2022 Volume 87 Issue 2 Pages 189-193
2022 Volume 87 Issue 2 Pages 189-193
The karyological results of Stevia rebaudiana introduced in the Primorye region (Far-east of Russia) are presented. S. rebaudiana has a chromosome number of 2n=2x=22 with a karyotype formula of 8m+2sm+1st. The chromosome size is very short (1.1–3.1 µm). The relative length of chromosomes varies from 5.1 to 16.16%. The centromere index (CI) varies from 15 to 49%. An interphase nucleus of S. rebaudiana has one or two nucleoli, which suggests the presence of one pair of nucleolar chromosomes. The clonal propagation method provides a high multiplication rate and also preserves these plants in vitro within a cold period. S. rebaudiana has adapted to the new ecological niche on a constant chromosomal number within the genotype reaction norm.
Sweetener plant Stevia rebaudiana Bertoni S. rebaudiana Hemsl. is a member of the Asteraceae, native to Paraguay. It produces a range of high potential low-calorie sweeteners in its leaves. These compounds possess up to 50–450 times the sweetness intensity of sucrose (Chang and Cook 1983, Curi et al. 1986, Pederson 1987). In addition, due to the antibacterial, diuretic, anti-inflammatory, anticarcinogenic, and other beneficial antioxidant properties of steviol glycosides of S. rebaudiana extracts, their field of application is expanding in the food industry and in the additional health treatment of diseases associated with metabolic syndrome and diabetes (Madan et al. 2010, Carrera-Lanestosa et al. 2017, Salar et al. 2020). Thus, the introduction of useful plants into new regions expands the cultural diversity of flora species and provides an additional source of natural foods in the human diet (Ferrazzano et al. 2016).
S. rebaudiana is an annual plant in moderate climate conditions. S. rebaudiana seeds cannot ripen under moderate zone environments. So, clonal propagation is one of the biotechnical methods which not only allows to obtain green cuttings in vitro but also provides division of rhizomes and has other many advantages compared with other propagation methods (Singh and Dwivedi 2014). Clonal propagation by stem-tip culture is an effective method to obtain a genetically uniform Stevia plant population for the production of sweet diterpene glucosides (Tamura et al. 1984). Plant reproduction by microcloning and tissue culture as well as genetic manipulations to identify the most valuable genotypes are often applied to obtain new varieties and polyploid plants (Singh and Dwivedi 2014, Yadav et al. 2011, Dyduch-Sieminska et al. 2020). This method also facilitates the preservation and propagation of useful diploid and polyploidy genotypes (Oliveira et al. 2004). In addition, the clonal propagation of S. rebaudiana may be applied not only to the massive propagation of clonal plants but also to the conservation of these plants in vitro within the winter period.
Seven strains of S. rebaudiana are currently being under introduction and agrotechnical investigation by Primorsky Scientific Research Institute on Agriculture. Plants of one variety were vigorous with large, thick leaves and represented high production results (Romashova et al. 2014). The plant introduction into moderate Far Eastern Primorye zones of Russia from the humid subtropic environment is considered to be similar to its adaptation to extreme conditions. In most cases, plants introduced into other regions lose their genetic stability and acquire a high level of polyploidy and aneuploidy (Mandák et al. 2003, Malakhova et al. 2008). High polyploid cytotypes of some species show better variability and adaptiveness to extreme conditions while grown in the condition of introduction along the borders of its natural range (Shang and Su 1985, Ekimova et al. 2012, Khrolenko et al. 2021). The karyological analysis could be used as evidence of genetic stability or variability of plants cultivated in the condition of introduction. The investigation of chromosomes and nucleolus-forming loci activity in S. rebaudiana based on the karyometric technique is discussed. The present study was undertaken to assess the stability of the chromosome set and the possibility of adaptation of S. rebaudiana to extreme conditions for a further genetic reproduction program, which includes micropropagation of the most valuable genotypes for agriculture.
Regenerated plantlets of Stevia rebaudiana Bertoni cultivated in Biotechnology Lab represent the studied material from Primorsky Scientific Research Institute on Agriculture. Plants were propagated by green cuttings in vitro. Growing plants in vitro were obtained according to the protocol developed by the staff of Russ. State Agr. Univ-Moscow Timiryazev Agr Acad (Kornilova and Kalashnikova 1996) and the Res. Inst. of Sugar Beet (Ilienko 1990), in a nutrient medium Murashige–Skoog (MS) (Murashige and Skoog 1962).
The rooted cuttings were transferred from in vitro conditions to artificial cultivation rooms with control conditions (16/8 h, 4,000 lx, 24°C and high humidity). The significant amount of micropropagated plants (height 10–12 cm) survived the transfer from artificial cultivation room conditions to compacted soil ridges by scheme 35×70 cm (Fig. 1A, B). The survived S. rebaudiana plants grew vigorously when transplanted to the soil after June 20th. The leaves were harvested after September 10 by hand. The uterine plants were dug out and transferred to the greenhouse before October 10 (Fig. 1C). The seedlings are planted onto the plantation annually.

Adventitious roots for the cytogenetic studies were collected from micro plants cultivated in vitro. The materials were prepared and analyzed according to generally accepted techniques for seed plants (Smirnov 1968) with some modifications (Khrolenko et al. 2012). The actively growing root tips were pretreated in 0.2% colchicine solution for 2–3 h at about 22°C and then fixed overnight in Farmer’s fluid (ethanol – 3 parts: acetic acid – 1 part). The material was then treated with 4% iron alum and stained with aceto-hematoxylin. The slides were prepared using the squash technique. The 1.5- to 2-mm-long root tips were cut out separately and placed in a drop of chloral hydrate solution on a microscope slide. The slide preparations were made from the specimen using a method of tissue squashing through the coverslip. The somatic chromosome number was studied in at least 20 well-prepared metaphase plates. Non-overlapping metaphase spreads with full chromosome complement were chosen and examined under a Zeiss Axioskop-40 microscope. Maximum intensity projection mode was used for the z-stack image summary. The slides were photographed by a Zeiss AxioCam (HRs) digital camera using an AxioVision version 4.8.3 program.
The images were selected for karyological analysis and assessment of morphometric characteristics. The chromosomes were classified on the centromere index using the criteria of Levan et al. (1964). For comparison with the data of Frederico et al. (1996), the absolute length of individual chromosome (µm); the relative length; the arm ratio of each chromosome (long arm/short arm) was defined. Each chromosome was cut out separately and chromosome pairs were arranged in decreasing order of their total length media. The length of the chromosomes was measured in micrometers at the images using an AxioVision 4.8.3. The centromere index was calculated as the ratio of the short arm length to the total length of the chromosome and multiplied by 100 for percentage. The relative length is the individual chromosome length divided by the total haploid chromosome length and multiplied by 100 for percentage.
Originally obtained for agricultural purposes, stevia plants were uniform. The divided cells with 2n=22 chromosomes formed a modal class (Fig. 2A). Figure 2 shows 11 chromosome pairs arranged in decreasing order of their total length media. Based on the obtained results, it was concluded that S. rebaudiana is a diploid with a basic chromosome number of x=11.
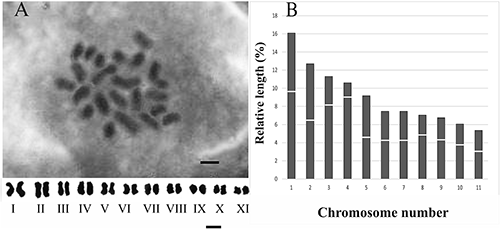
The relative length of chromosomes varied from 5.1 to 16.16% (Fig. 2B, histogram). Based on the position of the centromere, 11 chromosomes pairs in S. rebaudiana belonged to three morphological types: m (I–II, V–VII, IX–XI pairs), sm (III, VIII pairs), and st (IV pair). The centromere index (CI) varied from 15 to 49%.
Counting nucleoli in interphase nuclei of S. rebaudiana has shown that the number of nucleoli in cells varies from one to two (54.79% and 45.21%, respectively) (398 nucleuses studied) (Fig. 3B). Staining with aceto-hematoxylin may not reveal all constrictions in highly condensed metaphase chromosomes, thus distinguishing between centromere and secondary constrictions especially if those structures are closely located at chromosomes was not always possible. It is known that some of the secondary constrictions are nucleolar organizer regions (NORs) and the place of localization of ribosomal DNA. The connection between the nucleoli and the region of the secondary constrictions is shown in Fig. 3A. By the number of nucleoli formed in the telophase of mitosis, the number of nucleolar chromosomes can be determined. Therefore, the presence of one or two nucleoli in interphase nuclei of S. rebaudiana suggests the existence of one pair of nucleolar chromosomes.
The genus Stevia displays a great variation in chromosome number (Yadav et al. 2011). Stevia is defined as diploid and has 11 pairs of chromosomes that are typical for most South American members (Frederico et al. 1996, Li et al. 1982). The cross-species research into six Brazilian species of Stevia, inclusive of S. rebaudiana shows that its karyotypes are similar (Frederico et al. 1996). There are also strains of S. rebaudiana with 2n=33 and 2n=44 (representing triploid and tetraploid cytotypes) (Yadav et al. 2011). Our studies of S. rebaudiana were very similar to the results of Frederico et al. (1996) and Cimpeanu et al. (2006). According to the literature, the normal karyotype of S. rebaudiana consists of 7m+3sm+1st (Frederico et al. 1996), while S. rebaudiana cultivated in Iassy Botanical Garden showed the same chromosome number of 2n=22 with karyotype formula of 7m+3sm+M (Cimpeanu et al. 2006). In the present study, the chromosome arm ratio was found from 1.04 to 5.6 and the karyotype formula of 8m+2sm+1st was obtained. A comparison of our results with Frederico’s data indicates a difference in arm ratios for at least one chromosome pair. Furthermore, the sm and st pairs were located in different positions in karyotypes. Probably, these differences in the karyotypes reflect the ability of S. rebaudiana to respond to changing environments.
Many researchers applied the method of inducing polyploidy to Stevia (Oliveira et al. 2004, Zhuzhzhalova et al. 2013, Zhang et al. 2018). Some authors induced polyploidy in stevia by treating seeds with an aqueous solution of colchicine, using different periods and concentration, to obtain new varieties with higher yields, but the number of the polyploidy varieties was limited (Zhuzhzhalova et al. 2013, Zhang et al. 2018) like in the case of the in vitro-raised plants of S. rebaudiana. At the same time, the resulting microclonal plants were characterized by uniformity, large size, thick leaves, and a high content of stevioside (Romashova et al. 2014), as in the case of S. rebaudiana polyploid plants grown in vitro (Singh and Dwivedi 2014). Therefore, it is logical to assume the possibility of the appearance of polyploidy in S. rebaudiana in a temperate climate as under the conditions of introduction. Thus, being exposed to the same constant influence of the cultivation conditions in vitro, individual plant cells can adapt to them by changing the set of chromosomes. Stevia cells may tend to mixoploidy and aneuploidy.
However, the plant adapted to the new ecological niche on a constant chromosomal number within the normal range of the genotype reaction, 2n=22. The cells exposed to a wider range of stressful conditions of temperate climates in vivo tend to stabilize and maintain their chromosome numbers. In our opinion, when regenerated plantlets of S. rebaudiana transfer from in vitro conditions to the soil they back their genetic stability. The plant organism may restore control over all cells and processes, while changes occur based on the 2n=22 chromosome set at the level of individual genes and chromosomes.
The authors are grateful to the leading engineer of the biotechnological laboratory L. M. Timasheva for providing the Stevia rebaudiana microplants. The research was partially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the state assignment of the Federal scientific center of the East Asia terrestrial biodiversity, Russian Academy of Sciences, project N АААА-А17-117062710094-6.