| To whom correspondence should be addressed: Jiri Novotny, Department of Physiology, Faculty of Science, Charles University, Vinicna 7, 128 44 Prague 2, Czech Republic. E-mail: novotnj99@natur.cuni.cz Abbreviations: HEK293, human embryonic kidney cells 293; GPCR, G-protein coupled receptor; CN-PAGE, clear native polyacrylamide gel electrophoresis; TRH-R, thyrotropin releasing hormone receptor; GTP, guanosine triphosphate; GDP, guanosine diphosphate; DTT, dithiotreitol; IAA, iodoacetamide; LM, laurylmaltoside; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate. |
Specific protein-protein interactions play a key role in modulating the structure and function of proteins involved in signal transduction. Due to their ability to couple with other proteins, signal-transducing proteins are able to form high molecular weight complexes and thereby accurately regulate transfer of signals to next components of cell signaling networks (Wrana et al., 1992; Besset et al., 2000; Biondi and Nebreda, 2003; Eisold et al., 2009). However, receptor-mediated transmembrane signaling systems are quite complex and, in changed conditions, interactions of the same protein with other available interacting partners can result in termination of signaling by desensitization and down-regulation or in transduction of novel signals (Vieira et al., 1996; Luttrell and Lefkowitz, 2002). Currently there are two opposite concepts which describe the mechanism of signal transduction after activation of membrane-bound receptors (Vilardaga et al., 2009). In the first, signaling proteins are considered as freely diffusible molecules in the plasma membrane, which interact with each other after random collision (Gilman, 1987). In the second, signaling proteins are viewed as coupled in preassociated complexes (Tsunoda et al., 1997).
GPCRs are typical membrane-bound receptors characterized by seven transmembrane helices and their interaction with G-proteins represents one of the most fundamental biological processes. Ligand binding to a receptor alters the chemical conformation of the receptor molecule that can subsequently activate the α subunit of an appropriate G-protein (Neer, 1995). It is well known that the receptor coupling to G-proteins can be strongly influenced by treatment with specific ligands (agonists or antagonists). A common mechanism of G-protein-mediated signaling suggests that agonist activation of a GPCR promotes the GDP/GTP exchange on the α subunit of its cognate G-protein, which results in dissociation of trimeric G-protein into active α subunit and βγ heterodimer (Bourne, 1997). The generally accepted model supposes that receptor-G-protein coupling can occur only after the receptor activation, when conformation of the receptor is changed by separation of transmembrane helices and a G-protein can bind in this emergent cleft (Gilman, 1987; Cabrera-Vera et al., 2003). However, several recently published studies give support to the model in which receptors and G-proteins or effectors form preassociated complexes. Tight interactions have been described under control conditions between different GPCRs (α1-, α2- and β2-adrenergic, muscarinic acetylcholine M4, dopamine D2, adenosine A1, prostacyclin, bradykinin 2 and chemokine receptors) and trimeric G-proteins in HEK293 and other cell lines (Nobles et al., 2005; Gales et al., 2006; Philip et al., 2007; Lei et al., 2009; Levoye et al., 2009). Formation on such protein complexes appear to be dependent on the level of expression of their components. Nevertheless, it was demonstrated that some receptors interact with G-proteins even at low levels of overexpression and this interaction is not triggered by overexpression. On the contrary, overexpression of a specific receptor may sequester a population of its cognate G-proteins and thus dampen cell stimulation mediated by other GPCRs (Ayoub et al., 2007; Philip et al., 2007). The existence of preassociated receptor-G-protein complexes in resting state is not quite definite, because Hein and co-workers reported that in the case of the α2A-adrenergic receptor and Gi1 protein, there was no direct interaction between these proteins under resting conditions and the coupling of these signaling molecules occured just upon agonist binding to the receptor (Hein et al., 2005). Some observations indicate that GPCRs may form complexes with trimeric G-proteins due to their ability to form dimers or higher-order oligomers, because cytosolic surface of receptor monomers is too small to attach to both G-protein α and βγ subunits (Hamm, 2001; Nobles et al., 2005). Much information concerning receptor-G-protein interactions has been obtained by using fluorescence and bioluminescence resonance energy transfer (FRET and BRET) methods or co-immunoprecipitation (Georganta et al., 2010; Park et al., 2010).
In the present study, we aimed to explore the interactions between TRH receptor (TRH-R) and its cognate Gq/11 protein expressed in HEK293 cells by application of clear-native electrophoresis (CN-PAGE). Using variable experimental conditions, we have succeeded in identification of a putative TRH-R-Gq/11 protein complex with a molecular weight of approximately 140 kDa. Molecular complex formation between the receptor, Gq/11α and Gβ subunits was corroborated by the results of RNAi experiments using siRNA against Gβ1 and Gβ2. Importantly, the stability of this complex was found to be highly sensitive to hormone stimulation.
All materials for tissue culture were supplied by NUNC (Rochester, NY), Gibco (Carlsbad, CA) and PAA (Pasching, Austria). Complete Protease Inhibitor cocktail was from Roche (Basel, Switzerland). Nitrocellulose membrane was purchased from Schleicher-Schuell (Erdmannhausen, Germany), polyvinyldifluoride membrane from Bio-Rad (Hercules, CA), Whatman GF/C filters from Whatman Ltd. (Oxford, UK), acrylamide and bis-acrylamide from Serva (Heidelberg, Germany), LipofectamineTM RNAiMAX from Invitrogen (Carlsbad, CA) and secondary anti-rabbit antibody labeled with horseradish peroxidase from GE Healthcare (Chalfont St. Giles, UK). Preparation and characterization of rabbit polyclonal serum against Gq/11α protein was described previously (Matousek et al., 2004). All other primary antibodies (TRH-R1 sc-11574, G11α sc-394, Gβ sc-378, Gβ1 sc-379, Gβ2 sc-380, β-actin sc-1616), blocking peptides, secondary anti-goat antibody labeled with horseradish peroxidase and siRNA against Gβ1and Gβ2 and control siRNA were from Santa Cruz Biotechnology (Santa Cruz, CA).
HEK293 cells (clone E2 stably expressing the long isoform of the rat TRH receptor (TRH-R1) and clone E2M11 stably expressing both the long isoform of TRH-R1 and the mouse G11α protein) prepared in Dr. G. Milligan’s laboratory (University of Glasgow, U.K.) were obtained by courtesy of Dr. P. Svoboda (Institute of Physiology, Academy of Science of the Czech Republic). HEK293 cells were grown in DMEM supplemented with 10% heat-inactivated newborn calf serum, geneticin (0.8 mg/ml) and hygromycin B (0.2 mg/ml) at 37°C in 5% CO2 humified atmosphere (Svoboda et al., 1996).
Nearly confluent cell cultures grown in 80 cm2 tissue culture flasks (15 flasks per sample) were treated with or without 10 μM TRH for 10 min, 30 min, 1 h, 2 h, 4 h, 8 h or 16 h and subsequently harvested. After collection by centrifugation at 1,800 rpm for 10 min (Hettich Universal 30RF centrifuge), the cells samples were homogenized in 2 ml of chilled homogenization buffer H (750 mM aminocaproic acid, 50 mM Bis-Tris, 0.5 mM EDTA; pH 7.0) supplemented with Complete Protease Inhibitor Cocktail in a Teflon-glass homogenizer at 1,500 rpm for 5 min on ice. The homogenate was centrifuged for 3 min at 1,500 rpm at 4°C in a Hettich centrifuge and samples of the postnuclear supernatant (PNS) were used for fractionation on Percoll density gradient or frozen in liquid nitrogen and stored at –80°C. The resulting PNS was applied on the top of 25% (w/v) PercollR in TMEN buffer (20 mM Tris-HCl, 3 mM MgCl2, 1 mM EDTA, 150 mM NaCl; pH 7.4) and, after centrifugation in a Beckman Ti50.2 rotor at 50,000×g for 15 min (at 4°C), two layers of protein-rich opalescent material were separated. The upper fraction (with lower buoyant density) enriched with plasma membranes was diluted in TMEN buffer and centrifuged in a Beckman Ti 50.2 rotor at 150,000×g for 1 h (at 4°C). Membrane pellet was resuspended in a small volume of TMEN buffer. Samples were then frozen in liquid nitrogen and stored at –80°C.
Samples (50 μg protein) of the postnuclear supernatant or plasma membrane-enriched fraction were solubilized in sample buffer (75 mM Bis-Tris, 750 mM ε-aminocaproic acid, 0.5% Coomassie G-250) containing varying concentrations of different detergents (digitonin, lauryl maltoside, Brij 56, Triton X-100, CHAPS, and SDS) for 30 min on ice or at 37°C in some cases. The insoluble material was removed by centrifugation at 10,000 rpm for 10 min (at 4°C) and subsequent separation of protein complexes was performed on 6-15%, 10–15% or 12–15% linear gradient polyacrylamide slab gels cooled to 2°C using the Mighty Small SE 260 apparatus (GE Healthcare). A discontinuous buffer system using different anode (50 mM Bis-Tris, pH 7.0) and cathode (50 mM tricine and 15 mM Bis-Tris, pH 7.0) buffers was employed in the current CN-PAGE. Electrophoresis was carried out at a constant voltage 50 V for 30 min and then at a constant current 15 mA for 3 h till the Coomassie G-250 dye reached the end of the gel. After electrophoresis, proteins were transferred onto PVDF membrane at a constant voltage 100 V for 2 h. After blocking with 5% fat-free milk in TBS-T buffer (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20; pH 8.0), PVDF membranes were incubated with appropriate primary antibodies overnight and subsequently with horseradish peroxidase-conjugated secondary antibodies for 1 h. The blots were developed with the SuperSignal chemiluminiscent substrate (Pierce) and exposed to Kodak MXB film. Each experiment was carried out at least in triplicate and one representative blot was chosen for illustration.
After the first dimension CN-PAGE, the gel strips were cut and incubated with 1% dithiotreitol (DTT) in equilibration buffer (50 mM Tris-HCl, pH 6.8, 6 M urea, 0.1 mM EDTA, 2% SDS, 30% glycerol, 0.01% bromphenol blue) for 15 min and subsequently with 2.5% iodoacetamide (IAA) in equilibration buffer for 15 min. The strips were placed onto denaturating gels (4% stacking and 10% separating gel). The electrophoresis was performed in Laemmli running buffer (25 mM Tris, 192 mM glycin, 1% (w/v) SDS) at a constant voltage 200 V at room temperature. The separated proteins were transferred onto nitrocellulose membrane and probed with specific primary antibodies. Western blots for each detergent and antibody were replicated at least three times, one representative blot was chosen. The density of some selected spots was quantified by ImageQuantTM TL software (GE Healthcare, Chalfont St. Giles, UK).
E2M11 cells were plated in 6-well plates at 30% confluence 24 h before transfection. Eighty picomoles of each siRNA against Gβ1 and Gβ2 proteins or 160 picomoles of control siRNA were mixed with 5 μl of LipofectamineTM RNAiMAX in 500 μl of DMEM for 30 min at room temperature to produce the liposome-enclosed nucleotides, which were then added to each well containing 2 ml of serum-free medium. After 24-h incubation, cells were grown in serum-supplemented DMEM for next 48 h.
Immunochemical determination of GPCRs might often represent quite a difficult problem, because of lack of selective antibodies or their poor interaction with the receptors. Our initial experiments using standard SDS-PAGE followed by immunoblotting with chosen antibodies confirmed a high quality of primary antibody used for the detection of TRH-R1, Gq/11α and Gβ proteins (Fig. 1A). The major (43 kDa) and minor (40 kDa) signals observed on the blots after incubation with Gq/11α protein antibodies corresponded to the G11α and Gqα protein, respectively. When using G11α protein antibody, only a single band with lower mobility was detected. These observations are in line with earlier reports showing that G11α exhibits lower mobility than Gqα in polyacryalmide gels (Blank et al., 1991; Mullaney et al., 1993). Subsequently, the presumed effect of 10 μM TRH on the level of TRH-R was determined in various time intervals (Fig. 1B). The observed decrease of TRH-R signal after 1 h hormone treatment followed by its increase after prolonged treatment (4–16 h) well corresponds to earlier findings reported by Cook and Hinkle (Cook and Hinkle, 2004). In order to compare the levels of endogenously expressed Gq/11α and TRH-R1 with those in E2M11 cells, these proteins were concurrently assessed in samples of the postnuclear supernatant from rat brain cortex and HEK293 (clone E2M11) (Fig. 1C). Whereas the signal corresponding to Gq/11α was slightly higher in brain sample compared to HEK293 cells (clone E2M11), the level of the expression of TRH-R1 was approximately the same in both samples. Hence, these proteins are not excessively overexpressed in HEK293 cells (clone E2M11) and overexpression artefacts could be excluded.
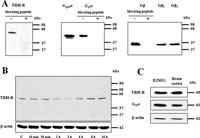 View Details | Fig. 1. Determination of selected GPCRs and G-protein subunits by SDS-PAGE and immunoblotting. For resolution of these proteins, 50 μg of PNS from control HEK293 cells (E2M11) was applied on standard 10% polyacrylamide gels. (A) In the case of availability of blocking peptide, the specificity of antibodies against TRH-R1, G11α and Gβ was proved by incubation with a synthetic peptide (0.5 μg/ml) corresponding to the protein epitope recognized by appropriate antibodies. Immunoblotting with specific antibodies against Gq/11α or G11α revealed two distinct bands corresponding to G11α (43 kDa) or Gqα (40 kDa). (B) The presumed effect of 10 μM TRH on the level of the receptor was determined at different time intervals and β-actin was used as a loading control. (C) The relative expression levels of TRH-R1 and Gq/11α were assessed in samples of PNS from HEK293 cells (E2M11) and rat brain cortex. β-Actin was used as a loading control. |
The postnuclear supernatant from control HEK293 cells (clone E2M11) was solubilized with different detergents (digitonin, lauryl maltoside, Brij 56, Triton X-100, CHAPS and SDS) at variable concentrations. After separation of protein complexes by CN-PAGE, two TRH receptor-containing complexes were identified using specific antibody against TRH-R1 (Fig. 2A). Interestingly, while the larger protein complex detected about 140 kDa was clearly visible after solubilization with all tested detergents, the smaller one (about 80 kDa) was detectable only after solubilization with different concentrations of digitonin, lauryl maltoside, Brij 56 or SDS. Perhaps not surprisingly, signal intensity reflecting the amount of solubilized complexes was dependent on the applied detergent concentration. As can be seen in the figure, some detergents appreciably influenced the complex mobility in the gel. For instance, treatment with lauryl maltoside or SDS resulted in increased or decreased TRH-R complex mobility, respectively. The shift in mobility of amphiphilic molecules induced by detergents and Coomassie G-250 was described previously (Helenius and Simons, 1977; Wittig et al., 2007).
 View Details | Fig. 2. Detergent solubilization and detection of protein complexes containing the TRH receptor. Protein complexes solubilized by different concentrations of digitonin (Dig), lauryl maltoside (LM), Brij 56 (Brij), Triton X-100 (TX-100), CHAPS and SDS from the postnuclear supernatant prepared from control HEK293 cells (E2M11) were separated on 6–15% linear gradient polyacrylamide gels under native conditions and immunoblotted with the indicated antibodies. In this way, two TRH-R complexes in the area about 140 kDa and 80 kDa were detected (A). Gq/11α and Gβ proteins were subsequently identified as the likely components of the larger receptor complex (140 kDa) (B, C). |
In the following experiments, molecular complexes containing the Gq/11 protein were observed under certain conditions. Using specific antibodies against Gq/11α or Gβ, a protein complex containing both these proteins was identified in the region about 140 kDa (Fig. 2B, C). Increased concentration (1%, 2%, and 4%) of lauryl maltoside resulted in separation of two distinct Gq/11α protein complexes in the 140-kDa region. Interestingly, a similar protein distribution pattern was observed also in the case of using sodium cholate as a solubilizing agent but not with any other detergents (data not shown). Our present identification of the TRH-R, Gq/11α and Gβ signals gathered in a band with a molecular weight around 140 kDa suggests the existence of a putative complex comprising these signaling proteins in control HEK293 cells (clone E2M11). Based on the above results, all next solubilization experiments were thus performed using 1% digitonin, LM, Brij 56, Triton X-100 or CHAPS and 0.05% SDS.
It has been previously reported that TRH-Rs can form dimers and substantial portion of these receptors was found to be glycosylated (Zhu et al., 2002). Molecular size of TRH-R dimers composed of glycosylated receptors was about 150 kDa and these dimers were apparently stable at 37°C. The observed size of TRH-R dimers rather decreased after deglycosylation of the receptors by PNGase F (Zhu et al., 2002). Therefore, it was of interest to check whether the TRH-R signal at 140 kDa observed on our immunoblots corresponded to glycosylated receptor dimers. Unfortunately, the methods used for protein deglycosylation are based on using incubation steps at increased temperatures which may cause protein denaturation and dissociation of molecular protein complexes. Results of our preliminary experiments indicated that 30-min incubation of samples at 37°C led to dissociation of observed complexes because no immunochemical signals of the receptor or Gq/11α protein were detectable in this area of immunoblots (Fig. 3). Based on these results it can be assumed that the TRH-R signal determined around 140 kDa under native conditions does not correspond to glycosylated receptor dimers because temperature about 37°C should not influence the stability of these receptor dimers.
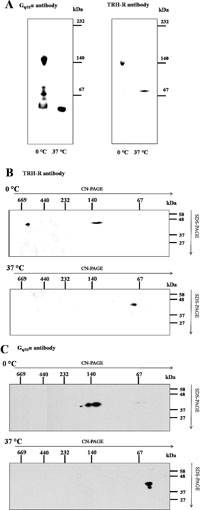 View Details | Fig. 3. Effect of temperature on the stability of the complex in the 140-kDa region. Samples of control cells were solubilized with 1% lauryl maltoside at 0°C or 37°C for 30 min. The solubilized protein complexes were separated by CN-PAGE and immunoblotted with TRH-R or Gq/11α antibody (A). The same antibodies were used for identification of the receptor (B) and Gq/11α (C) in protein complexes resolved by CN/SDS-PAGE. |
It can be speculated that the complexes detected by TRH-R or Gq/11α antibodies around 140 kDa may perhaps represent two distinct complexes which contain either TRH-R or Gq/11α proteins with other interacting partners. Therefore, in the next set of experiments we assessed the relative amount of these complexes containing TRH-R or Gq/11α in samples from E2 and E2M11 cells. The E2M11 cell line has been derived from the E2 cell line and it differs from the latter only by higher expression of the G11α protein. Firstly, samples of PNS from control E2 or E2M11 cells were resolved by SDS-PAGE to compare the levels of TRH-R and Gq/11α in both cell lines. As shown in Fig. 4A, the immunoblot signals of TRH-R were roughly the same in both samples, which corresponds to the assumption that the receptors should be expressed to the same extent in both cell lines. On the other hand, the amount of G11α was much higher in E2M11 than in E2 cells. Subsequently, samples of both cell lines were separated by CN/SDS-PAGE. The immunoblot signal of Gq/11α in the complex detected around 140 kDa was significantly lower in E2 than in E2M11 cells and a similar decrease was observed for TRH-R (Fig. 4B). These results suggest that the band with a higher mobility in gel below the 140 kDa marker corresponds to a single complex containing both TRH-R and Gq/11α. If this complex was composed of two distinct types of TRH-R and Gq/11α complexes, the immunoblot signal of TRH-R must have been similar in samples from both cell lines because the amount of other potential interacting receptor partners did not differ.
 View Details | Fig. 4. Determination of TRH-R and Gq/11α in E2M11 and E2 cells. Samples of PNS (50 μg) from both cell lines were resolved either by SDS-PAGE using standard 10% polyacrylamide gels (A) or separated on 12–15% linear gradient polyacrylamide gels without denaturing reagents in the first dimension and then on 10% gels by SDS-PAGE in the second dimension (B). After electrotransfer onto nitrocellulose membranes, proteins were probed with antibodies to TRH-R and Gq/11α. |
Our subsequent RNAi experiments indicated that transfection of E2M11 cells with siRNA against Gβ1 and Gβ2 caused a marked decrease in the expression of Gβ subunits but did not affect the expression of TRH-R1 and Gq/11α proteins (Fig. 5A). However, the lower expression of Gβ was reflected by substantially reduced levels of Gq/11α, Gβ and TRH-R1 in the putative TRH-R1-Gq/11α complex (Fig. 5B). These results conforms well with the aforementioned observations of altered amount of the TRH-R1-Gq/11 complex in HEK293 cells expressing different level of G11α and support the view that TRH-R1 and Gq/11 constitute common protein complexes.
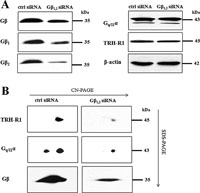 View Details | Fig. 5. Effect of decreased expression of Gβ on the level of the TRH receptor, Gq/11α and the putative TRH-R1-Gq/11 protein complex. E2M11 cells were transfected with control (ctrl) siRNA or siRNAs against Gβ1 and Gβ2 as described in Methods and the expression of Gβ, Gq/11α and TRH-R1 was assessed by immunoblotting (A). β-Actin was used as an internal loading control. The amount of Gq/11α, Gβ and TRH-R1 in the putative TRH-R1-Gq/11 protein complex in samples from control and RNAi-transfected cells was determined by CN/SDS-PAGE and immunobloting (B). |
In the next set of experiments, we focused on the stability of the putative TRH-R-Gq/11α complex. PNS from control or hormone-treated (10 μM TRH; 10 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h) E2M11 cells was solubilized with chosen detergents and protein complexes separated by CN-PAGE. The TRH-R-Gq/11 complex was identified with all the detergents applied for solubilization (Fig. 6A) using TRH-R antibody or with lauryl maltoside (Fig. 6B, C) using Gq/11α or Gβ antibodies. As shown in Fig. 6, the stability of the TRH-R-Gq/11 complex was strongly compromised by TRH treatment. Whereas short-term (30–60 min) hormone treatment resulted in a clear disruption of the complex, prolonged treatment (2–16 h) apparently led to partial complex reassociation in some cases.
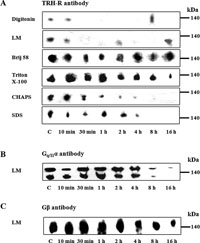 View Details | Fig. 6. Effect of TRH treatment on the stability of the putative TRH-R1-Gq/11 protein complex separated by CN-PAGE. Postnuclear supernatants prepared from control and TRH-treated (10 min, 30 min, 1h, 2 h, 4 h, 8 h, and 16 h) HEK293 cells (E2M11) were solubilized in sample buffer supplemented with 1% digitonin, 1% lauryl maltoside, 1% Brij 56, 1% Triton X-100, 1% CHAPS or 0.05% SDS for the detection of TRH-R (A) and with 1% lauryl maltoside for the detection of Gq/11α (B) or Gβ (C). Proteins were then separated on 6-15% linear gradient polyacrylamide gels without denaturing reagents followed by immunoblotting with the indicated antibodies. |
The structure of proteins in high molecular complexes can be extensively folded, which may prevent binding of antibodies to specific epitopes. Therefore, it appeared advantageous to apply denaturing conditions for more precise estimation of the presumed changes in total quantity of the studied TRH-R complex. Hence, in the following experiments we analyzed the samples of PNS from control and TRH-treated (30 min, 2 h, 4 h, 8 h, 16 h) cells also by CN/SDS-PAGE. The determination of TRH-R was accomplished by immunoblotting with specific receptor antibody subsequently to electrophoretical separation of proteins after sample solubilization with digitonin, lauryl maltoside, Brij 56 and Triton X-100 (Fig. 7A and Fig. 9A). In this setting, a major decrease in chemiluminiscence signal of TRH-R was found after short-term (30 min) treatment with the hormone. As noted above, increased signal intensity after long-term hormone treatment (8–16 h) indicated a partial return of TRH-R into the TRH-R-Gq/11α complex. The Gq/11α and Gβ proteins were detected not only after solubilization with lauryl maltoside as in the case of CN-PAGE, but with digitonin or Triton X-100 as well (Fig. 7A and Fig. 9C). The treatment of HEK293 cells (clone E2M11) with TRH resulted in a gradual time-dependent reduction of both the Gq/11α and Gβ protein signals, the most significant decline being found especially after long-term hormone treatment (8–16 h).
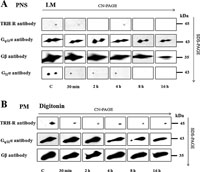 View Details | Fig. 7. Effect of TRH treatment on the stability of TRH-R-Gq/11 protein in complex separated by CN/SDS-PAGE in PNS or PM-enriched fraction. Samples of postnuclear supernatants (A) or PM-enriched fraction (B) prepared from control and TRH-treated (10 min, 30 min, 1h, 2 h, 4 h, 8 h, and 16 h) HEK293 cells (E2M11) were solubilized in sample buffer supplemented with 1% LM or 1% digitonin, respectively. Proteins were separated on 12–15% linear gradient polyacrylamide gels in the first dimension. The excised gel strips incubated with 1% DTT and then with 1% IAA were applied on standard 10% polyacrylamide gels. After SDS-PAGE, dissociated components of the TRH-R-Gq/11 protein complex were detected by immunoblotting with specific antibodies. |
Because GPCRs and trimeric G-proteins predominantly reside in the plasma membrane, it was of interest to find whether TRH receptor and Gq/11 protein complexes could be detectable in the plasma membrane (PM)-enriched fraction isolated by Percoll density gradient centrifugation of the postnuclear supernatant from control HEK293 cells (clone E2M11). After solubilisation with different detergent and resolution of proteins by CN/SDS-PAGE, alterations in the amount of TRH-R1, Gq/11α or Gβ subunits in the TRH-R-Gq/11α complex induced by TRH treatment were similar to those observed in the postnuclear supernatant (Fig. 7B and Fig. 9B, D).
Our preceding experiments showed that the TRH-R-Gq/11α complex dissociated at 37°C, but even under these conditions only immunochemical signal of TRH-R sized about 80 kDa was detectable by CN-PAGE (Fig. 3). This complex broke up into receptor monomers only after CN/SDS-PAGE (Fig. 3B). The supposition that this complex represents TRH-R dimers is supported by previous observations (Zhu et al., 2002) indicating that increased temperature does not influence the stability of TRH-R dimers. It could be expected that this complex composed of TRH-R monomer and some other interacting protein(s) would disintegrate at increased temperatures, as observed in the case of Gq/11 proteins in the TRH-R-Gq/11 complex (Fig. 3). The existence of TRH-R homodimers was previously confirmed using BRET in control HEK293 cells transfected to express this receptor (Kroeger et al., 2001).
Similarly as in the case of the TRH-R-Gq/11 complex, TRH significantly influenced the stability of the presumed homodimer of TRH receptor obtained after solubilization with different detergents of PNS. Clear time-dependent changes were observed in the signal intensity corresponding to TRH-R dimer discerned by CN-PAGE after solubilization with any of five used detergents or by CN/SDS-PAGE after solubilization with lauryl maltoside (Fig. 8). The amount of TRH-R dimer markedly increased after 10 min of TRH treatment and roughly the same signal intensity was detectable also after 30-min, as well as after 1- or 2-h hormone treatment. Prolonged (8–16 h) TRH treatment resulted in a decrease of the TRH-R signal, which can be related to partial reassociation of the TRH-R-Gq/11 complex observed in the same time interval. Using CN/SDS-PAGE, a strong signal of TRH-R dimer was determined in samples after 2- or 4-h hormone treatment (Fig. 8B).
 View Details | Fig. 8. Effect of TRH treatment on the stability of TRH-R1 dimer. Postnuclear supernatants prepared from control and TRH-treated (10 min, 30 min, 1h, 2 h, 4 h, 8 h, and 16 h) HEK293 cells (E2M11) were solubilized in sample buffer supplemented with 1% digitonin, 1% lauryl maltoside, 1% Brij 56, 1% Triton X-100 or 1% CHAPS, and after protein resolution by CN-PAGE (A) or CN/SDS-PAGE (B), the presumed TRH homodimer was determined by immunoblotting with selective receptor antibody. |
 View Details | Fig. 9. Densitometric analysis of the spots corresponding to TRH-R and Gq/11α immunoblot signals in complexes resolved by CN/SDS-PAGE. The density of spots from Fig. 7A, B and other experiments with different detergents was quantified by ImageQuantTM TL software (Amersham Biosciences) and the data expressed as percentage of signal intensity determined in the corresponding control samples (100%). The effect of TRH treatment on the levels of TRH-R and Gq/11α was determined in samples of PNS (A and C) as well as of the PM-enriched fraction (B and D). Data represent the mean±S.E.M. of three separate experiments; *p<0.05 compared with control in each group. |
In many previous studies investigating the interactions between GPCRs and trimeric G-proteins in HEK293 cells by using FRET, BRET or co-immunoprecipitation, usually at least a certain amount of a particular receptor was demonstrated to be in a direct connection with its cognate G-protein, or these signaling molecules were found to localize together in specific microdomains (Martin Shreeve, 2002; Nobles et al., 2005; Gales et al., 2006; Ayoub et al., 2007; Philip et al., 2007; Audet et al., 2008). However, the current evidence favouring the existence of precoupled receptor and G-protein complexes is less than fully conclusive, because in the case of the α2A-adrenergic receptor and Gi1α protein Hein and co-workers failed to see direct connection of these molecules in living cells (Hein et al., 2005).
In the present study, we aimed to detect the presumed complexes of the TRH receptor and its cognate Gq/11 protein in HEK293 cells stably expressing high level of these proteins. After solubilization with different detergents (digitonin, lauryl maltoside, Brij 56, Triton X-100, CHAPS or SDS) of the postnuclear supernatant or the plasma membrane-enriched fraction prepared from control naïve cells, we were able to identify a putative TRH-R-Gq/11 signaling complex using native electrophoresis. Application of this technique allowed us to detect also some other molecular complexes containing either TRH-R or Gq/11α or Gq/11α along with Gβ, but none of them comprised all these proteins together. These observations indicate that TRH-R forms several complexes, which may or may not contain the Gq/11 protein and that the Gq/11α and Gβ subunits can interact with other suitable partners. The levels of some of these detected molecular complexes increased after TRH treatment suggesting a partial relocation of TRH-R or Gq/11 from the complexes detected around 140 kDa to the complexes containing other interacting partners of these proteins. High molecular complexes of THR-R without the Gq/11 protein and complexes of the Gq/11 protein without TRH-R have not been examined in the present study. Substantial attention has been paid primarily to the TRH-R-Gq/11 complex in the 140-kDa region and partly also to the complex in the 80-kDa region apparently representing TRH-R homodimer.
Dimers or oligomers of various GPCRs have been described in several previous studies and they are believed to play an important role in many processes, such as modulation of ligand binding, receptor signaling, trafficking and internalization (Jordan and Devi, 1999; Franco et al., 2000; George et al., 2000; Rocheville et al., 2000; Terrillon and Bouvier, 2004). Dimerization of truncated mutants of several GPCRs was found to occur early in secretory pathway (Grosse et al., 1997; Zhu and Wess, 1998; Karpa et al., 2000; Shioda et al., 2001). TRH-R1 has been demonstrated to form homodimers or heterooligomers with TRH-R2, but not with other GPCRs, such as GnRH-R or β2-adrenergic receptor (Hanyaloglu et al., 2002; Pfleger et al., 2004). Because HEK293 cells do not express endogenous TRH receptors and they were transfected to express TRH-R1, the identified complex in the 80-kDa region most likely corresponds to TRH-R1 homodimer.
To confirm the assumed molecular composition of the putative TRH-R-Gq/11 complex, experiments with different expression levels of Gq/11 protein subunits were conducted. Results of these experiments indicated that TRH-R and Gq/11 represent one complex and are not components of two different molecular complexes.
The presumed receptor-G-protein complexes might be considered as dynamic structures whose stability and composition can apparently be affected by ligand binding (Christopoulos and Kenakin, 2002; Tuteja, 2009). Therefore, we also examined the consequences of short as well as prolonged hormone treatment on the complex stability. For these explorations, HEK293 cells were treated with TRH for different time intervals and the solubilized complexes resolved by CN-PAGE or CN/SDS-PAGE were probed with TRH-R and Gq/11 selective antibody. In this way, a noticeable decrease in the TRH-R-Gq/11 complex signal was observed after 10-min TRH treatment, which was even more pronounced in cell samples treated for 30-min with the hormone. In parallel, a symmetric TRH-R signal increase was noticed in the presumed receptor homodimer after such short-term hormone treatments. Interestingly, it has been reported previously that treatment of cells with TRH can elevate the fraction of TRH receptors behaving as dimers (Angers et al., 2002; Hanyaloglu et al., 2002; Zhu et al., 2002). Our present results conform well to previous experiments on cells expressing PARs-YFP (PAR-1 and PAR-2) and Gi1α RLuc where a strong basal BRET signal determined between these proteins in resting state was markedly diminished after receptor activation with thrombin or peptides mimicking the tethered protein (Ayoub et al., 2007). Whereas there was a distinct signal drop after 10 min, the BRET signal almost totally disappeared after 30 min of agonist treatment.
The observed decrease in the amount of the putative TRH-R-Gq/11 protein complex and the concomitant increase in TRH-R homodimer may indicate that the TRH-R-Gq/11 complex probably dissociates into TRH homodimer and trimeric Gq/11 protein and/or G-protein subunits as a consequence of short-term hormone treatment. These findings support the notion that TRH-R may occur in the form of homodimer in the presumed TRH-R-Gq/11 protein complex. In such a pentameric complex composed of receptor homodimer and trimeric G-protein, one receptor molecule could bind the Gα subunit and the other could contact the Gβγ heterodimer. This description fully corresponds to the concept emphasizing the essential role of receptor dimers in the interactions of GPCRs with G-protein α and βγ subunits (Nobles et al., 2005).
Using CN/SDS-PAGE and immunoblotting, a moderate increase in TRH-R signal was observed in the TRH-R-Gq/11 protein complex after prolonged (8–16 h) hormone treatment, which may reflect a partial reassociation of the complex. Interestingly, this change was detectable only after solubilization of samples of the postnuclear supernatant with Brij 56 or Triton X-100, but not with digitonin or lauryl maltoside. It is well known that solubilizing effectiveness of different detergents obviously depends on detergent-to-protein ratio. This assumption was supported by our results from experiments with different concentrations of detergents used for solubilization of membrane complexes. Therefore, it appeared reasonable to assess the extent of dissociation/reassociation of the putative TRH-R-Gq/11 complex in the course of hormone treatment also in the plasma membrane-enriched fraction. The ratio between the amount of receptor complexes and detergent concentration would be expected to increase in this fraction, compared to that in the postnuclear supernatant. When using digitonin as a detergent under these conditions, a clear diminution of the TRH-R signal was detectable after short-term (up to 2 h) hormone treatment, whereas a certain increase was determined after longer treatment. These observations indicate that short- and long-term hormone treatment can dynamically regulate the stability of the TRH-R-Gq/11 complex. The apparent partial reassociation of this complex after prolonged hormone treatment might appear to be in contradiction with the general opinion that GPCRs usually undergo down-regulation after sustained agonist treatment (Li et al., 2000). However, it is important to note that in the case of THR receptors rather up-regulation of these receptors after hormone treatment was observed in some previous studies (Drmota et al., 1999; Cook and Hinkle, 2004). Therefore, it is quite conceivable that TRH-R can at least partially restore its presumed complex with its cognate Gq/11 protein.
It seems that a significant amount of Gq/11 may form preassociated complexes with their cognate GPCRs, but complex stability is strongly compromised by hormone stimulation. Our finding of a gradually declining signal intensity of Gq/11α and Gβ in both molecular complexes under investigation during hormone treatment well corresponds to previous reports demonstrating that long-term agonist stimulation usually leads to desensitization of the relevant receptors and their signaling pathways, which may be accompanied by a large subcellular redistribution of G-protein subunits (Ransnas et al., 1991; Negishi et al., 1992; Milligan, 1993; Wedegaertner et al., 1996). Similar observations have also been made on HEK293 cells where TRH induced a partial transfer of G11α into the light-vesicular and cytosolic fraction along with its down-regulation (Drmota et al., 1998).
Another interesting finding of the present study was the resolution of two high molecular complexes in the 140 kDa region containing the Gq/11α protein. As shown in Fig. 7, a clear signal of the Gβ subunit was present in both these Gq/11α complexes, but there was detected only a single relevant signal of TRH-R corresponding to the complex with a somewhat higher electrophoretic mobility. This observation may suggest that other endogenous GPCRs found in HEK293 cells (e.g., metabotropic glutamate receptor type 4, ACTH receptor, serotonin 1D receptor) may also form receptor-G-protein complexes of similar size. Indeed, this assumption was corroborated by our finding that Gq/11-coupled α1B-adrenergic receptor (α1B-AR) also occurs in the form of two distinct bands of around 140 kDa on immunoblots (unpublished results). Intriguingly, this signal pattern very closely resembled the one seen for Gq/11α. The precoupling of Gq/11 with different GPCRs may explain the different patterns of TRH-R1, Gq/11α and Gβ observed after long-term TRH treatment when the intensity of Gq/11α and Gβ bands were decreasing in contrast to the TRH-R1 signal.
Several previous studies using BRET and FRET experiments indicated that GPCRs can be precoupled with their cognate G-proteins, especially in the case of receptors linked to Gq/11 and Gs proteins (Gales et al., 2005; Philip et al., 2007; Lei et al., 2009). However, studies preoccupied with Gi-coupled receptors provided rather contradictory results, mainly for α2A-AR (Hein et al., 2005; Gales et al., 2006; Qin et al., 2008). This may be explained by lower affinity of Gi proteins towards their cognate receptors and thus decreased formation of precoupled complexes when compared with Gq/11 or Gs proteins. Our present identification of the TRH-R1-Gq/11 protein complex in samples from cells under resting conditions conforms well to the results of previous studies showing the ability of Gq/11 to form molecular complexes with GPCRs. Our preliminary experiments focused on the presumed molecular complexes of Gi proteins in HEK293 cells revealed only a very faint band corresponding to the putative precoupled α2A-AR-Gi complex (unpublished data), which is line with the above mentioned poor coupling properties of Gi proteins. These findings along with earlier observations support the view that different G-proteins may considerably differ in their ability to form molecular complexes with their cognate receptors.
In conclusion, this is the first report about application of native electrophoresis for successful resolution of a high molecular receptor-G-protein complex. We identified a putative TRH-R-Gq/11 protein complex in various samples of HEK293 cells prepared under different solubilization conditions. The observed dissociation and partial reassociation of the TRH-R-Gq/11 complex during TRH treatment vindicates the notion about high sensitivity of such GPCRs signaling complexes to hormone action. We believe that application of this approach in future studies focused on protein complexes of GPCRs and their signaling partners might shed a new light on complex organization of these transmembrane signaling systems.
We are very grateful to Dr. P. Svoboda for allowing us to use HEK293 cell line expressing TRH receptors and G11α protein. This work was supported by the Grant Agency of Charles University (6409), the Grant Agency of the Czech Republic (305/08/H037) and by Ministry of Education of the Czech Republic (MSM0021620858).
|