2018 Volume 65 Issue 1 Pages 63-73
2018 Volume 65 Issue 1 Pages 63-73
Ghrelin functions as a neuroprotective agent and saves neurons from various insults include ischemic injury. However, it remains to be elucidated whether ghrelin protects neuronal cells against ischemic injury-induced excessive autophagy. Autophagy is required for the maintenance of neural stem cell homeostasis. However, regarding autophagic cell death, it is commonly assumed that excessive autophagy leads to self-elimination of mammalian cells. The purpose of this study was to investigate the potential neuroprotection effects of ghrelin from excessive autophagy in adult rat hippocampal neural stem cells (NSCs). Oxygen-Glucose Deprivation (OGD) strongly induces autophagy in adult rat hippocampal NSCs. Ghrelin treatment inhibited OGD-induced cell death of adult rat hippocampal NSCs assessed by cell-counting-kit-8 assay. Ghrelin also suppressed OGD-induced excessive autophagy activity. The protective effect of ghrelin was accompanied by an increased expression levels of Bcl-2, p-62 and decreased expression level of LC3-II, Beclin-1 by Western blot. Furthermore, ghrelin reduced autophagosome formation and number of GFP-LC3 transfected puncta. In conclusion, our data suggest that ghrelin protects adult rat hippocampal NSCs from excessive autophagy in experimental stroke (oxygen-glucose deprivation) model. Regulating autophagic activity may be a potential optimizing target for promoting adult rat hippocampal NSCs based therapy for stroke.
GHRELIN is a 28-amino acid peptide hormone type of growth hormone (GH) secretagogues (GHS) was first isolated from stomach in 1999 by Kojima [1]. Ghrelin with n-octanoyl modification at Ser3 act through the GHS-receptor 1a (GHS-R) which is a G protein-coupled receptor and stimulates the release of GH [2]. Ghrelin also has physiological actions throughout the body, including effects on exocrine and [3], carbohydrate metabolism [4]. Moreover, ghrein shows nonendocrine effects to control differ brain functions, such as protection of neuronal cells [5-8]. In addition, ghrelin improves functional recovery after traumatic by inhibition of apoptotic cell death pathway [9]. The neuroprotective effect of ghrelin be associated with its inhibition of inflammatory activity [10], oxidative stress [5]. However, the exact protective mechanisms of ghrelin in neuroprotection remain to be further clarified.
Autophagy is the cellular process of self-digestion, acting to remove targeted proteins and organelles under various cellular stresses and/or extrinsic stimuli. In fact, dysregulated autophagy has been involved in many human disease states. Also as a bulk cellular degradation pathway, autophagy has been widely reported in its neuroprotective potential [11-13] and autophagy is thought to be a protective mechanism that sustain cell survival under stress conditions. By contrast, altercation exist whether excessive autophagy activity contribute to autophagy induced cell death [14-16], when autophagy is excessively induced, it can result in autophagic cell death, type II programmed cell death (PCD). The majority of the cell death due to cerebral ischemia has been attributed to apoptosis; type I PCD [17]. However there is increasing evidence that excessive autophagy is involved in mediating neuronal cell death during cerebral ischemia [18, 19].
In the present study, in order to study the role of ghrelin in hypoxia/ischemia-induced injury, we investigated the effect of ghrelin on the survival of adult rat hippocampal NSCs exposed to hypoxia. Our results suggest that the neuroprotective properties of ghrelin during hypoxic injury are associated with the inhibition of LC3-II, Beclin-1 or activation of Bcl-2, p-62 through the suppression of excessive autophagosome formation. Moreover, our data also suggest that regulation of autophagy activity is associated with the neuroprotective effect of ghrelin.
Rat ghrelin was obtained from Peptides International (Louisville, KT, USA). D-Lys-3-GHRP-6 was purchased from Bachem (Torrance, CA, USA). NSC expansion media, DMEM/F12, and B27 supplement were obtained from Gibco/Invitrogen. B-27 is an optimized serum substitute developed for low-density plating and long-term viability and growth of central nervous system (CNS) neurons (Brewer et al. 1993). All tissue culture reagents were obtained from Gibco/Invitrogen, and all other reagents were obtained from Sigma unless otherwise indicated.
Adult rat hippocampal NSCs cultures and treatmentsAdult rat hippocampal NSCs were obtained from Chemicon (Catalog No. SCR022, Billerica, MA, USA). These cells are ready-to-use primary NSCs isolated from the hippocampus of adult Fisher 344 rats. They were grown in a NSCs expansion medium containing Dulbecco’s modified Eagles’s medium (DMEM)/F12 medium (Gibco/Invitrogen, Carlsbad, CA) with L-glutamine, B27 supplement, 1× solution of penicillin, streptomycin and fungizone, and basic fibroblast growth factor (bFGF, 20 ng/mL). Tissue culture plastic- or glass wares that were used to culture hippocampal NSCs were coated with poly-L-ornithine (10 μg/mL) and laminin (5 μg/mL). The hippocampal NSCs were maintained at 37°C in a 5% CO2 humidified incubator and passaged once every 3–4 days. To determine whether ghrelin stimulates the proliferation of hippocampal NSCs, cells were treated with ghrelin (100 nM) for 24 h. All experiments were performed three times in duplicate.
Oxygen-Glucose Deprivation (OGD)To induce ischemia, cells were exposed to OGD as previously described, with some modifications [5]. On the day of the experiment, the DMEM/F12 medium was replaced with OGD medium (glucose-free DMEM). Cultures were then placed in a humidified 37°C incubator within the Hypoxic Workstation (Daiki Sciences Co. Ltd. by Ruskinn Technology, Bridgend Mid Glamorgan, UK) containing a gas mixture of 1% O2, 5% CO2, and 94% N2 for 3 h to initiate the ischemic insult. OGD was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cultures were incubated for an additional 24 h under normoxic conditions.
Evaluation of cell proliferation using Cell Counting Kit-8 (CCK-8)Experiments for cell viability were performed in 24-well plates. Cells were seeded at a density of 1 × 105 cells/mL of DMEM/F12 medium containing bFGF. After 24 h, the media were replaced with fresh media with ghrelin (100 nM) or a vehicle and incubated for 24 h. The cell viability was determined using the cck-8 method (Enzo life science Icn. Lausen, Switzerland) according to the manufacturer’s instructions. In brief, 20 μL of cck-8 solution was add into each well (containing 200 μL of medium), and further cultured for 2 h at 37°C. The absorbance of each group at 450 nm was detected (n = 3) using an absorbance microplate reader (Molecular devices LLC., CA, USA) and it was directly proportional to the number of living cells. All experiments were performed three times in triplicate.
Cyto-ID® Green autophagy dyeTo stain cells with Cyto-ID® (Enzo life science Icn. NY, USA) we followed the manufacturer’s protocol with modifications. In brief, cells were first washed with PBS (supplemented with 0.2% B27) and then mixed with either Cyto-ID® solution. The cells were then incubated at 37°C for 30 min in the dark, then washed twice with PBS to remove the free dyes. Cells were fixed in 4% paraformaldehyde for 20 min at room temperature then cells were washed twice with PBS, followed by mounting with fluorescence mounting media (Dako, CA, USA). Images were acquired by the Carl Zeiss LSM 700 Meta confocal microscope and analyzed using Carl Zeiss ZEN image software. Stained samples were analyzed by flow cytometric analysis using FACSCaliburTM (Becton Dickinson Biosciences, CA, USA). All experiments were performed three times in duplicate.
Transmission Electron MicroscopyCells were fixed in 1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7). Fixed monolayers were scraped; post fixed in 2% osmium tetroxide, 100 mM cacodylate buffer; dehydrated with increasing concentrations of ethanol; and gradually infiltrated with Epon resin (Pelco, CA, USA). Thin sections were stained with uranyl acetate and lead citrate and examined using the Olympus EM 900 transmission electron microscope.
Transient transfection and identification of autophagyGFP-tagged LC3 expression vector was utilized to demonstrate the occurrence of autophagy. Adult rat hippocampal NPCs were seeded (9 × 103 cells/well) in 96-well plates and cultured overnight, then GFP-LC3 expression plasmids were transiently transfected into the cells using Lipofectamin 2000 transfection reagent (Invitrogen, CA, USA), according to the manufacturer’s instructions. The cells were subjected to the indicated treatments 24 h after transfection. At the end of the treatments, cells were fixed in 4% paraformaldehyde for 20 min at room temperature then cells were washed twice with PBS, followed by mounting with fluorescence mounting media (Dako, CA, USA). The puncta were observed under the Carl Zeiss LSM 700 Meta confocal microscope and analyzed using Carl Zeiss ZEN image software. A minimum of 100 cells per sample was counted in triplicate for each experiment.
Fluorescence-activated cell sorting (FACS) analysisCell viability was examined by FACS analysis. 1 × 106 cells were collected and fixed with 3.7% PFA. After fixation, the propidium iodide (PI, 50 μg/mL)-staining solution with RNase A (BD Biosciences, CA, USA) was added. After 30 min of incubation, and then the cells were filtered on a nylon mesh filter. Stained samples were analyzed by flow cytometric analysis using FACSCaliburTM (Becton Dickinson Biosciences, CA, USA). All experiments were performed three times in duplicate.
Western blot analysisCells were lysed in a buffer containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 140 mM NaCl, 1% (w/v) Nonidet P-40, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, and 10 μg/mL aprotinin. Cell lysates were separated by 12% SDS-PAGE and electrotransfered onto polyvinylidene difluoride membranes (Bio-Rad, CA, USA). The membranes were soaked in a blocking buffer (1× Tris-buffered saline, 1% BSA, and 1% nonfat dry milk) for 1 h and incubated overnight at 4°C with the primary antibodies against LC3-II, Beclin-1 (Novus Biologicals, Littleton, USA; 1:500) and p-62 (MBL, Nagoya, Japan; 1:500) and Bcl-2 (Cell Signaling, Danvers, MA, USA; 1:1000) and β-actin (Santa Cruz Biotechnology, CA, USA). Blots were developed using a peroxidase-conjugated anti-rabbit IgG and a chemiluminescent detection system (Santa Cruz Biotechnology, CA, USA). The bands were visualized using a ChemicDoc XRS system (Bio-Rad) and quantified using Quantity One imaging software (Bio-Rad, CA, USA). All experiments were performed three times in duplicate.
Statistical analysisData are presented as mean±s.e.m. of three different experiments (each experiment was performed in duplicate). Statistical analysis between groups was performed using one-way ANOVA and the Holm–Sidak method for multiple comparisons using SigmaStat for Windows Version 3.10 (Systat Software, Inc., Point Richmond, CA, USA). p < 0.05 was considered statistically significant.
In previous papers, we reported the protective effect of ghrelin on oxygen-glucose deprivation induced apoptosis [5] and tunicamycin-induced endoplasmic reticulum (ER) stress in neuronal cells [20]. In this study we investigated the effect of ghrelin on hypoxia induced autophagic cell death. After exposure to OGD 1% O2, 5% CO2, and 94% N2 for 3 hours, followed by an additional 24 h under normoxic conditions then cell viability of OGD group was significantly reduced when compared with the normoxic group (Fig. 1A). Cells are stained with PI and analyzed with a flow cytometer (Fig. 1 B). The percentage of viable cells compared with normoxic controls decreased to 75.7% (OGD) and was significantly increased to 93.7% (p < 0.05 vs. OGD-vehicle control) by pretreatment with ghrelin. It has been reported that the adult rat hippocampal NSCs express GHS-R1a [21]. The exposure of cells to the receptor-specific antagonist D-Lys-3-GHRP-6 (10–6 M) abolished the protective effect of ghrelin against OGD insult. Cell viability assessed by cell counting kit-8 assay. We found that ghrelin treated cells showed protective effect against OGD-induced cell death.

Ghrelin protects adult rat hippocampal NSCs against OGD insult
Cells were then placed in a humidified 37°C incubator within the Hypoxic Workstation containing a gas mixture of 1% O2, 5% CO2, and 94% N2 for 3 h to initiate the ischemic insult. OGD was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cells were incubated for an additional 24 h under normoxic conditions. (A) OGD insulted cells were treated with ghrelin (100 nM) for 27 h and the CCK-8 methods was used to analyze the viability of cells. (B) OGD insulted cells were harvested by stained with propidium iodide (PI, 50 μg/mL)-staining solution with RNase A (BD Biosciences). Results were expressed graphically and quantified as the fraction of cells in normoxia, OGD insult treated vehicle or ghrelin using CellQuest software (Beckman Coulter). The data are expressed as the mean ± S.E.M. of three different experiments (each experiment was performed in duplicate). **p < 0.01 vs. the Normoxia or OGD- vehicle.
To further determine the OGD induced autophagy activity in the adult rat hippocampal NSCs, we examined the autophagy activity using Cyto-ID analysis. OGD strongly induces autophagy activity in adult rat hippocampal NSCs (Fig. 2A). It has been reported that hypoxic injury triggered the expression of a number of markers for autophagy markers, such as microtubule-associated protein 1 light chain 3B (LC II), Beclin-1 and p62 in neuronal cells. As shown in Fig. 2B, OGD induced the expression of these markers in the adult rat hippocampal NSCs. LC3-II (16 KD) level was remarkably increased, while LC3-I (18 KD) level unchanged under the hypoxic conditions. The ratio of LC3-II/LC3-I was also increased in an OGD insult time dependent manner. The expression levels of p62 protein, a well-known autophagic substrate, were significantly decreased in the OGD-insulted cells. One of the key mechanisms for control of autophagy is the modulation of the autophagic protein Beclin-1 and the anti-apoptotic Bcl-2 protein expression [22]. In addition, Bcl-2 level was decreased but Beclin-1 level was significantly increased under the hypoxic conditions.

OGD strongly induces autophagy markers in adult rat hippocampal NSCs
Cells were then placed in a humidified 37°C incubator within the Hypoxic Workstation containing a gas mixture of 1% O2, 5% CO2, and 94% N2 for 3 h to initiate the ischemic insult. OGD was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cells were incubated for an additional 0, 2, 4, 24 h under normoxic conditions. (A) To further determine the OGD induced autophagy activity in the adult rat hippocampal NSCs, we examined the autophagy activity using Cyto-ID® assay. The cells were then incubated at 37°C for 30 min in the dark, then washed twice with PBS to remove the free dyes. Cells were fixed in 4% paraformaldehyde for 20 min at room temperature then cells were washed twice with PBS, followed by mounting with fluorescence mounting media. Stained samples were analyzed by flow cytometric analysis using FACSCaliburTM. All experiments were performed three times in duplicate. (B) Protein lysates were prepared and assessed by western blot analysis using anti-LC3-II, anti- Beclin-1, anti-p62, anti-Bcl-2 and anti-β-actin antibodies. The band intensities of LC3-II, Beclin-1, p62, Bcl-2 were normalized to the band intensities of β-actin and they are expressed as relative band intensities. The results are representative of three independent experiments. The data are expressed as the mean ± S.E.M. of three different experiments (each experiment was performed in duplicate). *p < 0.05 , **p < 0.01 vs. the Normoxia.
LC3 is correlated with the autophagosomal membranes that exist in two molecular forms: LC3-I (18 kDa) and LC3-II (16 kDa). The cytoplasmic LC3-I form is converted into the LC3-II form, which is recruited to autophagosomal membranes during autophagy activation. Beclin-1 is one component of a protein complex with PI3K, which has an important role in membrane trafficking and restructuring involved in the formation of autophagosomes [23]. Ghrelin significantly attenuated OGD induced LC3-II (Fig. 3A) and Beclin-1 (Fig. 3B) protein level upregulation. Also, ghrelin treated cells showed an increased OGD induced p62 (Fig. 3C) and Bcl-2 (Fig. 3D) protein level downregulation.
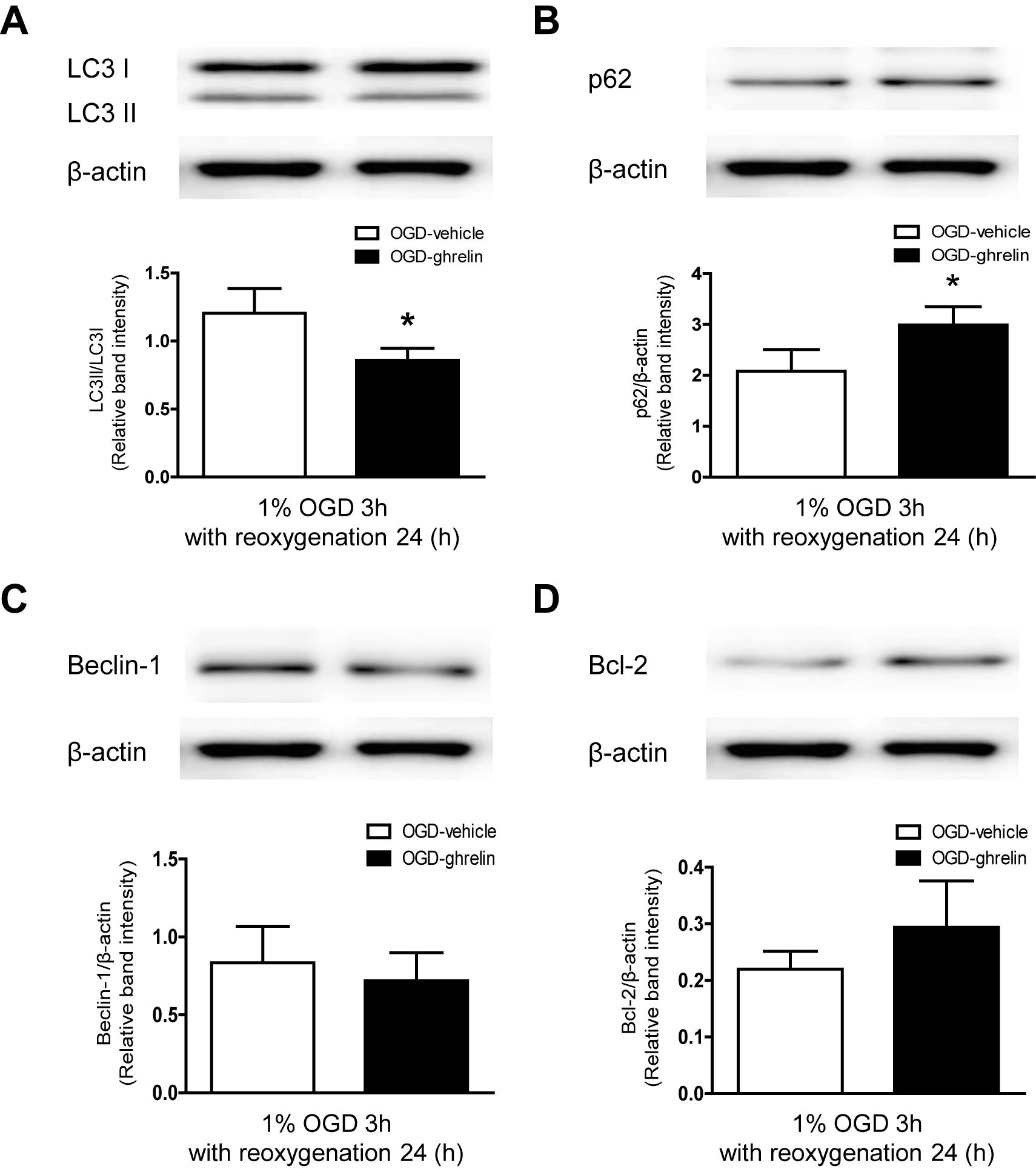
Ghrelin inhibits OGD induced excessive autophagy markers
OGD (1% O2, 5% CO2, and 94% N2 for 3 h) was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cells were incubated for an additional 24 h under normoxic conditions. OGD insulted cells were treated with ghrelin (100 nM) for 27 h. Protein lysates were prepared and assessed by western blot analysis using anti- LC3-II, anti-Beclin-1, anti-p62, anti-Bcl-2 and anti-gβ-actin antibodies. The band intensities of LC3-II, Beclin-1, p62, Bcl-2 were normalized to the band intensities of β-actin and they are expressed as relative band intensities. The results are representative of three independent experiments.
Cyto-ID® assay is based on the usage of a specific dye that Flow Cytometric Analysis or fluorescence microscopy of autophagic activity with Cyto-ID® Green autophagy dye staining in cells. Here, we investigated the effect of ghrelin on OGD induced autophagy activity in adult rat hippocampal NPCs as determined by flow cytometric analysis or confocal microscope. Ghrelin suppressed OGD induced autophagy activity using flow cytometric analysis (Fig. 4A). In addition, ghrelin treated cells showed a dramatically decreased green fluorescence dye using Carl Zeiss LSM 700 Meta confocal microscope (Fig. 4B).
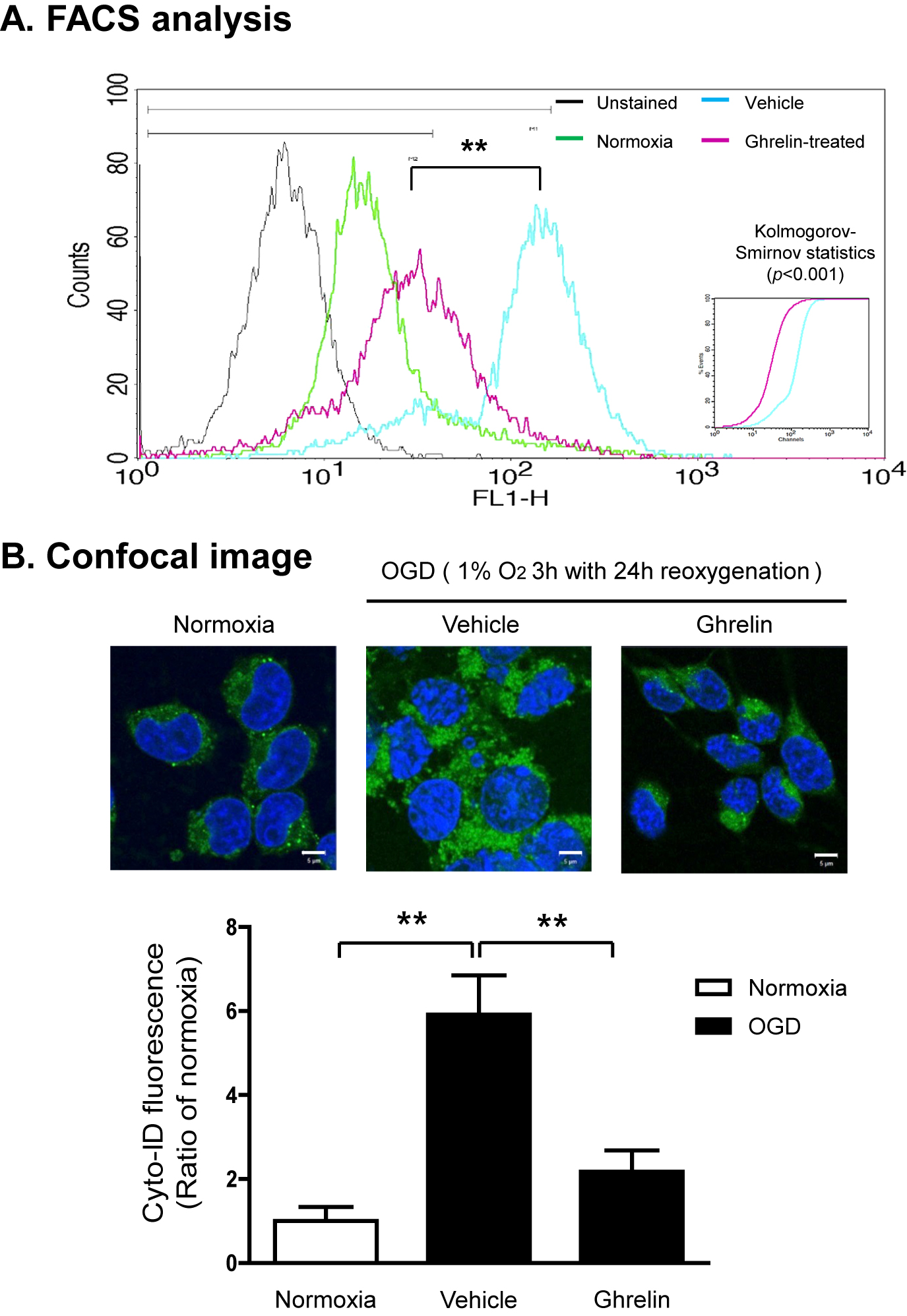
Ghrelin suppresses OGD induced excessive autophagy activity
OGD (1% O2, 5% CO2, and 94% N2 for 3 h) was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cells were incubated for an additional 24 h under normoxic conditions. OGD insulted cells were treated with ghrelin (100 nM) for 27 h. To further determine the OGD induced autophagy activity in the adult rat hippocampal NSCs, we examined the autophagy activity using Cyto-ID® assay. (A) Stained samples were analyzed by flow cytometric analysis using FACSCaliburTM. (B) Stained samples were analyzed by Carl Zeiss LSM 700 Meta confocal microscope. The results are representative of three independent experiments.
Transmitted electron microscopy (TEM) also showed that vacuolar structures containing cytoplasm were clearly encircled by double membrane structures, resembling autophagosomes and autophagolysosome, in OGD-insulted adult rat hippocampal NSCs (Fig. 5A). There was rare or no autophagosome or autophagolysosome in the control. Ghrelin significantly attenuated OGD induced autophagosomes and autophagolysosome formation in adult rat hippocampal NSCs. In addition, ghrelin remarkably reduced number of GFP-LC3 transfected puncta (Fig. 5B).

Ghrelin reduces autophagosome formation and number of GFP-LC3 transfected puncta
OGD (1% O2, 5% CO2, and 94% N2 for 3 h) was terminated by replacing the OGD medium with DMEM/F12 medium containing 4.5 mg/mL glucose, and cells were incubated for an additional 24 h under normoxic conditions. OGD insulted cells were treated with ghrelin (100 nM) for 27 h. (A) Cells were fixed in 1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7). Fixed monolayers were scraped; post fixed in 2% osmium tetroxide, 100 mM cacodylate buffer; dehydrated with increasing concentrations of ethanol; and gradually infiltrated with Epon resin (Pelco). Thin sections were stained with uranyl acetate and lead citrate and examined using the Olympus EM 900 transmission electron microscope. Accumulation of GFP-LC3 transfected puncta occurs upon OGD induced adult hippocampal NPCs. (B) Cells were transfected with GFP-LC3 and after 24 h cells were incubated under hypoxic conditions. At the end of the treatments, cells were fixed in 4% paraformaldehyde for 20 min at room temperature then cells were washed twice with PBS, followed by mounting with fluorescence mounting media. The puncta were observed under the Carl Zeiss LSM 700 Meta confocal microscope and analyzed using Carl Zeiss ZEN image software. A minimum of 100 cells per sample was counted in triplicate for each experiment. The data are expressed as the mean ± S.E.M. of three different experiments (each experiment was performed in duplicate). *p < 0.05 vs. the OGD-vehicle.
In the present study, we demonstrated that ghrelin protects adult hippocampal NSCs from the OGD-induced excessive autophagy cell death via the inhibition of autophagy activity markers and suppression of autophagosomes and autophagolysosome formation. The neuroprotective effect of ghrelin be associated with the inhibition of OGD-induced autophagy activity and the suppression of autophagy marker, such as LC3-II, Beclin-1 and activation of p62 and Bcl-2 during OGD stress responses. In this study, we showed for the first time that ghrelin rescues adult rat hippocampal NSCs from OGD stress-induced excessive autophagy cell death.
Ghrelin is a newly discovered peptide with various functions, including regulation energy metabolism[24], food intake and glucose homeostasis [25], promoting cell proliferation. In addition, ghrelin has been recently been shown to have, including CNS to control neuronal function[26] and various brain injury [27, 28]. Several growth factors, including bFGF and insulin like growth factor have been implicated in neuroprotective effect. The major finding of this study is that the potent neuroprotective agent ghrelin protects the OGD induced cell death of adult rat hippocampal NSCs via regulating autophagic activity.
Autophagy is activated by nutrient starvation and growth factor deprivation when cells are unable to take up external nutrients. In this study, autophagy was observed within 4 hours under hypoxic condition, and the number of autophagosomes further increased during the reperfusion phase (0, 2, 4, 24 h). LC3-II and Beclin-1 are essential components in the process of autophagy. Beclin-1 and LC3-II were reported to be increased during reperfusion in fibrillated mouse hearts [29]. And recently reported to OGD/reoxygenation-induced increased expression of Beclin-1 and LC3-II in hippocampal neurons [30]. LC3, the rat microtubule-associated protein 1A light chain 3, is normally located throughout the cytoplasm but becomes concentrated in the membranes of autophagosomes after cleavage of a few amino acid at the C-terminus (LC3-II) during maturation of autophagy [31]. Beclin-1 was first described as a Bcl-2-interacting protein and was formerly found to promote autophagy [32]. Beclin-1 was expressed in many areas including cerebral cortex, hippocampus, and cerebellum in the rat brain [33]. As expected, we found that after OGD, LC3-II and Beclin-1 expression in adult rat hippocampal NPCs were up regulated.
It is well-known that ghrelin is mainly produced by endocrine cells in the gastric oxyntic mucosa [1]. Given the fact that ghrelin can access the brain via diffusion [34] and the serum ghrelin levels were increased in middle cerebral artery occluded rats [35], these findings suggest that peripherally synthesized ghrelin may contribute to the protection of adult hippocampal NSCs during OGD. Moreover, plasma ghrelin levels are elevated [36] and survival of newborn cells in the dentate gyrus of the hippocampus is increased [37] in calorie-restricted animals. In contrast, there is still much room for debate if ghrelin is synthesized in the brain because the evidences of central production of ghrelin have been inconsistent. Earlier immunocytochemical studies suggested the presence of ghrelin in some brain areas, such as the hypothalamus [38] and the cortex [39]. However, it has been reported that no ghrelin-specific staining is found and ghrelin receptor-expressing neurons do not have adjacent ghrelin immunoreactive terminals in the brain or spinal cord of the rat and mouse [40]. Recently, Cabral et al. [41] provided a comprehensive review of all available data related to the distribution of ghrelin in the CNS and concluded that there are no indisputable and reliable evidences to support the idea that ghrelin is present in the brain at physiologically significant levels.
In conclusion, our data demonstrated that ghrelin protected against OGD induced autophagy cell death which regulated the excessive autophagy in adult rat hippocampal NSCs. Furthermore, the OGD induced excessive autophagy activity downregulation may be responsible for the neuroprotective agent. Taken together, our results for the first time suggested that the protective effect of ghrelin under hypoxic condition was regulated by autophagy. Regulating autophagic activity may be a potential optimizing target for promoting adult hippocampal NSCs based therapy for stroke.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030072) (to S.P.) and (No. 2014R1A1A2058316) (to H.C.)