| To whom correspondence should be addressed: Sumio Ishijima, Department of Bioengineering, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, O-okayama, Tokyo 152-8551, Japan. Tel: +81–3–5734–2571, Fax: +81–3–5734–2946 E-mail: sishijim@bio.titech.ac.jp |
Cilia and flagella have fundamentally the same 9+2 structure, but their movements are different (Jahn and Bovee, 1967; Sleigh, 1974). The difference in their movements between cilia and flagella must occur by the different patterns of localized active sliding between the doublet microtubules in the axoneme. As a first step towards understanding the regulation of the localized active sliding between the doublet microtubules, the change in flagellar movement of mammalian spermatozoa has been analyzed in detail (Ishijima and Mohri, 1985; Ishijima et al., 2002; Ohmuro and Ishijima, 2006; Ishijima et al., 2006; Kaneko et al., 2007; Ishijima, 2007), because the mammalian spermatozoa remarkably change their flagellar movements in the female reproductive tract and its change in beating pattern is necessary for successful fertilization (Ishijima, 2007). Detailed examinations of the flagellar movements of mammalian spermatozoa revealed that there were two beating modes in flagellar movements under the constant rate of microtubule sliding; i.e., a constant sliding displacement mode generally observed in the normal spermatozoa and a constant frequency mode in the hyperactivated spermatozoa (Ohmuro and Ishijima, 2006; Ishijima, 2007). This change in flagellar movement of the hyperactivated spermatozoa resulted from a substantial increase in sliding displacement at the midpiece of the spermatozoa (Ohmuro and Ishijima, 2006).
Examinations of the direction of rotational movements of the spermatozoa (Ishijima and Hamaguchi, 1992; Ishijima et al., 1992) and Ca2+ regulation of chirality of the three-dimensional components of the flagellar waves of the demembranated, reactivated sea urchin spermatozoa (Ishijima and Hamaguchi, 1993) revealed that chirality of the three-dimensional components of the planar waves, right-handed or left-handed, was regulated by the intracellular Ca2+ concentrations and determined by the propagation direction of the localized active sliding between the doublet microtubules around the axoneme; namely, the localized active sliding between doublets propagated from the base of the flagellum towards the tip along the axoneme, and at the same time, spread around the axoneme. The localized sliding transmitted in the sequence of doublets 1, 9, 8, etc., formed a right-handed helical wave at high Ca2+ concentrations, whereas the sliding in the sequence of doublets 1, 2, 3, etc., formed a left-handed helical wave at low Ca2+ concentrations (Ishijima and Hamaguchi, 1993). Therefore, more perfect right-handed and left-handed helical waves of the sperm flagella have been expected under certain mechanical conditions.
Careful observations of the effects of increased viscosity on the flagellar waves of sea urchin spermatozoa revealed that planar waves on the sea urchin sperm flagella changed to dextral helical waves when the spermatozoa entered seawater with the increased viscosity of 1500 cP (Woolley and Vernon, 2001). Furthermore, it has been reported that the tunicate Ciona spermatozoa generate both left-handed and right-handed helical waves in the presence of both thiourea and methyl cellulose (Brokaw, 1966). These findings suggest that the spatio-temporal pattern of the localized sliding between the doublet microtubules has mechanically changed during the conversion from planar into helical waves. However, in these reports, the conversions from planar to helical waves only rarely occurred, and accordingly, the condition under which the conversion of planar to helical waves occurred was not clearly specified and, furthermore, the mechanisms which underlie the change in flagellar waves were still not fully understood.
In the present study, the change in flagellar waves of the tunicate and sea urchin spermatozoa was examined using high-speed video microscopy in order to clarify the condition under which the planar waves convert to helical waves and the chirality of the resultant helical waves. The flagellar movement of spermatozoa freely swimming near a lower surface of a coverslip was also examined in order to estimate the intracellular Ca2+ concentrations. A mechanical constraint, suppression of rotation of the sperm head around its long axis by its attachment to a glass surface or by increased viscous resistance, induced helical waves of the tunicate and sea urchin sperm flagella, but the chirality of the helical waves was different between the tunicate and the sea urchin spermatozoa, which was determined by the pattern of localized sliding between the doublet microtubules and possibly by intracellular Ca2+ and cAMP concentrations.
Spermatozoa of a tunicate, Ciona intestinalis, and two sea urchins, Clypeaster japonicus and, Hemicentrotus pulcherrimus, were used in this study. The spermatozoa of Ciona were obtained from the sperm duct after dissecting the animal and oozed out. The obtained undiluted semen was stored at 0°C until used. The semen was diluted 100 times and activated with artificial seawater (ASW) (Jamarin U, Jamarin Laboratory, Osaka, Japan) containing 10 mM theophylline for two minutes (T0179, Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan). The spermatozoa of Clypeaster and Hemicentrotus were obtained by intracoelomic injection of 1 mM acetylcholine (A0084, Tokyo Kasei Kogyo) dissolved in ASW and placed in a plastic culture dish (35 mm×10 mm) then kept in a refrigerator until used. The obtained semen was diluted to approximately 103 times with ASW.
A 5-μl aliquot of the Ciona sperm suspension was added to 50 μl of working solution in an observation chamber (0.3 mm deep, 15 mm wide, and 15 mm long) made of vinyl tape attached to a slide and mixed well with the working solution: ASW or ASW with an increased viscosity using methyl cellulose. These were 2% solutions of methyl cellulose in ASW that gave viscosities of 20–30 cP (M0291, Tokyo Kasei Kogyo), 80–120 cP (M0292, Tokyo Kasei Kogyo), 350–550 cP (M0293, Tokyo Kasei Kogyo), 1500 cP (M0387, Sigma Chemical Co., St. Louis, MO, USA), and 4000 cP (M0512, Sigma Chemical). The transition from planar to helical waves on the sea urchin sperm flagella was induced by the method of Woolley and Vernon (2001). Approximately 50 μl of a working solution was transferred into the observation chamber and a 10-μl aliquot of the sperm suspension was placed next to the working solution, so as to meet each other. To avoid spermatozoa sticking to the glass surface, a coverslip surface was coated with 0.3% BSA (126575, Calbiochem Corp., La Jolla, CA, USA). The movements of the sperm and their flagella were observed and recorded using a Nikon Eclipse E600 microscope (Nikon Corp., Tokyo, Japan) with a phase-contrast condenser and a 20× or 40× BM objective. The obtained images were directly stored on disk by a computer using a high-speed camera (Hi-Dcam, NAC Image Technology, Inc., Tokyo, Japan) at the rate of 250 images per second or a Panasonic CCD video camera (WV-BL 730, Matsushita Communication Industrial Co., Ltd., Yokohama, Japan) at the rate of 60 images per second. The images were recorded for more than two seconds. The shutter speed was 1/500 s.
For the detailed field-by-field analyses, the sperm and their flagellar movements were analyzed using image analysis software (Bohboh, Bohboh Soft, Tokyo, Japan; Ohmuro and Ishijima, 2006). The amplitude of the flagellar waves was defined as the maximum transverse displacement of the flagellum from the wave axis, which was drawn through the center of the beat envelope. The wavelength of the planar waves and pitch of the helical waves were determined by reference to the wave axis. The beat frequency of the flagellar waves were determined from the period required for one complete propagation of the planar waves of the free-swimming spermatozoa or the helical waves of the spermatozoa that firmly attached to the glass surface by their heads. The swimming speed of the free-swimming spermatozoa was determined by measuring the distance between the positions of the sperm head at the beginning and the end of a 0.1 sec. interval.
When Ciona spermatozoa moved near the lower surface of a coverslip that was coated with BSA, the spermatozoa moved in relatively large circular paths with fairly planar waves (Fig. 1A and Fig. 2). The percentage of the spermatozoa yawing clockwise or counterclockwise was nearly identical (clockwise; 46.7±10.5%, n=394). The planar waves of the spermatozoa yawing counterclockwise were compared to those of the spermatozoa yawing clockwise (Fig. 2). The planar waves of these spermatozoa were fairly symmetrical; however, the difference in curvature between these planar waves clearly explained the difference in direction of the yawing motion of the spermatozoa.
 View Details | Fig. 1. Trajectories of the heads of Ciona and Hemicentrotus spermatozoa yawing near the lower surface of a coverslip in seawater. Twenty video fields at intervals of 1/60 have been superimposed to show the direction of the sperm progression and the curvature of their trajectories. (A) The Ciona spermatozoa moved in large circular paths. Four of nine spermatozoa yawed clockwise (arrows). (B) The Hemicentrotus spermatozoa moved in small circular paths. Ten spermatozoa all yawed clockwise (arrows). Scale bar, 20 μm. |
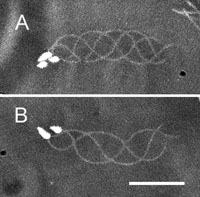 View Details | Fig. 2. Planar waves of Ciona spermatozoa yawing near the lower surface of a coverslip in seawater. Images at intervals of 1/125 s have been superimposed. The spermatozoa yawed counterclockwise (A) and clockwise (B). Scale bar, 20 μm. |
When the Ciona spermatozoa were transferred to an observation chamber, some spermatozoa became attached to the coverslip surface by their heads. Such spermatozoa beat with helical (Fig. 3A) or planar (Fig. 3C) waves, but almost all the spermatozoa beating with the planar waves were constantly interrupted by the three-dimensional irregular waves (Fig. 3B). This change in flagellar wave probably results from obstruction by the glass surface because the spermatozoa attached to the coverslip surface by the tips of their heads beat and rolled continuously with the planar waves (data not shown).
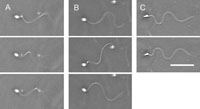 View Details | Fig. 3. Flagellar waves of Ciona spermatozoa attached to a coverslip by their heads in seawater. Most spermatozoa beat with planar waves interrupted with a three-dimensional wave (B), but there were some spermatozoa beat with helical waves (A) and planar waves (C). Scale bar, 20 μm. |
When the Clypeaster and Hemicentrotus spermatozoa moved near the lower surface of a coverslip that was coated with BSA, the spermatozoa moved in small circular paths with fairly planar waves (Fig. 1B). All the Hemicentrotus spermatozoa (100%, n=327) yawed clockwise when observed from above. This result was identical to that of our earlier report (Ishijima and Hamaguchi, 1992) and fundamentally different from that of the Ciona spermatozoa. The sea urchin spermatozoa beat with planar waves regardless of their attachment to the glass surface by their heads, although the movement parameters of their planar waves slightly changed, but their planarity was conserved (Ishijima and Hiramoto, 1994).
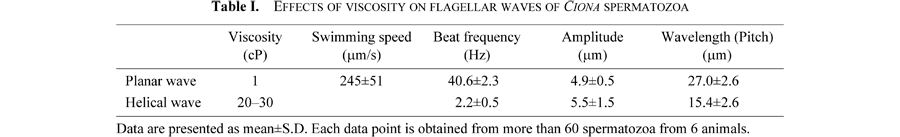
When Ciona spermatozoa moved in seawater with a viscosity of 20–30 cP, the free-swimming spermatozoa had planar or helical waves. On the other hand, when the spermatozoa attached to the coverslip surface by their heads, more than 80% of the flagellar waves were helices, regardless of the manner of their attachment to the glass surface (Fig. 4A). The chirality of the helical waves was left-handed (200 spermatozoa observed) (Fig. 4B, C). Table I presents sample data for motility parameters of the helical waves. In seawater with viscosities higher than 80–120 cP, the attachment of the heads to a coverslip surface was not necessary for inducing the helical waves, even though these waves were restricted to the proximal parts of the flagella and remarkably decreased their amplitude (Fig. 5A). The increase in viscosity of the seawater did not change the fundamental feature of the helical waves (Fig. 5), but the swimming speed gradually decreased with the viscosity; namely, 4.81±0.40 μm/s in 80–120 cP, 3.02±0.36 μm/s in 350–550 cP, 1.86±0.63 μm/s in 1500 cP, and 1.41±0.33 μm/s in 4000 cP, obtained from 10 spermatozoa.
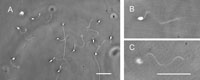 View Details | Fig. 4. Helical waves of Ciona spermatozoa attached to a coverslip by their heads in seawater with viscosity of 20–30 cP. (A) Most Ciona spermatozoa generated helical waves. The helical waves were left-handed as determined by differential focusing; namely, the flagellar parts were focused on the upper focal plane (B) and on the lower focal plane (C). The sperm heads were in focus (B) and out of focus (C). Scale bar, 20 μm. |
 View Details | Fig. 5. Effects of increased viscosity on sperm flagellar waves. Helical waves of Ciona sperm flagella at viscosity of 80–120 cP (A), 350–550 cP (B), 1500 cP (C), and 4000 cP (D). Planar waves of Hemicentrotus sperm flagellum at viscosity of 4000 cP (E). Scale bar, 20 μm. |
When the Hemicentrotus spermatozoa moved in seawater with the increased viscosity, conversion of the planar waves into helical waves could not be observed as well as in ordinary seawater. However, it could be observed sometimes when the Clypeaster spermatozoa entered from ordinary seawater into the seawater with a viscosity of 1500 cP (Fig. 6). The chirality of the helical waves of the Clypeaster spermatozoa was right-handed (30 spermatozoa observed) (Fig. 6B), which was opposite to that of the Ciona spermatozoa. This change in flagellar waves did not occur in the Hemicentrotus spermatozoa under this condition.
 View Details | Fig. 6. Helical waves of Clypeaster spermatozoa. (A) The helical wave of a free-swimming spermatozoon. (B) The helical wave was right-handed as determined by focusing on the upper focal plane. (C) The helical wave of a spermatozoon attached to a coverslip by its head. Scale bar, 20 μm. |
There were several differences in the effects of the increased viscosity on the flagellar waves between the Ciona and the Hemicentrotus spermatozoa. At the viscosity of 4000 cP, the Ciona spermatozoa had a small flagellar wave that was restricted to the proximal region of the flagella (Fig. 5D), whereas the Hemicentrotus spermatozoa had several small waves of increased curvature over the whole flagellum (Fig. 5E). Taking into account the results in the present study, this apparent difference in flagellar waves between the Ciona and the Hemicentrotus spermatozoa in 4000-cP seawater results from the essential difference between the planar and helical waves.
Almost twenty years ago, we predicted the three-dimensional components of the flagellar waves of various spermatozoa based on the examinations of the rotational movements of the spermatozoa around their long axes (Ishijima et al., 1992), because the sperm flagella having a 9+0 axoneme, for example, sperm flagella of the annelid Myzostomum, the arthropod Asian horseshoe crabs, and the fish eel, generate almost complete helical waves (Gibbons et al., 1985; Ishijima et al., 1988, 1994), and the mammalian spermatozoa that have the nine outer dense fibers surrounding the 9+2 axoneme generate fairly large three-dimensional components of flagellar waves (Woolley, 1977; Ishijima et al., 1986). Therefore, the sperm flagella with a 9+2 axoneme have been expected to generate helical waves under certain mechanical conditions (Brokaw, 1966).
When the Ciona spermatozoa are attached to a coverslip surface by their heads in ordinary seawater, almost all the planar waves were interrupted by an irregular three-dimensional wave that was generated at the base of the flagella. When the viscosity of the seawater was increased (20–30 cP), helical waves were predominant and stable, although planar waves have been seen in the free-swimming spermatozoa and the spermatozoa attached to a coverslip surface by the tips of their heads and rolled around their longitudinal axes (data not shown). These results suggest that mechanical constraints, obstruction of rotation of the sperm head around its long axis by attaching its sperm head to the glass surface and viscous resistance increased with a high viscosity medium, induce the conversion of the planar waves into helical waves. The sea urchin spermatozoa from Echinus esculentus (Woolley and Vernon, 2001) and C. japonicus in the present study generated the helical waves at the interface between the ordinary seawater and seawater with the increased viscosity of 1500 cP. The suppression of rotation of the sperm head by the strong resistance due to the increased viscosity seems to also induce the helical waves in these cases.
To generate the helical waves of the sperm flagella, localized active sliding between the doublet microtubules continuously propagates around the axoneme (Ishijima and Hamaguchi, 1993). On the other hand, to generate the planar waves, the localized sliding discontinuously switches from one group of doublets to another. Therefore, it is reasonable to hypothesize that there is some mechanical factor which inhibits the localized active sliding from continuously spreading around the axoneme in the planar waves. One of the most plausible candidates for this role is the central pair complex in the axoneme because of its anisotropic structure and function (Okuno and Hiramoto, 1979; Ishijima and Hiramoto, 1994). Therefore, if the active sliding force increased by obstruction of the sperm head rotation and by a high viscosity medium overcomes the mechanical resistance generated by the central pair complex, then the localized active sliding that does not behave very much in the planar waves would begin to continuously propagate around the axoneme to generate helical waves. On the other hand, we found in the present study that induction of the helical waves in the Ciona spermatozoa was much easier than in the Clypeaster spermatozoa. This difference in the spermatozoa of different species must be caused by changes in the intracellular environment (below).
The chirality of the helical waves of the sea urchin spermatozoa was right-handed, which is identical to the observation by Woolley and Vernon (2001). On the other hand, the Ciona spermatozoa generated left-handed helical waves. There were also significant differences in the percentage of spermatozoa yawing clockwise and the curvature of the circular swimming path made by the sperm head (Fig. 1) between the sea urchin and tunicate spermatozoa. In our earlier papers (Ishijima and Hamaguchi, 1992, 1993; Ishijima et al., 1992), we revealed that the proportion of spermatozoa yawing (or rolling) clockwise to those yawing (or rolling) counterclockwise was related to the Ca2+ concentrations in the cell. Furthermore, the curvature of the circular swimming path or the asymmetrical flagellar waves was shown to correlate with the Ca2+ concentration in the Ciona (Brokaw, 1997) and the sea urchin (Brokaw and Gibbons, 1975; Gibbons, 1982; Ishijima and Hamaguchi, 1993) spermatozoa in the same manner. In fact, the shear angle curves of the flagellar beating of the Ciona spermatozoa at the Ca2+ concentrations of 2×10–8 M and 10–6 M (Fig. 14A, B in Brokaw, 1997) agree closely with those of the sea urchin spermatozoa at the Ca2+ concentrations of less than 10–8 M and 2.4×10–5 M (Fig. 11 and Fig. 13 in Gibbons, 1982), and curvatures of the swimming paths of the Ciona spermatozoa calculated from Fig. 14 A, B (Brokaw, 1997) lie on the line obtained from the sea urchin spermatozoa (Fig. 3 in Ishijima and Hamaguchi, 1993). Based on these relationships, the Ca2+ concentrations of the sea urchin and the Ciona spermatozoa can be estimated to be approximately 10–4 M and 10–8 M, respectively, because a least-squares regression line of the curvature (κ) of the swimming path of the spermatozoa yawing clockwise was κ=0.0065+0.93/c2, where c is the concentration of Ca2+ in logarithm (Fig. 3 in Ishijima and Hamaguchi, 1993) and the average curvatures of the swimming path of the sea urchin and the Ciona spermatozoa were 0.078±0.021 μm–1 (12 spermatozoa measured) and 0.023±0.01 μm–1 (14 spermatozoa measured), respectively. Therefore, the right-handed helical waves in the sea urchin spermatozoa will be caused by high Ca2+ concentrations, whereas the left-handed helical waves in the Ciona spermatozoa will be caused by low Ca2+ concentrations (Ishijima and Hamaguchi, 1993).
Brokaw (1966) reported that the combination of methyl cellulose and thiourea, an inhibitor of metabolic reactions, was necessary to induce the helical waves. Furthermore, both left-handed and right-handed helical waves were found under this condition. In the present study, the Ciona spermatozoa, which were activated with 10 mM theophylline, more easily changed their waves from planar to helical waves and generated only the left-handed helical waves. Therefore, thiourea and cAMP increased by theophylline may determine the occurrence rate of the right-handed helical waves.
Taking into account of the geometry of the helical waves, the right-handed helical waves are generated by the localized sliding which propagates from the base of the flagellum towards the tip along the axoneme, and at the same time, spreads around the axoneme in the sequence of doublets 1, 9, 8, etc., whereas the left-handed helical waves are generated by the localized sliding, which propagates distally along the axoneme, and simultaneously spreads around the axoneme in the sequence of doublets 1, 2, 3, etc. (Ishijima and Hamaguchi, 1993).
The physiological meaning of the change in the chirality of the beating cilia and flagella is unclear (Ishijima et al., 1992). As shown in the present study, sperm flagella have both chiralities. Furthermore, cilia also have both chiralities; namely, the cilia of the Paramecium living in freshwater generate left-handed waves, whereas those of the Mytilus living in seawater generate right-handed waves (Ishijima et al., 1992). There seems to be no difference in a function between these chiralities. However, nodal cilia have a counterclockwise rotation when viewed from the base (Hirokawa et al., 2006), and this rotational movement produces a unidirectional nodal flow, which triggers the L-R axis determination in the mammalian development.
The Ciona adults were provided by Y. Satou (Kyoto University) and M. Yoshida (The University of Tokyo) through the National Bio-Resource Project of the MEXT, Japan.
|