consequently, RUN domains have often been regarded as a binding domain for small GTPases, especially Rab and Rap, in the literature. Nevertheless, even though determination of the Rab binding activity of RUN domains would be an important step toward understanding the function of RUN proteins, the Rab binding activity of most RUN domains has never been investigated. In addition, if any of the RUN domains functioned as a specific Rab effector domain (i.e., binding of specific GTP-Rab), it would be an ideal tool for trapping specific GTP-Rab and blocking specific Rab-mediated membrane trafficking event in cells if overexpressed.
In this study we used yeast two-hybrid assays (Fukuda et al., 2008; Itoh et al., 2006; Tamura et al., 2009) to systematically investigate the Rab binding activity of 19 RUN proteins that are found in the human genome or mouse genome. The results showed that only six of them, i.e., DENND5A/B, PLEKHM2, RUFY2/3, and RUSC2, interact with specific Rab isoforms with different Rab binding specificity, i.e., Rab6A/B, Rab1A, Rab33, and Rab1/Rab35/Rab41, respectively. We also identified the minimal Rab35-binding site of RUSC2 and developed a novel RUN-domain-based GTP-Rab35-specific trapper, named RBD35 (Rab-binding domain specific for Rab35). In addition, we showed that expression of enhanced green fluorescent protein (EGFP)-tagged RBD35 in PC12 cells strongly inhibited nerve growth factor (NGF)-induced neurite outgrowth, in which Rab35 has recently been shown to be involved (Chevallier et al., 2009; Kanno et al., 2010).
Horseradish peroxidase (HRP)-conjugated anti-FLAG tag (M2) mouse monoclonal antibody and anti-FLAG tag antibody-conjugated agarose were obtained from Sigma-Aldrich Corp. (St. Louis, MO). HRP-conjugated anti-T7 tag mouse monoclonal antibody and anti-T7 tag antibody-conjugated agarose were purchased from Merck Biosciences Novagen (Darmstadt, Germany). Anti-green fluorescent protein (GFP) rabbit antibody, HRP-conjugated anti-GFP rabbit antibody, HRP-conjugated anti-red fluorescent protein (RFP) rabbit antibody, and HRP-conjugated anti-Myc tag rabbit antibody were from MBL (Nagoya, Japan). HRP-conjugated anti-GST (glutathione S-transferase) antibody was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-Rab1A rabbit polyclonal antibody and anti-Rab35 rabbit polyclonal antibody were from Abnova (Taipei, Taiwan) and ProteinTech Group (Chicago, IL), respectively. The specificity of each anti-Rab antibody was confirmed by using recombinant FLAG-tagged Rab1A/B, Rab35, and Rab41 expressed in COS-7 cells (data not shown). All other reagents used in this study were analytical grade or the highest grade commercially available.
cDNAs encoding human and mouse RUN proteins (see Fig. 1 and Table I for details) were amplified from Marathon-Ready human/mouse whole brain, testis, and/or fetal brain cDNA (BD Biosciences, San Jose, CA) by PCR with the following pairs of oligonucleotides containing a restriction enzyme site (underlined: BamHI, BglII, or SalI) and/or a stop codon (in bold), essentially as described previously (Fukuda et al., 1999): 5'-GGATCCATCCAGGAAGCCAGGACTAT-3' (hDENND5A-RUN1-5' primer, sense) and 5'-TCACCTCTCATCCACCTCAGGCT-3' (hDENND5A-RUN1-3' primer, antisense); 5'-GGATCCCCATGCCGGACCCCGCCGCT-3' (hDENND5A-RUN2-5' primer, sense) and 5'-TCAGATGTCGATGCCCTTGA-3' (hDENND5A-RUN2-stop primer, antisense); 5'-GGATCCATGCAAGAGGCACGAAGTTT-3' (hDENND5B-RUN1-5' primer, sense) and 5'-CTACTTTACTAGATCTTCATCTG-3' (hDENND5B-RUN1-3' primer, antisense); 5'-GGATCCCAGTGTCGGACTCCACCCCA-3' (hDENND5B-RUN2-5' primer, sense) and 5'-TTACACATCCACTCCTTTGA-3' (hDENND5B-RUN2-stop primer, antisense); 5'-GGATCCAAACACGTATGGGTACGCAC-3' (hRUTBC1-RUN-5' primer, sense) and 5'-TCACTGGACCAGCTCATCAGCAG-3' (hRUTBC1-RUN-3' primer, antisense); 5'-GGATCCGCGGCTGGCTTCCTACGCAG-3' (hRUTBC2-RUN1-5' primer, sense) and 5'-CTACCGCACGTGGGAGCTGTGGA-3' (hRUTBC2-RUN2-3' primer, antisense); 5'-GGATCCGAGGAGCTGCTCTACCGGGC-3' (hRUTBC3-RUN-5' primer, sense) and 5'-TCACTCCTTCAGGGGCTGCTGCG-3' (hRUTBC3-RUN-3' primer, antisense); 5'-GGATCCTTCGCGCAGGTGCAGTTCCG-3' (mRUNDC1-N1 primer, sense) and 5'-TCAAAAGGCATCTTTGATGT-3' (mRUNDC1-stop primer, antisense); 5'-GGATCCATGAGCGGATCACAGAACAA-3' (hRUNDC2A-Met primer, sense) and 5'-TTACTTCTCGGGCGACTCCG-3' (hRUNDC2A-stop primer, antisense); 5'-AGATCTATGGAAGCGAGCTTTGTCCA-3' (hRUNDC3A-Met primer, sense) and 5'-TCAGCTGGGGCTCAGTGCTG-3' (hRUNDC3A-stop primer, antisense); 5'-AGATCTATGGCCTCCCGGAGCCTGGG-3' (hRUNDC3B-Met primer, sense) and 5'-TCAGGATGGAGTTAGGCCTG-3' (hRUNDC3B-stop primer, antisense); 5'-AGATCTATGCTTTCAGTGGTGGAGAA-3' (hPLEKHM1-Met primer, sense) and 5'-TCACTTATGGCCCGAGTGATGGA-3' (hPLEKHM1-RUN-3' primer, antisense); 5'-AGATCTATGGAGCCGGGGGAGGTGAA-3' (hPLEKHM2-Met primer, sense) and 5'-TCAGGAGGACACAGTCTCTGCCG-3' (hPLEKHM2-RUN-3' primer, antisense); 5'-ACCTCCACCAACCTGGAGTG-3' (hPLEKHM2-N1 primer, sense) and 5'-AAGTCGCAAAGCGGCGGCCT-3' (hPLEKHM2-C1 primer, antisense); 5'-CCTTTCGGACCGGCTCTCC-3' (hPLEKHM2-N2 primer, sense) and 5'-GCCTCACACTTCGATTCTTG-3' (hPLEKHM2-C2 primer, antisense); 5'-GAGAAGCTGGCACTGGCCAA-3' (hPLEKHM2-N3 primer, sense) and 5'-TCAGCACCAGGGGTCTCGGG-3' (hPLEKHM2-stop primer, antisense); 5'-GGATCCATGCAGAGCATCCTCTATCA-3' (hKIAA0226-Met primer, sense) and 5'-TCACAGGTACCGACCACCTTCAT-3' (hKIAA0226-C1 primer, antisense); 5'-GGATCCATGAAACTCAGCATTAAGGT-3' (mRUFY1-RUN-5' primer, sense) and 5'-TCATTCATGCTCTCTGCCGCTGT-3' (mRUFY1-RUN-3' primer, antisense); 5'-GGATCCAGCGGCAGAGAGCATGAA-3' (mRUFY1-C-5' primer, sense) and 5'-TCACTTCTCGCTTCTTTC-3' (mRUFY1-stop primer, antisense); 5'-GGATCCATGGCTACAAAGGACCCC-3' (mRUFY2-Met primer, sense) and 5'-TCATGGCATGTTAGAGGA-3' (mRUFY2-stop primer, antisense); 5'-GGATCCATGTCTGCCCTGACGCCT-3' (mRUFY3-Met primer, sense) and 5'-TTAATGGTGCTTTGGGAT-3' (mRUFY3-stop primer, antisense); 5'-AGATCTATGAGCTTCATATGCTCA-3' (mRUFY4-RUN-5' primer, sense) and 5'-TCAGGTGTCCTGGATTTC-3' (mRUFY4-stop primer, antisense); 5'-GGATCCATGGCTTCTAGCAGCACTGA-3' (mFYCO1-Met primer, sense) and 5'-TCAAATGCTGTCCTCGACTGC-3' (mFYCO1-CC1-3' primer, antisense); 5'-GCGGATCCATGGCAGAAGCCCAGAGT-3' (mRUSC1-RUN-5' primer, sense) and 5'-GCGTCGACCTATCGGAGACTCTGAGCCAA-3' (mRUSC1-RUN-3' primer, antisense); and 5'-GCAGATCTTCTCCCGATGGCAACTCG-3' (mRUSC2-RUN-5' primer, sense) and 5'-GCGTCGACTCACAGACAGAACTCAGCGGG-3' (mRUSC2-RUN-3' primer, antisense) or 5'-gcGTCGACTCAgttttggctgctccc-3' (mRUSC2-stop primer, antisense). cDNAs encoding the mouse DENND1A/connecdenn (Allaire et al., 2010; Marat and McPherson, 2010) were similarly amplified from Marathon-Ready mouse whole brain and testis cDNA by PCR using the following pairs of oligonucleotides with a BglII site (underlined) or with a stop codon (in bold): 5'-AGATCTATGGGCTCCAGGATCAAGCA-3' (DENND1A-Met primer, sense) and 5'-CTAACCTTCGCCGGAATTGAGAA-3' (DENND1A-C1 primer antisense); 5'-ATGTTGCATCTGTACGCCAG-3' (DENND1A-N1 primer, sense) and 5'-TCTAGGCTGCTGAAGACATC-3' (DENND1A-C2 primer antisense); and 5'-GACCGAGCTGCCAGCATTGA-3' (DENND1A-N2 primer, sense) and 5'-TCACTCAAAGGTCTCCCACT-3' (DENND1A-stop primer, antisense). Purified PCR products were directly inserted into the pGEM-T Easy vector (Promega, Madison, WI) and verified with an automated sequencer. cDNA encoding an open reading frame of DENND1A was constructed on the pGEM-T Easy vector by using appropriate restriction enzyme sites. cDNAs containing each of the RUN domain-containing fragments (or DENND1A) were excised from the pGEM-T Easy vector with appropriate restriction enzymes and then subcloned into the pGAD-C1 vector (James et al., 1996), pmStr-C1 vector, pEGFP-C1 vector (BD Biosciences), and/or pEF-T7/Myc tag mammalian expression vectors modified from pEF-BOS as described previously (Fukuda et al., 1999). The pEF-Myc tag (MEQKLISEEDLNEGS; an N-terminal Myc tag sequence is shown in bold) expression vector was constructed by PCR essentially as described previously (Fukuda et al., 1999). Deletion mutants of RUFY1 (i.e., RUN, C, CC1, and CC2+FYVE; see Fig. 1), RUFY2 (i.e., RUN, C, CC1, and CC2+FYVE; see Fig. 1 and Fig. 5A), RUFY3 (i.e., RUN, C, RUN+CC1, CC1, and CC2; see Fig. 4A), and RUSC2 (i.e., RUN, ΔC1-ΔC5, ΔN1-ΔN4, ΔN3/C2, and ΔN4/C2; see Fig. 6A) were also constructed by conventional PCR techniques, and the resulting cDNAs were transferred to the pGAD-C1 vector, pmStr-C1, and/or pEGFP-C1 vector as described above. The sequences of the oligonucleotides used for the construction of these deletion mutants are available from the authors on request. A constitutive active (CA) mutant (Q67L) and a constitutive negative (CN) mutant (S22N) of Rab35 were prepared by site-directed mutagenesis (Itoh et al., 2006; Tamura et al., 2009) and subcloned into the pEF-FLAG tag mammalian expression vectors as described previously (Fukuda et al., 1999). Other expression plasmids, including pEF-FLAG-Rab1A, pEF-FLAG-Rab1B, pEF-FLAG-Rab4A, pEF-FLAG-Rab33A, pEF-FLAG-Rab35, pEF-FLAG-Rab41, and pEF-T7-TBC1D10C/mFLJ00332, were also prepared as described previously (Fukuda, 2003; Itoh and Fukuda, 2006).
 View Details | Fig. 1. Schematic representation of human (h) and mouse (m) RUN domain-containing proteins. Amino acid numbers are given on both sides. Solid lines indicate the constructs used for the yeast two-hybrid assays (see Fig. 2), and the name of each construct is shown above (or below) each line. CC, coiled-coil; C1, protein kinase C conserved region 1 domain (Cys-rich domain); DENN, differentially expressed in normal and neoplastic cells; FYVE, Fab1, YOTB/ZK632.12, Vac1, and EEA1; PH, pleckstrin homology; PLAT, polycystin-1, lipoxygenase, alpha-toxin; RUN, RPIP8, UNC-14, and NESCA; SH3, Src homology domain 3; and TBC, Tre-2/Bub2/Cdc16. The DENN domain is composed of three subdomains, an upstream subdomain (u-DENN), a core subdomain (DENN), and a downstream subdomain (d-DENN) (Levivier et al., 2001). |
Yeast two-hybrid assays were performed by using pGBD-C1-Rab(CA)/(CN) lacking the C-terminal geranylgeranylation site (ΔCys) and pGAD-C1-RUN proteins/domains (in Fig. 2) as described previously (Fukuda et al., 2008; Itoh et al., 2006; Tamura et al., 2009). In Fig. 4, Fig. 5, and Fig. 6, only pGBD-C1-Rab(CA)ΔCys constructs were used for two-hybrid assays. The yeast strain, medium, culture conditions, and transformation protocol used were as described in James et al. (1996).
 View Details | Fig. 2. Rab binding specificity of RUN domain-containing proteins as revealed by yeast two-hybrid assays. Rab binding activity of the RUN domains of DENND5A/B (A), RUTBC1-3 (B), RUNDC1-3 (C), PLEKHM1/2 and KIAA0226 (D), RUFY1-4 (E), FYCO1 (F), and RUSC1/2 (G). Yeast cells containing pGBD plasmid expressing a constitutive active form (CA, which mimics the GTP-bound form) (Fukuda et al., 2008; Itoh et al., 2006) or a constitutive negative form (CN, which mimics the GDP-bound form) (Tamura et al., 2009) of Rab (positions indicated in the left panels) and pGAD plasmid expressing the RUN domain indicated were streaked on SC-AHLW medium and incubated at 30°C for one week. Positive patches are boxed. Note that only six RUN proteins bound specific Rab isoforms. |
COS-7 cells were maintained at 37°C in DMEM containing 10% fetal bovine serum and antibiotics under a 5% CO2 atmosphere. PC12 cells were cultured at 37°C on poly-L-lysine-coated or collagen type IV-coated dishes in DMEM containing 10% horse serum, 10% fetal bovine serum, and antibiotics with or without NGF (100 ng/ml; Merck KGaA, Darmstadt, Germany) under a 5% CO2 atmosphere. Plasmids were transfected into COS-7 cells (for GST pull-down assays and co-immunoprecipitation assays) by using Lipofectamine Plus or Lipofectamin LTX Plus (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s instructions. Plasmids were also transfected into PC12 cells (for immunofluorescence analyses and neurite outgrowth assays) by using Lipofectamine 2000 (Invitrogen Corp.) according to the manufacturer’s instructions.
The RBD35 fragment was subcloned into the pGEX-4T-3 vector (GE Healthcare Ltd., Little Chalfont, UK), and GST-RBD35 was expressed in Escherichia coli BL21(DE3)pLysS and purified by the standard protocol. Nucleotide exchange of Rab and GST pull-down assays were performed as described previously (Fukuda and Kanno, 2005; Kanno et al., 2010). Briefly, glutathione-Sepharose beads (GE Healthcare Ltd.) coupled with 6–10 μg of GST-RBD35 were incubated for 1 h with COS-7 cell lysates containing FLAG-Rab35, FLAG-Rab35(Q67L), FLAG-Rab35(S22N), FLAG-Rab35+T7-DENND1A, or FLAG-Rab35+T7-TBC1D10C in the presence of nothing, 1 mM GDP, or 0.5 mM GTPγS. After washing the beads three times, proteins bound to the beads were analyzed by 10% SDS-PAGE and then immunoblotted with HRP-conjugated anti-FLAG tag antibody and HRP-conjugated anti-GST antibody as described previously (Fukuda and Kanno, 2005). Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; GE Healthcare Ltd.). GTP-Rab35 pull-down assays from PC12 cell lysates were performed in a manner similar to that described above.
Co-immunoprecipitation assays in COS-7 cells were performed as described previously (Fukuda et al., 1999; Fukuda and Kanno, 2005). In brief, agarose beads coupled with nothing (Mock) or FLAG-Rabs were incubated with the COS-7 cell lysates expressing T7/Myc-tagged Rab-binding proteins in the presence of 0.5 mM GTPγS. Alternatively, a complex between FLAG-Rab and T7-Rab-binding protein in COS-7 cell lysates was immunoprecipitated with anti-FLAG tag antibody-conjugated agarose beads in the presence of 0.5 mM GTPγS (for Fig. 3C). After washing the beads, proteins bound to the beads were analyzed by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-Myc tag antibody (1/5000 dilution), HRP-conjugated anti-T7 tag antibody (1/10,000 dilution) and/or HRP-conjugated anti-FLAG tag antibody (1/10,000 dilution). Immunoreactive bands were visualized by enhanced chemiluminescence. Associations between EGFP-Rab33A and mStr (monomeric Strawberry)-RUFY2/3 truncated mutants in COS-7 cells were similarly evaluated by immunoprecipitation with 1 μl of anti-GFP antibody and 10 μl of anti-rabbit IgG dynabeads (Invitrogen Corp.). The blots shown in this paper are representative of at least two independent experiments.
 View Details | Fig. 3. Rab binding activity of RUN domain-containing proteins (PLEKHM2, RUFY2, RUFY3, and RUSC2) as revealed by co-immunoprecipitation assays (A–C) and immunofluorescence analyses (D–G). (A) Interaction between PLEKHM2 (Full and RUN) and Rab1A, (B) between RUFY2/3 and Rab4A/33A, and (C) between RUSC2-C (see Fig. 1) and Rab1A/35 in COS-7 cells. Co-immunoprecipitation assays in COS-7 cells were performed in the presence of 0.5 mM GTPγS as described under Materials and Methods. After washing the beads, proteins bound to the beads were detected with anti-Myc tag or anti-T7 tag antibody (middle panels), and anti-FLAG tag antibody (bottom panels). The open arrowheads and closed arrowheads indicate the positions of Myc-PLEKHM2-Full and Myc-PLEKHM2-RUN, respectively, in A and of T7-RUFY2 and T7-RUFY3, respectively, in B. Input means 1/100 volume of the reaction mixture used for the immunoprecipitation (top panels). The positions of the molecular mass markers (Mr in kilodaltons) are shown on the left. (D) Co-localization between EGFP-PLEKHM2 (Full and RUN) and mStr-Rab1A, (E) between mStr-RUFY2 and EGFP-Rab4A/33A, (F) between mStr-RUFY3 and EGFP-Rab33A, and (G) between EGFP-RUSC2-C and mStr-Rab1A/35 in PC12 cells. PC12 cells co-expressing EGFP-Rab and mStr-Rab-binding protein (or mStr-Rab and EGFP-Rab-binding protein) were analyzed with a confocal fluorescence microscope as described under Materials and Methods. The arrowheads in the insets indicate the co-localization points. The small arrows in (G) indicate the co-localization points between RUSC2-C and Rab35 at the tips of neurites. Insets show magnified views of the boxed area. Scale bars, 10 μm. |
PC12 cells transiently expressing EGFP-Rab and mStr-tagged Rab-binding protein (or mStr-Rab and EGFP-tagged Rab-binding protein) were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer for 20 min at room temperature. The cells were examined for fluorescence with a confocal fluorescence microscope (Fluoview FV1000; Olympus, Tokyo, Japan). The images were processed with Adobe Photoshop software (CS5).
The length of the neurites of NGF-differentiated PC12 cells was measured essentially as described previously (Fukuda and Mikoshiba, 2000). In brief, pEGFP-C1-RBD35, pEGFP-C1-RUSC2-ΔC4, or pEGFP-C1 alone was transiently expressed in PC12 cells by using Lipofectamine 2000, and the cells were treated with 100 ng/ml NGF 24 hours after transfection. After exposure to NGF for 36 hours, the cells were fixed, and images of the cells were captured at random (n>100 in three different dishes for each construct) with a confocal fluorescence microscope, and the length of the neurites was measured with MetaMorph software (Molecular Devices, Sunnyvale, CA).
RUN proteins in the human genome and mouse genome were obtained by using the SMART (Simple Modular Architecture Research Tool) program (available at http://smart.embl-heidelberg.de/) or the Blastp program (available at http://blast.ncbi.nlm.nih.gov/). Sequence alignment and depiction of the phylogenetic tree of the RUN domains from humans and mice were performed by using the ClustalW program (available at http://clustalw.ddbj.nig.ac.jp/top-e.html) set at the default parameters.
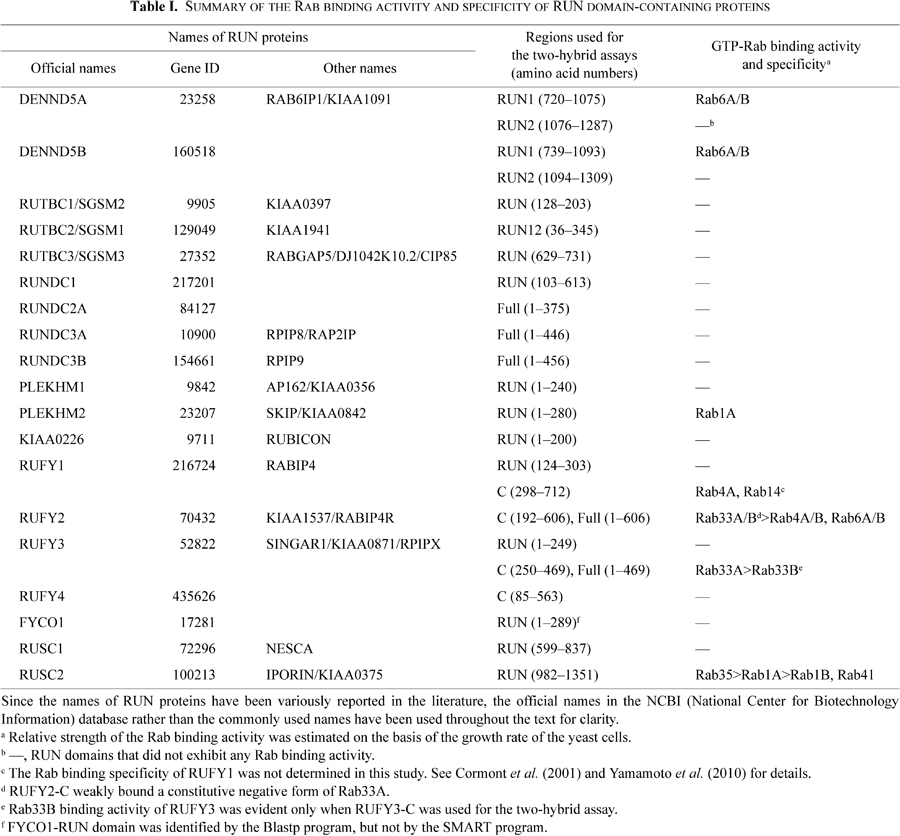
Nineteen RUN domain-containing proteins were identified in the human (or mouse) genome by using the SMART program or the Blastp program (Fig. 1 and Supplemental Fig. S1), and we classified them into the following six groups based on their domain structures and sequence similarities: RUN protein containing a DENN/Rab-GEF (guanine nucleotide exchange factor) domain (DENND5A/B; green boxes in Fig. 1), RUN proteins containing a TBC/Rab-GAP domain (RUTBC1-3; blue boxes), RUN proteins containing a pleckstrin homology (PH) domain (PLEKHM1/2 and KIAA0226/RUBICON; purple boxes), RUN proteins containing conserved CC domains (RUFY1-4 and FYCO1; black boxes), RUN proteins containing a SH3 domain (RUSC1/2; triangles), and others (RUNDC1-3). cDNA fragments encoding each RUN protein (or RUN domain-containing region) were amplified by PCR (solid lines in Fig. 1) and constructed into the pGAD-C1 vector, a prey vector for yeast two-hybrid assays. All of the possible interactions between the 19 RUN proteins and 60 constitutive active (CA)/negative (CN) mutants of mammalian Rabs were investigated by using yeast two-hybrid panels (Fig. 2) that we recently developed (Fukuda et al., 2008; Itoh et al., 2006; Tamura et al., 2009). Although most of the RUN proteins (or RUN domains) did not exhibit any Rab binding activity (Fig. 2B and C), six of them clearly interacted with a CA form of specific Rab isoforms, suggesting that these RUN domains function as a Rab effector domain (summarized in Table I, and white boxes in Fig. 2). The first RUN domain of DENND5A (i.e., DENND5A-RUN1), but not its second RUN domain (RUN2), interacted specifically with the CA form of Rab6A and Rab6B, consistent with a previous report (Recacha et al., 2009). DENND5B, a DENND5A-related protein, also interacted specifically, but weakly, with the CA form of Rab6A and Rab6B through the RUN1 domain (Fig. 2A). The RUN domain of RUSC2/IPORIN, originally described as a Rab1-binding domain (Bayer et al., 2005), interacted with a CA form of Rab35 and Rab41, in addition to Rab1A/B (Fig. 2G), and based on the growth rate of the yeast cells in the selection medium, the RUSC2 RUN domain seemed to preferentially interact with Rab35 rather than with Rab1A/B. Three additional interactions, a RUFY2-Rab33A/B interaction, a RUFY3-Rab33A interaction (Fig. 2E), and a PLEKHM2-RUN-Rab1A interaction (Fig. 2D), were also identified, but this last interaction seemed to be very weak (i.e., the growth rate was very slow). These previously uncharacterized interactions observed in yeast cells (Fig. 2) were confirmed in mammalian cultured cells both by co-immunoprecipitation assays in COS-7 cells (Fig. 3A-C) and by immunofluorescence analyses in PC12 cells (Fig. 3D-G). As an example, consistent with the results of the yeast two-hybrid assays (Fig. 2E), RUFY2 interacted with both Rab4A and Rab33A in COS-7 cells (Fig. 3B, lanes 2 and 3) and co-localized with both Rabs in PC12 cells (Fig. 3E), whereas RUFY3 interacted with Rab33A, but hardly at all with Rab4A (Fig. 3B, lanes 5 and 6), in COS-7 cells, and co-localized with Rab33A, not with Rab4A (Fig. 3F). We then focused on the three strong, previously uncharacterized interactions, i.e., the RUFY2-Rab33A interaction, RUFY3 (originally described as SINGAR1; single axon-related 1 (Mori et al., 2007))-Rab33A interaction, and RUSC2-Rab35 interaction, and attempted to map the Rab-binding site of RUFY2/3 and RUSC2 in greater detail by deletion analysis.
The RUFY family consists of four members (RUFY1-4) both in humans and mice, and they share an N-terminal single RUN domain and one or two CC domains in their C-terminal portion (Fig. 1). In contrast to RUFY3, RUFY1, originally described as RABIP4 (Rab4 interacting protein) (Cormont et al., 2001; Mari et al., 2001), RUFY2, and RUFY4 contain an additional FYVE (Fab1, YOTB/ZK632.12, Vac1, and EEA1) domain at their C-terminal end. Since RUFY1 has recently been shown to function as a dual Rab effector (i.e, a dual effector for Rab4 and Rab14) through its C-terminal region, not its N-terminal RUN domain (Yamamoto et al., 2010), we first attempted to determine whether the RUN domain of RUFY3 functions as a Rab33A-binding domain by yeast two-hybrid assays with five truncated mutants (Fig. 4A). Interestingly, however, the results of the deletion analysis under our experimental conditions indicated that Rab33A interacted with the first CC domain (named CC1 below), not with the N-terminal RUN domain (Fig. 4B). We further found that the CC1 domain of RUFY3 is involved in the specific Rab33A binding activity (Fig. 4C), although, in contrast to the full-length protein, the CC1 domain of RUFY3 interacted with Rab33B weakly (Fig. 2E). The interaction of Rab33A with the C-terminal domain of RUFY3 was confirmed by two independent approaches using mammalian cultured cells. In the first approach, we showed by immunofluorescence analyses that mStr-RUFY3-C clearly co-localized with EGFP-Rab33A in PC12 cells, whereas no co-localization between mStr-RUFY3-RUN and EGFP-Rab33A was observed in PC12 cells (Supplemental Fig. S2C). In the second approach, we showed by co-immunoprecipitation assays in COS-7 cells that mStr-RUFY3-C actually interacted with EGFP-Rab33A (Supplemental Fig. S2A, lane 4 in the middle panel, open arrowhead), although, unexpectedly, interaction between T7-RUFY3-C and FLAG-Rab33A was hardly observed by co-immunoprecipitation assays in COS-7 cells for an unknown reason (data not shown). Interestingly, mStr-RUFY3-RUN alone also interacted with EGFP-Rab33A by co-immunoprecipitation assays, although, unlike RUFY3-C, a significant amount of the RUFY3 RUN domain non-specifically bound to the control GFP beads (asterisk in the middle panel of Supplemental Fig. S2A). However, since no co-localization between mStr-RUFY3-RUN and EGFP-Rab33A was observed in living cells (Supplemental Fig. S2C), the interaction between them observed by the co-immunoprecipitation assays may have been an artifact caused by the adhesive nature of the RUFY3 RUN domain. Alternatively, the RUFY3 RUN domain may function as a second Rab33-binding site and contribute to the Rab33A recognition in a whole protein. In any event, since the RUFY3-C interacted with Rab33A by all three different approaches described above (Fig. 4, and Supplemental Fig. S2A and C), RUFY3 is likely to interact with Rab33A mainly through the first coiled-coil domain.
 View Details | Fig. 4. Mapping of the site responsible for the Rab33A-binding site of RUFY3. (A) Schematic representation of the deletion mutants of RUFY3 used in this study. RUN contains amino acid residues 1–249 of mouse RUFY3; C, amino acid residues 250–469; RUN+CC1, amino acid residues 1–329; CC1, amino acid residues 250–329; and CC2, amino acid residues 330–469. (B) Specific interaction between the CC1 domain of RUFY3 and Rab33A as revealed by yeast two-hybrid assays. (C) The CC1 domain of RUFY3 is responsible for specific interaction with Rab33. The yeast two-hybrid assays and description of the data are described in the legend for Fig. 2. Positive patches are boxed. |
Since RUFY2 also interacted with Rab33A/B (Fig. 2E), we performed a deletion analysis (Fig. 5A) to investigate whether the CC1 domain of RUFY2 functions as a Rab33-binding site, the same as the RUFY3 CC1 domain does, and, as expected, the RUFY2 CC1 domain alone interacted with Rab33A/B by yeast two-hybrid assays (middle panel of Fig. 5B). In contrast to the RUFY2-C construct, however, the RUFY2 CC1 domain alone did not interact with Rab4A/6A/6B, indicating that an additional domain of RUFY2 contributes to the binding of Rab4A/6A/6B. Actually, the CC2+FYVE construct of RUFY2 was found to interact with Rab4A/6A (right panel of Fig. 5B). These results, together with the fact that RUFY1, which is structurally related to RUFY2, interacts with Rab4A via the region between the CC2 domain and the FYVE domain (Cormont et al., 2001; Mari, et al., 2001; Yamamoto et al., 2010), indicated that RUFY2 contains two Rab-binding sites, a Rab33-binding site (i.e., CC1 domain) and a Rab4A/6A/6B-binding site, the latter of which is presumably located between the CC2 domain and the FYVE domain (Fig. 5C). Thus, RUFY2 is likely to function as a dual Rab effector, the same as RUFY1 (Yamamoto et al., 2010), whereas RUFY3 functions as a Rab33A-specific effector, because it lacks a domain corresponding to the region between the CC2 domain and the FYVE domain of RUFY2. Since no co-localization between mStr-RUFY2-RUN and EGFP-Rab33A was observed in PC12 cells (Supplemental Fig. S2D) and since mStr-RUFY2-C, but not mStr-RUFY2-RUN, interacted with EGFP-Rab33A (Supplemental Fig. S2B, lane 4 in the middle panel, open arrowhead), we concluded that the RUFY2 CC1 domain functions as the Rab33A-binding site, the same as the RUFY3 CC1 domain does.
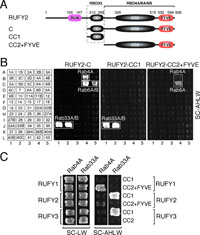 View Details | Fig. 5. Mapping of the site responsible for the Rab33-binding site of RUFY2. (A) Schematic representation of the deletion mutants of RUFY2 used in this study. RUFY2-C contains amino acid residues 192–606 of mouse RUFY2; CC1, amino acid residues 192–271; and CC2+FYVE, amino acid residues 272–606. (B) Distinct domains of RUFY2 contribute to Rab33 binding and Rab4A/6A/6B binding as revealed by yeast two-hybrid assays. Note that the CC1 domain of RUFY2 is necessary and sufficient for specific interaction with Rab33, whereas CC2+FYVE interacts with Rab4A/6A. The yeast two-hybrid assays and description of the data are described in the legend for Fig. 2. Positive patches are boxed. (C) Distinct Rab4A/Rab33A binding activity of RUFY1-3. RUFY1 interacts with Rab4A through the region between CC2 and FYVE (Cormont et al., 2001; Yamamoto et al., 2010), whereas RUFY3 interacts with Rab33A through the CC1 domain. By contrast, RUFY2 possesses both properties. |
We next attempted to identify the minimal Rab-binding site of RUSC2 by a systematic deletion analysis (Fig. 6A), because the original RUN fragment that we used for the yeast two-hybrid assays (Fig. 2G) contained both N-terminal and C-terminal flanking regions (i.e., more than 120 amino acids flank the RUN domain on each side). As shown in Fig. 6B, the N-terminal deletion constructs (ΔN1-ΔN4) had no binding activity toward any of the four Rabs. The results obtained with the C-terminal deletion constructs (ΔC1-ΔC5), however, were somewhat complicated. Deletion of the 32 C-terminal amino acids from the original RUN construct (ΔC5) mostly abrogated Rab1A, Rab1B, and Rab41 binding activities, whereas C-terminal deletion of 72 or 112 amino acids (ΔC4 or ΔC3) completely abrogated all Rab binding activity. To our surprise, however, additional C-terminal deletion (deletion of 152 or 174 C-terminal amino acids; ΔC2 or ΔC1) unexpectedly restored Rab35 binding activity, but not Rab1A, Rab1B, or Rab41 binding activity, suggesting that a region containing amino acids 1200-1279 has some inhibitory effect on the Rab35 binding activity of RUSC2. Since the ΔC1 construct of RUSC2 exhibited very weak Rab35 binding activity, we concluded that the ΔC2 construct of RUSC2 is the minimal functional Rab35-binding site.
 View Details | Fig. 6. Mapping of the minimal Rab35-binding site of RUSC2. (A) Schematic representation of the deletion mutants of RUSC2 used in this study. Amino acid numbers are shown on both sides of each construct. The minimal high affinity Rab35-specific-binding site is indicated by a dotted box and referred to as RBD35 (Rab-binding domain specific for Rab35) in the text. Relative Rab binding activities (±, +, ++, or +++) are indicated at the right of each construct based on the growth rate of the yeast cells (see B). (B) Rab1A/B, Rab35, and Rab41 binding activities of the RUSC2 deletion mutants. Note that the RUN domain of RUSC2 alone did not recognize Rab35 and that an additional N-terminal region adjacent to the RUN domain was required for the Rab35 binding activity. The yeast two-hybrid assays and description of the data are described in the legend for Fig. 2. |
A report of the identification of a specific effector domain in the Rab research literature has often significantly advanced our understanding of the involvement of a specific Rab protein in membrane trafficking (Fukuda, 2010). As an example, a Rab27-specific effector domain (i.e., synaptotagmin-like protein homology domain; SHD) can trap and sense the level of endogenous GTP-Rab27A in cells (i.e., GTP-Rab27 pull-down assay) (Imai et al., 2009; Itoh and Fukuda, 2006), and the same construct can be used to inhibit the function of endogenous Rab27 protein (e.g., melanosome transport in melanocytes and secretion by secretory cells) (Holt et al., 2008; Kuroda et al., 2003) by trapping GTP-Rab27 (i.e., dominant negative assay) (Fukuda (2008) and references therein). We therefore attempted to develop a RUN-domain-based GTP-Rab trapper. Among the Rab-RUN protein interactions identified above (Fig. 2), we focused on the ΔC2 construct of RUSC2, which specifically and strongly interacted with Rab35 in yeast cells (Fig. 6), because the Rab6-DENND5A-RUN1 interaction has already been structurally investigated (Recacha et al., 2009) and a CC-domain-based GTP-Rab33 trapper (i.e., the Atg16L1 CC domain) has recently been reported (Itoh et al., 2008). In addition, considerable attention has recently been directed toward Rab35, because it was recently shown to be involved in a variety of cellular events, including cytokinesis (Kouranti et al., 2006), endocytic recycling (Patino-Lopez et al., 2008; Sato et al., 2008), actin remodeling (Shim et al., 2010; Zhang et al., 2009), and neurite outgrowth (Chevallier et al., 2009; Kanno et al., 2010). However, only a very few Rab35-binding proteins have been identified (Fukuda et al., 2008; Kanno et al., 2010; Zhang et al., 2009), and the Rab binding specificity (or effector domain) of most of them has never been thoroughly investigated. We therefore further characterized the Rab35 binding ability of the ΔC2 construct of RUSC2, which we refer to as RBD35 (Rab-binding domain specific for Rab35) below, by using mammalian cell cultures.
We did so by using beads coupled with GST-RBD35 to investigate whether the RUSC2-ΔC2 construct could specifically trap a GTP-bound form of FLAG-Rab35 in COS-7 cells. Consistent with the results of the yeast two-hybrid assays (Fig. 6B), GST-RBD35 trapped FLAG-Rab35 only in the presence of GTPγS, and not in the presence of GDP (compare lanes 3 and 4 in the middle panel of Fig. 7A). Similarly, GST-RBD35 trapped the CA form of Rab35 (i.e., Rab35(Q67L)), but not its CN form (i.e., Rab35(S22N)) (compare lanes 3 and 4 in the middle panel of Fig. 7B). GST-RBD35 is likely to sense the level of GTP-Rab35 in cells, because manipulation of the cellular level of GTP-Rab35 either by expression of TBC1D10C, a specific GAP, an inactivator for Rab35 (Patino-Lopez et al., 2008; Hsu et al., 2010), or by expression of DENND1A/connecdenn, a specific GEF, an activator for Rab35 (Allaire et al., 2010; Marat and McPherson, 2010; Yoshimura et al., 2010), altered the amount of GTP-Rab35 trapped by the beads without altering the total level of Rab35 expression (Fig. 7C). Expression of TBC1D10C dramatically decreased the level of GTP-Rab35 trapped by the beads (lane 2 in the second panel from the top in Fig. 7C), whereas expression of DENND1A dramatically increased the level of GTP-Rab35 trapped by the beads (lane 3 in the second panel from the top in Fig. 7C). Moreover, GST-RBD35 specifically trapped recombinant Rab35, but did not trap Rab1A, Rab1B, or Rab41, in COS-7 cells (lane 7 in the middle panel in Fig. 7D), and it also trapped endogenous Rab35, but not Rab1A, in PC12 cells (lane 4 in the middle panel in Fig. 7E and F).
 View Details | Fig. 7. Characterization of RBD35, a specific trapper of GTP-Rab35. (A) GTP-dependent binding of GST-RBD35 with FLAG-Rab35. Glutathione-Sepharose beads coupled with GST alone (lanes 1 and 2) or GST-RBD35 (lanes 3 and 4) were incubated with COS-7 cell lysates expressing FLAG-Rab35 in the presence of 1 mM GDP (lanes 1 and 3) or 0.5 mM GTPγS (lanes 2 and 4). Proteins bound to the beads were analyzed by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-FLAG tag antibody (1/10,000 dilution; middle panel) and HRP-conjugated anti-GST antibody (1/5000 dilution; bottom panel). Input means 1/200 volume of the reaction mixture used for the pull down-assay (top panel). (B) GTP-dependent binding of GST-RBD35 with FLAG-Rab35(Q67L). Glutathione-Sepharose beads coupled with GST alone (lanes 1 and 2) or GST-RBD35 (lanes 3 and 4) were incubated with COS-7 cell lysates expressing FLAG-Rab35(S22N) in the presence of 1 mM GDP (CN; lanes 1 and 3) or FLAG-Rab35(Q67L) in the presence of 0.5 mM GTPγS (CA; lanes 2 and 4). Proteins bound to the beads were analyzed by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-FLAG tag antibody (1/10,000 dilution; middle panel) and HRP-conjugated anti-GST antibody (1/5000 dilution; bottom panel). Input means 1/200 volume of the reaction mixture used for the pull down-assay (top panel). Note that GST-RBD35 specifically trapped the GTP-bound form of Rab35 (lane 4 in A and B). (C) Effect of Rab35-GAP (TBC1D10C) and Rab35-GEF (DENND1A) on the GTP-Rab35 level in cultured cells. COS-7 cells were co-transfected with pEF-FLAG-Rab35 and control pEF-BOS vector (Mock; lane 1), pEF-T7-TBC1D10C (lane 2, open arrowhead), or pEF-T7-DENND1A (lane 3, closed arrowhead). COS-7 cell lysates were incubated with glutathione-Sepharose beads coupled with GST-RBD35, and proteins bound to the beads were analyzed by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-FLAG tag antibody (1/10,000 dilution; second panel from the top) and HRP-conjugated anti-GST antibody (1/5000 dilution; bottom panel). Input means 1/100 volume of the reaction mixtures used for the GST pull-down assay (top panel and third panel from the top). Note that the amount of GTP-Rab35 trapped by the RBD35 beads was increased by co-expression of DENND1A and decreased by co-expression of TBC1D10C. (D) Rab binding specificity of RBD35. Glutathione-Sepharose beads coupled with GST alone (lanes 1–4) or GST-RBD35 (lanes 5–8) were incubated with COS-7 cell lysates expressing FLAG-Rab1A, FLAG-Rab1B, FLAG-Rab35, or FLAG-Rab41 in the presence of 0.5 mM GTPγS and 10 mM MgCl2. Proteins bound to the beads were analyzed by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-FLAG tag antibody (1/10,000 dilution; middle panel) and HRP-conjugated anti-GST antibody (1/5000 dilution; bottom panel). Input means 1/100 volume of the reaction mixtures used for the GST pull-down assay (top panel). Note that GST-RBD35 specifically trapped Rab35 (lane 7), but not Rab1A, Rab1B, or Rab41. (E) and (F) GST-RBD35 was capable of trapping endogenous Rab35 molecules, but not endogenous Rab1A molecules, in PC12 cells. PC12 cell lysates were incubated with 2.5 mM EDTA and either 1 mM GDP (lanes 1 and 3) or 0.5 mM GTPγS (lanes 2 and 4) on ice for 30 min, and MgCl2 was then added to a final concentration of 10 mM. Glutathione-Sepharose beads coupled with GST alone (lanes 1 and 2) or GST-RBD35 (lanes 3 and 4) were incubated for 2 hours with the above PC12 cell lysates. (View PDF for the rest of the caption.) |
Lastly, we investigated the effect of RBD35 on NGF-induced neurite outgrowth of PC12 cells, a process in which Rab35 has recently been shown to be involved (Chevallier et al., 2009; Kanno et al., 2010). Expression of EGFP-tagged RBD35 in PC12 cells was found to strongly inhibit neurite outgrowth of PC12 cells when compared to control EGFP-expressing cells (Fig. 8A and B). We also tested the effect of RUSC2-ΔC4, which lacked Rab35 binding activity by yeast two-hybrid assays (Fig. 6), on neurite outgrowth of PC12 cells. The result showed that expression of EGFP-RUSC2-ΔC4 had little effect on neurite outgrowth in comparison with the EGFP-RBD35-expressing cells (compare left and right shaded bars in Fig. 8B), although it exhibited a weak inhibitory effect on neurite outgrowth in comparison with the control EGFP-expressing cells. This weak inhibitory effect of RUSC2-ΔC4 may be attributable to its degradation product, whose molecular mass almost coincides with that of RBD35 (data not shown). Moreover, the strong inhibitory effect of RBD35 on neurite outgrowth of PC12 cells was almost completely abolished by co-expression of Rab35 (Fig. 8C), indicating that RBD35 is likely to inhibit NGF-induced neurite outgrowth of PC12 cells by trapping endogenous Rab35. On the basis of these findings, together with the fact that RBD35 specifically trapped GTP-Rab35 (Fig. 7), we concluded that EGFP-RBD35 functions as a dominant negative construct by trapping endogenous Rab35 molecules in living cells and inhibits neurite outgrowth of PC12 cells as a result.
 View Details | Fig. 8. Overexpression of EGFP-tagged RBD35 in PC12 cells inhibited neurite outgrowth of PC12 cells. (A) Typical images of EGFP-RBD35-expressing cells (middle panel), EGFP-RUSC2-ΔC4 (right panel), and EGFP-expressing control cells (left panel). Scale bars, 30 μm. (B) Effect of expression of EGFP-RBD35, EGFP-RUSC2-ΔC4, and EGFP alone on neurite outgrowth of PC12 cells. Total neurite length values (mean and S.E.) of EGFP-RBD35-expressing cells, EGFP-RUSC2-ΔC4-expressing cells, and control cells are shown. Note that expression of EGFP-RBD35 strongly inhibited neurite outgrowth of PC12 cells (**, p<0.01, in comparison with the control EGFP-expressing cells or EGFP-RUSC2-ΔC4-expressing cells; Student’s unpaired t-test). (C) Co-expression of mStr-Rab35 with EGFP-RBD35 in PC12 cells attenuated the inhibitory effect of RBD35 on neurite outgrowth of PC12 cells (**, p<0.01, in comparison with the control EGFP-expressing cells or EGFP-RBD35+mStr-Rab35-expressing cells; Student’s unpaired t-test). |
Because a RUN domain is often found in proteins that are involved in the regulation of small GTPase Rab (e.g., Rab1, Rab4, Rab5, Rab6, and Rab7) (Fig. 1) (Bayer et al., 2005; Bernards, 2003; Callebaut et al., 2001; Cormont et al., 2001; Fukuda, 2011; Haas et al., 2005; Mari et al., 2001; Pankiv et al., 2010; Recacha et al., 2009; Tabata et al., 2010; Yamamoto et al., 2010; Yang et al., 2007), RUN domains have often been considered to be the binding site of some Rabs in the literature, even though their Rab binding activity had never been thoroughly investigated. In the present study we for the first time performed a genome-wide analysis of the Rab binding activity of RUN domains in humans and mice by yeast two-hybrid assays in which panels of CA/CN mutants of 60 Rabs were used as bait (Fig. 2). The results showed that only four of the 19 RUN proteins interacted with specific Rab isoforms via their RUN domain-containing region, i.e., PLEKHM2 with Rab1A, DENND5A/B with Rab6A/B, and RUSC2 with Rab1A/B, Rab35, and Rab41 (Fig. 2 and Supplemental Fig. S1A). The RUN domain alone of even DENND5A and RUSC2, however, was insufficient to recognize specific Rabs, i.e., a PLAT (polycystin-1, lipoxygenase, alpha-toxin) domain adjacent to the RUN1 domain of DENND5A and an N-terminal adjacent region of the RUN domain of RUSC2 were also required for Rab6A binding activity (Recacha et al., 2009) and Rab35 binding activity (Fig. 6), respectively. These results indicated that RUN domains alone do not function as general Rab-binding motifs and that only a few RUN domains partly contribute to specific Rab binding (i.e., the RUN domain is just part of the Rab-binding site for DENND5A and RUSC2, and an additional region adjacent to the RUN domain is required for Rab binding activity).
Although RUSC2 was initially identified as a Rab1A/B-, Rab35-, and Rab41-binding protein by yeast two-hybrid assays (Fig. 2G), we succeeded in identifying the minimal functional Rab35-specific-binding site of RUSC2 (amino acid residues 982–1199) by a systematic deletion analysis (Fig. 6). We then developed a novel RUN-domain-based GTP-Rab35 trapper, which we named RBD35 (Fig. 7). GST-RBD35 specifically trapped both recombinant and endogenous Rab35 in a GTP-dependent manner (Fig. 7A, B, and E), but it did not trap Rab1A at all (Fig. 7D and F) despite the original RUSC2 construct being bound to Rab1A (Fig. 2G). Because of the specific GTP-Rab35 binding ability of RBD35, overexpression of RBD35 with an EGFP-tag in PC12 cells strongly inhibited NGF-induced neurite outgrowth (Fig. 8), the same phenotype as overexpression of Rab35(CN) in PC12 cells (Chevallier et al., 2009; Kanno et al., 2010). We therefore consider fluorescently labeled RBD35 to be an ideal tool for investigating the function of Rab35 in membrane trafficking, and the effect of RBD35 on other types of Rab35-dependent events, including cytokinesis, receptor recycling, and actin remodeling, is now under investigation in our laboratory.
What function(s) do RUN domains have in common? This is still an open question, because our findings clearly rule out the possibility that Rab is a common ligand of all RUN domains. Since some RUN domains have been shown to bind Rap, another type of small GTPase (Janoueix-Lerosey et al., 1998; Kukimoto-Niino et al., 2006; Yang et al., 2007), Rap may be a common ligand of RUN domains. However, we think that this possibility is also unlikely because of the very low sequence conservation among the known RUN domains (only one Tyr residue is invariant in all RUN domains; asterisk in Supplemental Fig. S1B). Actually, the Arg-168 of RUFY3/RPIPX in the positively charged surface of the RUN domain, which has previously been proposed to be involved in Rap2 binding (Kukimoto-Niino et al., 2006), is not conserved in all other RUN domains (# in Supplemental Fig. S1B). Moreover, the minimal Rap2-binding site of RUFY3 has been shown to be 40 amino acids larger than the predicted RUN domain (Kukimoto-Niino et al., 2006), indicating that the RUN domain alone is insufficient to recognize Rap2. This observation is very similar to our own results showing that the minimal Rab35-binding site of RUSC2 is larger than the predicted RUN domain (Fig. 6). Since we basically used larger fragments (or full-length proteins) than the predicted RUN domains in the yeast two-hybrid assays, the lack of Rab binding activity of most of the RUN proteins should not be attributable to the truncation of the Rab-binding site. Since RUN domains are highly diversified at the amino acid level, it would be interesting to investigate whether the RUN domain-containing region of RUN proteins functions as a binding site of small GTPases other than Rab and Rap in the future.
In summary, we systematically investigated the Rab binding activity of 19 human/mouse RUN proteins and identified four novel interactions, i.e., interactions between PLEKHM2 and Rab1A, RUFY2 and Rab33A/B, RUFY3 and Rab33A, and RUSC2 and Rab35. We also developed RBD35, a novel RUN-domain-based GTP-Rab35 trapper, and demonstrated that overexpression of EGFP-RBD35 strongly inhibits Rab35-dependent neurite outgrowth of PC12 cells. We therefore think that RBD35 will be a useful tool that will make it easy to probe the specific involvement of Rab35 in cellular phenomena and possibly shed light on the molecular mechanism of Rab35-dependent membrane trafficking.
We thank Megumi Aizawa, Miho Inaba, and Namiko Kamiyama for technical assistance, and members of the Fukuda Laboratory for valuable discussions. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to N.O. and M.F.) and by a grant from the Global COE Program (Basic & Translational Research Center for Global Brain Science) of the MEXT of Japan (to N.O. and M.F.). H.K. and K.I. were supported by the Japan Society for the Promotion of Science.
|