| To whom correspondence should be addressed: Takeshi Nishioka, Department of Biomedical Sciences and Engineering, Faculty of Health Sciences, Hokkaido University, N12-W5, Kita-ku, Sapporo 060-0812, Japan. Tel/Fax: +81–11–706–3411 E-mail: trout@hs.hokudai.ac.jp |
LOX is not a novel molecule. It is described in classic molecular biology textbooks such as “Molecular Biology of The Cell”. Furthermore there have been numerous reports of the role of LOX or LOXL in cancer biology. The LOX family encode genes for copper-dependent amine oxidases, which catalyze the covalent cross-link of the component side chains of collagen and elastin. The extracellular matrix (ECM) is stabilized by these enzymes. There are five members in the LOX family (LOX, LOXL1, LOXL2, LOXL3, and LOXL4).
The C-terminal end of LOX encode the enzyme domain and is highly conserved between the family members. The N-terminal end encodes for variable pro-peptide regions with variable sequences (Fig. 1). Cysteine-rich scavenger receptor domains are found in LOXL2, LOXL3, and LOXL4. These are assumed to play an amine oxidase role (Kim et al., 2003).
 View Details | Fig. 1. Schematic representations of LOX and its family, LOXL. Si: signal peptide aa: amino acid residue number Cu++: Copper containing catalytic domain CRL: cytokine receptor-like domain SRCR: scavenger-receptor cysteine-rich domains LOXL3 and 4 have similar compositions seen in LOXL2 |
Recent studies showed that LOX is not only involved in ECM stabilization but also influences cell proliferation (Saad et al., 2010; Baker et al., 2011). LOX is also important in bone formation. LOX gene deficiency results in decreased osteoblastic differentiation (Pischon et al., 2009). However, functions of the LOX family are not fully understood except for LOX and LOXL2. Studies have shown that these molecules promote tumor cell invasion/metastasis. To understand the role of other members, creating knockout mice is required. For example, mice lacking LOXL1 do not deposit normal elastic fibers in the uterus (Liu et al., 2004). Enlarged airspaces of the lung, loose skin and vascular abnormalities were also observed. LOXL1 was located at the sites of elastogenesis and interacts with fibulin-5. Since the LOX family require copper ions for their function, deficiency can lead to diseases such as Menkes’ syndrome, which is characterised by sparse and coarse hair, growth failure, mental retardation, and developmental delay (Price et al., 2007).
Functions of the LOX family became clear mainly from the study of the cardiovascular system where fibrosis is strongly associated with the LOX family. Among the cytokines that up-regulate LOX, the most widely known is transforming growth factor-beta (TGF-β). LOX mRNA and protein were over-expressed with the presence of TGF-β (Voloshenyuk et al., 2011a). The effect of TGF-β on LOX expression was also confirmed by other researchers (van Meeteren and Ten Dijke, 2011). Tumor necrosis Factor-α (TNF-α) also stimulates LOX expression (Voloshenyuk et al., 2011b). It is interesting that our irradiation-surviving cells expressed higher IL6- (36.0-fold) and LOX- mRNA (4.8-fold) compared to un-irradiated parental cells in vitro (Nishioka et al., 2011). IL6 might have an influence on LOX expression. The cell line we used was QRsP a mouse sarcoma, a kind gift from Dr. Okada (Okada et al., 1992). Furthermore, these irradiation-surviving cells showed a significant mitotic activity and a marked invasion into the surrounding tissues in mice. Cells from the irradiation-surviving derived tumor showed increased TNF-α (2.5-fold) compared to un-irradiated parental tumor cells. These experimental results may explain the un-controllable behaviour of recurrent tumours often seen in the clinic.
To understand the expression of the LOX family, signal transduction must be examined. The increase of LOX protein in response to TGF-β1 was prevented by inhibitors of Smad3, p38-MAPK, JNK and ERK1/2 (Voloshenyuk et al., 2011a). Blockade of PI3K also decreased TGF-β1 induced phosphorylation of Smad3. The authors concluded that PI3K/Akt and Smad pathways may be integrated in TGF-β1 signaling. Platelet derived growth factor (PDGF) may also influence LOX. There is evidence that activation of ERK 1/2 and expression of LOX are involved in the effects of PDGF-BB and/or TGF-β1 on cellular migration and proliferation of endothelial and smooth muscle cells during the process of vascular remodeling (Qi et al., 2011). Cardiomyopathy has been created in mice by infection of T. Cruse, resulting in the up-regulation of LOX, other ECM-related genes, tissue inhibitor of metalloproteinase (TIMP-1), and TGF-β (Soares et al., 2010). In cardiac fibroblasts, Rac1 GTPase was shown to mediate up-regulation of fibronectin via LOX and connective tissue growth factor (CTGF). Also, the same study showed inhibition of the signaling pathway reduced LOX expression, and a LOX inhibitor appeared to prevent signal transduction, fibronectin expression and collagen cross-linking (Adam et al., 2011). The physiological role of LOX in the cardio-vascular system forms part of the stress-response. The heart is a dynamic organ in nature, and thus the myocardial structure of the collagen network can be reversibly modified to adapt to transient cardiac injuries. In the case of persistent injuries, the heart goes through a maladaptive collagen remodeling, and this non-reversible damage is caused by the excessive interstitial and perivascular deposition of collagen types I and III fibers. This maladaptation leads to compromised heart functions such as left ventricular failure. Its major causative molecules were reported to be TGF-β and LOX (López et al., 2010). Taken together, expression of LOX and its family members is a double-edged sword; it is effective for minor tissue injuries, but damaging for functionality in major injuries.
Hypoxia is a characteristic of many malignancies arising from various sites. Cancer cells are better adapted to survive at a low oxygen level than normal cells (Cassavaugh and Lounsbury, 2011). In hypoxic cancer cells, the hetero diametric hypoxia transcription factor hypoxia inducible factor-1 (HIF-1) binds to the hypoxia responsive element (HRE) in the promoter region (Postovit et al., 2008) of many target genes including LOX (Erler et al., 2006). Chromatin immune-precipitation (ChIP) using rabbit polyclonal antiserum to HIF-1α clearly showed that LOXL2 is also under the influence of HIF-1α (Schietke et al., 2010). HIF-1α binding activity was a 3.1-fold (i.e. HRE enrichment) compared to the value before ChIP. Such HIF-1α regulation on LOXL2 was mentioned by other researchers (Pez et al., 2011). For the rest of the LOX family, the role of HIF-1 is ambiguous. Cancer cells that express higher LOX or LOXL2 protein have a more proliferative and invasive nature (Brekhman and Neufeld, 2009; Schietke et al., 2010). Brekman and Neufeld clearly demonstrated that the number of cells which invaded into collagen gel was significantly reduced by sh-LOXL2. More recently, a group from the University of Lyon showed that LOX over-expression enhanced HIF-1α expression under hypoxia. Interestingly, LOX up-regulates HIF-1α, meaning they potentiate each other to foster tumor progression (Pez et al., 2011). They also reported that LOX activated PI3K/AKT pathway, thereby up-regulating HIF-1α. These seem to be an adaptation mechanism for cancer cells to survive in hypoxia. A group from the National Cancer Institute in the United States found that Pdcd4, a novel tumor suppressor, reversed hypoxia-induced LOX expression in T47D breast cancer cells. However, Pdcd4 did not directly affect HIF-1α protein expression. Results of a HRE luciferase assay suggested that Pdcd4 regulates LOX in a HIF independent manner (Santhanam et al., 2010). Given these results, it is natural to ask how LOX or LOXL gives tumor cells such an invasive and metastatic nature. A recent breast cancer study showed that LOXL2 promotes invasion by regulating the expression and activity of the extracellular proteins tissue inhibitor of metalloproteinase-1 (TIMP1) and matrix metalloproteinase-9 (Barker et al., 2011). In an experiment using fluorescent staining, a renal cell carcinoma cell line exhibited clear E-Cadherin expression in normoxia whereas in hypoxia no fluorescent signals were observed (Schietke et al., 2010). The authors demonstrated quantitatively that E-cadherin expression is directly controlled by LOX or LOXL2. Either decreased function or expression of E-cadherin leads to EMT and metastasis (Elloul et al., 2010; Baranwal and Alahari 2009; Peinado et al., 2008). This finding is supported by other studies (Sahlgren et al., 2008; Peinado et al., 2005). LOX has another target molecule involved in EMT, Snail. LOXL2 promotes malignant transformation by Snail-dependent pathways (Peinado et al., 2008). Earlier on, they did in vitro analysis of Snail in mouse skin and LSCC tumors. Snail was stabilized as tumor progressed, and LOXL2 was associated with this event (Peinado et al., 2005). Other groups suggested LOXL2-stabilized Snail causes E-cadherin repression. Loss of E-cadherin is a major factor for EMT (MacPherson et al., 2010; Rückert et al., 2010). One of the attractive hypotheses is that hypoxia (1% oxygen) and LOX expression promote expansion of cancer stem cells (CSC). This was shown in glioblastoma multiform neurospheres isolated from freshly resected tumors (Bar et al., 2010).
Table I summarizes articles that clearly stated LOX or LOXL detection methods and endpoints for the past five years. In general, LOX or LOXL expression correlates with a poor prognosis. It is of note that the majority of clinical studies employed more objective methods such as quantitative fluorescence analysis or real-time quantitative PCR. Automated quantitative fluorescence analysis enables fluorescent staining limited to tumor areas, and thus the obtained results are supposed to be accurate. A lung study demonstrated that high-intensity LOX staining was correlated with the extent of invasion in a cohort of 166 surgically treated patients with lung adenocarcinoma (Wilgus et al., 2011). Interestingly, LOX status was a significant prognostic factor for 5-year survival in stage I patients (low: 80%, high: 45%), a subgroup which is generally thought to have a good prognosis. Another interesting result came from a Spanish group. They used fluorescence in situ hybridization and showed that increased copies of the LOXL4 gene locus on chromosome 10q24 were associated with a higher invasiveness and frequency of lymph-node metastasis in head and neck cancer (Görögh et al., 2007).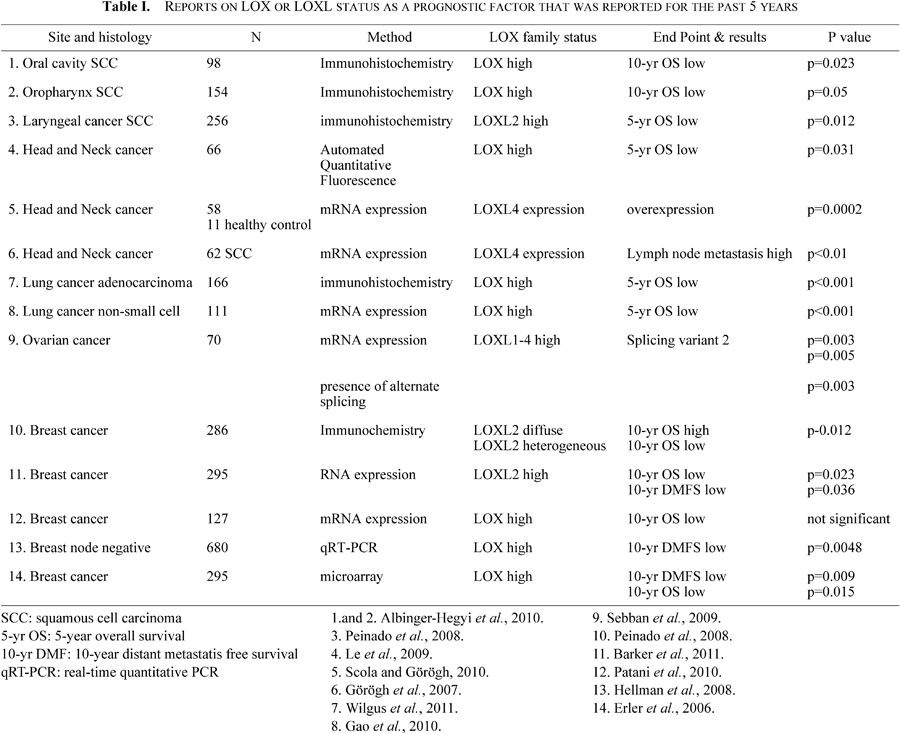
Recently, several new attempts have been made for cancer treatments. Included are vascular endothelial growth factor (VEGF) blockade (Bhargava and Robinson, 2011) and inhibition of epidermal growth factor receptor (Fojo and Parkinson, 2010; Wheeler et al., 2009). Precise high-dose radiotherapies such as stereotactic body radiotherapy (Onishi et al., 2011) or intensity modulated radiotherapy (Miah et al., 2011) also have been developed. Due to these new types of treatment, improved local control rates of cancers have been reported (Argiris et al., 2011; Nieder and Andratschke, 2012). However, distant metastases remain a problem, and limit further gains in control probability. A typical example is nasopharyngeal carcinoma. The use of intensity modulated radiotherapy (IMRT) provides excellent high-dose coverage of primary lesions and lymph-node areas, but distant metastases occur frequently after radiotherapy (Xiao et al., 2011), suggesting the presence of radio-tolerant and highly metastatic cells at the end of the therapy. An attractive hypothesis is that such malignant clones, possibly with a high HIF-1α, and LOX or LOXL2, are present from the beginning or develop during a course of radiotherapy. If this hypothesis is the case, suppressing LOX or LOXL2 could be a next generation molecular targeting therapy. A group from California demonstrated that a monoclonal antibody (AB0023) against LOXL2 was efficacious in both primary and metastatic xenograft models (Barry-Hamilton et al., 2010). A group from Ohio State University reported that tetrathiomolybdate (TM; a potent copper chelator) administration decreased lung metastasis significantly in mouse experiments (Kumar et al., 2010). Inhibiting copper-dependent LOX activity, TM treatment decreased tumor cell motility and invasiveness in vitro. They hypothesized that inhibition of focal adhesion kinase (FAK) leads to an up-regulation of molecules that affect tumor neo-angiogenesis and metastasis. A random cell motility assay showed that TM treatment significantly inhibited tumor cell motility. It inhibited FAK activation, which is closely associated with focal adhesion formation necessary for cell motility. The study group also showed that TM treatment significantly inhibited oral squamous cell invasion in a matrigel invasion assay. They concluded that TM-mediated inhibition of LOX decreased FAK, resulting in decreased metastasis. An experiment by a Canadian research team demonstrated that β-aminopropionitrile (BAPN), an irreversible inhibitor of LOX, significantly reduced distant metastasis of MDA-MB-231 breast cells. Of note was that the timing of drug delivery was important; the number of metastases was reduced by half when BAPN was administered simultaneously with a tumor cell injection whereas BAPN had no effect if the injected tumor cells had already developed palpable masses. They stated that LOX blockade is effective during the extravasation and/or initial tissue colonization stage of MDA-MB-231 cell circulation (Bondareva et al., 2009).
In conclusion, a LOX or LOXL suppressor is a promising anti-metastatic drug. As described at the beginning of this section, several molecularly targeted drugs have been developed, but their effects have not reached our expectations. Cancer cells seem to know how to deal with the effect of such new drugs (i.e. VEGF or EGFR blockade). “Life finds a way” (Spielberg, 1993). The paleontologist in Jurassic Park was right in believing the potentiality of life. However, we strongly hope that this time we will be on the winning side with a new generation molecular targeting drug that has a completely different type of anti-cancer mechanism.
The authors thank Mr. Neil Colley for helping in manuscript preparation. This work was supported in part for “Creation of Innovation Centers in Advanced Interdisciplinary Research Areas Program” in Project for Developing Innovation Systems, by the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.